b Key Lab of Mesoscopic Chemistry MOE, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, China
Since the first successful synthesis of M41S-type mesoporous silicates, mesoporous materials with pore size ranged from 2 to 50 nm have attracted increasing attention for its unique characters and potential applications. Among them, carbonaceous materials with uniform mesoporous structure [1-3] have attracted considerable interest and have long been one of the most popular research areas in materials science for their unique characters of open-accessed structure, uniform pore size, large surface area, chemical inertness and good mechanical stability, offering wide applications in many fields e.g. sorption [4, 5], catalysis, electrochemistry [6-10], etc. To date, soft template method [11-14] involving a co-assembly between amphiphilic surfactant and the precursor by hydrogen bonding or electrostatic interactions is a prevailing approach to prepare mesoporous carbon. Recently, great efforts have been devoted to functionalize the carbon surface or framework with the aim to further enhance/expand the properties and applications. However, to date, functional mesostructured carbon materials [15-17] are mainly obtained via nanocasting method and indirect post functionalization method. In this regard, developing an easy-accessed method towards the synthesis of functionalized mesoporous carbon is of great importance.
Herein, as shown in Fig. 1-left, we developed a simple in situ functionalization strategy to fabricate sulfonic acid group functionalized mesoporous carbon (SMC) by a newly designed evaporation induced self-assembly/carbonization (EISAC) approach. In our strategy, the precursor of sucrose and template (P123) was firstly dissolved in diluted sulfuric acid and then the obtained mixture solution underwent an evaporation induced selfassembly to form a mesophase, in which the precursor of sucrose was partial carbonized by sulfuric acid as the solvents evaporation. The partial carbonized mesophase was further carbonized by calcinations in inert atmosphere to remove the template. After template removal, a carbonaceous material with uniform mesostructures, graphitic pore walls and rich sulfonic acid group was obtained. This EISAC process to form mesoporous carbon possesses a mechanism of nanoblock assembly and is similar to the nanoparticle assembly reported by Stucky, which has been successfully applied to the synthesis of mesoporous metal oxide [18]. Different from nanocasting method [19] which suffers fussy and costly problems, our method is simple and cheap, making it suitable for mass production. Moreover, the soft template method based on organic-organic self-assembly with resins as precursor, presented an excellent soft template method to synthesis carbonaceous materials, this method is limited to that the organic precursor should have crossing-linking and thermosetting feature. Whereas, the current method utilizes the condensation among sucrose molecules under strong acidic conditions for the coassembly of sucrose and surfactant. Cross-linking property among expensive organic resin for FDUs type mesoporous carbon materials is not needed. Investigation on the electrochemical supercapacitive test shows that the resultant SMC achieves a superior electrochemical capacitive performances (216 F/g) to phenolic resin derived mesoporous carbon (OMC, 152 F/g) and commercial activated carbon (AC, 119 F/g).

|
Download:
|
| Figure 1. Schematic illustration (left) towards the synthesis of SMC by EISAC method and TEM (right, inset is the selected area electron diffraction) micrograph of SMC-700. | |
2. Results and discussion
The morphology of the obtained mesoporous carbon synthesized by EISAC method was firstly characterized by transmission electron micrograph (TEM) and shown in Fig. 1-right. The TEM image of SMC-700 reveals the final product possess a wormlike mesoporous structure with the uniform size of ~3.6 nm. The corresponding selected area electron diffraction (SAED) patterns (inset in Fig. 1-right) of SMC-700 shows two bright diffraction rings assigned to the (002) and (101) planes of graphite, indicating that the derived SMC-700 has a graphitic structure. The structure and the crystallinity of the obtained carbon materials were further characterized by X-ray diffraction patterns (Fig. 2). The small angle XRD pattern of SMC-400 in Fig. 2a shows a weak diffraction peaks around 2.0°, indicative of the existence of mesoporous structure. While, the small angle XRD pattern of SMC-700 exhibits a wide peak around 1.5°, illustrating the obtained mesoporous carbon possesses a weak ordered mesostructures after template removal. The wide angle XRD pattern (inset in Fig. 2a) of SMC-400 shows two inconspicuous diffraction peaks around 25° and 45°, showing an amorphous structure. For SMC-700, the wide angle XRD pattern (inset in Fig. 2b) exhibits two obvious diffraction peaks around 25° and 45°, indexed to the (002) and (101) planes of graphitic carbon [20], further confirming the graphitic structure, which is in accord with the result of SAED analysis. The graphitic structure of the SMC-700 was also characterized by Raman spectrum shown in Fig. S1 in Supporting information. The Raman spectrum of SMC-700 shows two peaks around 1350 and 1560 cm-1, ascribed to the 'D-band' and 'G-band' of graphitic carbon.

|
Download:
|
| Figure 2. Small angle XRD and wide angle XRD (inset) patterns of (a) SMC-400; and (b) SMC-700 | |
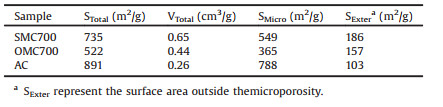
The textural properties of the obtained mesoporous carbon (SMC-700) were confirmed by N2 adsorption-desorption isotherms shown in Fig. 3a. The isotherms of SMC-700 shows a typical type-Ⅳ curves with an obvious condensation step, indicative of the existence of uniform mesopores. As shown in Table 1, the calculated BET surface area and pore volume for SMC-700 are 735 m2/g and 0.65 cm3/g, respectively.

|
Download:
|
| Figure 3. (a) N2 sorption isotherms and pore size distribution curve (PSD inset) of SMC700; (b) TG-DSC curves of the as-made precursor/P123 composite measured under Ar atomosphere. | |
|
|
Table 1 Structural parameters of SMC700, OMC700 and AC. |
The Barrett-Joyner-Halenda (BJH) pore size distribution curve (inset in Fig. 3a) shows a narrow pore size distribution with the mean pore diameter of 3.7 nm, consistent with the TEM measurement. To examine the effect of sulfuric acid towards the template removal during the carbonization, a Thermogravimetric and Differential Scanning Calorimetry analysis (TG-DSC) was done under air flow as shown in Fig. 3b. TG curve of the as-made precursor/P123 composite shows that the mass loss mainly occurs at the temperature of 220 ℃, at which a sharp exothermic peak emerged on the DSC curve, which is attributed to the pyrolysis of the template. The lower decomposition temperature than the one (315 ℃) without the aid of sulfuric acid [21] suggests that the introduction of sulfuric acid in the assembly process favors the decomposition of template under low temperature. The surface chemistry of the obtained SMC-700 is studied by FT-IR spectroscopy (Fig. S2 in Supporting information) in which the bands at 1043cm-1 is attributed tothe-SO3 vibrations [22], suggesting that the surface of the resultant mesoporous carbon is functionalized by sulfonic acid group. The total acid density confirmed by back chemical titration is 0.47mmol/g.
The carbonaceous materials with open-accessed mesopores have many potential applications such as catalysis, sorption, electrochemical supercapacitors [23], etc. Here, the electrochemical supercapacitive (EC) performances of the obtained SMC-700 compared to the phenolic resin derived OMC-700 (522m2/g) fabricated by an EISA method [1] and commercial activated carbon (AC, 891m2/g) were investigated by cyclic voltammetry and galvanostatic charge/discharge curves (GDC). The cyclic voltammograms (CVs) of SMC-700 (Fig. 4a), OMC-700 (Fig. S3a) and AC (Fig. S4a) under various scan rates all display a near rectangulartype curves, indicative of an electric double layered capacitive (EDLC) behavior. Figs. 4b, S3b and S4b show the galvanostatic charge/discharge curves of SMC-700, OMC-700 and AC under various current densities and all the curves exhibits a regular triangular shaped pattern, revealing good columbic efficiency. Fig. 4c shows that the specific capacitance of the three carbons calculated from GDC curves. For SMC-700, the capacitances (Fig. 4c, from 0.5 to 20A/g) calculated from GDC curves (Fig. 4b) are 216, 213, 208, 205, 201 and 198F/g, the values are higher than those of OMC-700 (maximum capacitance of 152F/g) and AC (maximum capacitance of 119F/g) at every current density. The higher capacitance of SMC-700 may be attributed to the sulfonic acid functionalities introduced by sulfonation reaction during carbonization. The sulfonic group favors the enhancement of surface wettability, leading to a larger capacitance.

|
Download:
|
| Figure 4. Electrochemical supercapacitive performance tested in 6.0 mol/L KOH. (a) Cyclic voltammogram of SMC-700; (b) galvanostatic charge/discharge curves of SMC-700 under various current densities (c) specific capacitance of the three carbons under various current densities; (d) cycle stability test of SMC-700 under current density of 10 A/g. | |
In order to evaluate the electrochemical cyclic stability of SMC-700, galvanostatic charge/discharge investigations were performed at a high current density of 10.0A/g. As shown in Fig. 4d, after 5000 cycles, the SMC-700 remains a high specific capacitance of 197F/g and the retention is about 98%, indicative of an excellent electrochemical cycleability. The superior EC performances of SMC-700 to OMC-700 and AC may be attributed to chemical doping and graphitic structure, providing improved electrolyte wettability and conductivity.
3. ConclusionAn evaporation induced self-assembly/carbonization (EISAC) method was developed and applied to the synthesis of sulfonic acid functionalized mesoporous carbon. The obtained mesoporous carbon possesses wormlike mesostructure, uniform size (3.6nm), large surface area (735m2/g), graphitic pore walls, rich sulfonic acid group and achieves a superior electrochemical capacitive performances (216F/g) to phenolic resin derived mesoporous carbon (OMC, 152F/g) and commercial activated carbon (AC, 119F/ g). Moreover, the rich surface acidic group offers the possibilities for the application of the obtained mesoporous carbon as solid acid catalyst on hydrolysis of cellulose, esterification of high fatty acids, biodiesel synthesis and etc.
4. Experimental 4.1. SynthesisTypically, 2.0g P123 (Aldrich) was dissolved in 20.0mL anhydrous ethanol and 0.02mol sucrose was dissolved in 20.0mL diluted sulfuric acid (25wt%). The above solution were mixed to get a homogenous solution with stirring, followed by dipped coating to a Petri dish to undergo a solvent evaporation for 24h at 40 ℃ to obtain a dark film. The obtained film was aged at 100 ℃ for 24h to get as-made product. Template removal was operated by direct calcination with multiple steps in an Ar flow, first at 400 ℃ for 4h with the ramp rate of 1 ℃/min, then to 700 ℃ for 2h with the same ramp rate. The obtained sample was denoted as SMC-T (SMC: sulfonated mesoporous carbon, T: calcination temperature/℃).
4.2. CharacterizationsTransmission electron micrograph (TEM) images were performed on a JEOL 2100 microscope. Power X-ray diffraction (XRD) measurements were carried out using a Philips X'Pert X-ray diffractometer with a Cu Ka radiation (40kV, 40mA). N2 sorption isotherms were measured using a Micromeritics ASAP 2020 analyzer at-196 ℃. All samples were degassed at 300 ℃ for 4h before measurements were taken. The specific surface area is calculated by Brunauer-Emmett-Teller (BET) theory and the pore size distribution is determined by adsorption curve by Barrett-Joyner-Halendar (BJH) method. FTIR spectra were recorded on a VECTORTM 22 spectrometer. Thermogravimetric and Differential Scanning Calorimetry analysis (TG-DSC) was recorded on NETZSCH STA 449C under Ar conditions with the heating rate of 10 ℃/min.
4.3. Electrochemical testElectrochemical supercapacitive performances were measured in a three-electrode configuration with an Hg/HgO reference electrode and a platinum coil counter electrode in 6.0mol/L KOH aqueous electrolyte. The working electrode was prepared by mixing the obtained material, carbon black and polytetrafluorethylene (PTFE) together at a mass ratio of 80:10:10, and dipping the resulting mixture onto nickel foam before being pressed togetherat10.0MPa.The supercapacitive performances of samples were determined by cyclic voltammetry (CV) and galvanostatic charge/discharge curves. The specific capacitance was calculated by the discharge curve by the formula of Cm= IΔt/mΔV (F/g), where I is the current density (A), Δt is the discharge time (s), m is the weight of active material, ΔV is the potential window of discharging (V).
AcknowledgmentsThis work was supported by the Ministry of Science and Technology of China (No. 2009CB623504), the National Science Foundation of China (Nos. 20773062, 20773063, 21173119, and 21273109), the Fundamental Research Funds for the Central Universities.
Appendix A. Supplementary dataSupplementary data associatedwith this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.004.
| [1] | M. J. Xie, H. H. Dong, D. D. Zhang, X. F. Guo, W. P. Ding, Simple synthesis of highly ordered mesoporous carbon by self-assembly of phenol-formaldehyde and block copolymers under designed aqueous basic/acidic conditions, Carbon 49 (2011)2459-2464. |
| [2] | Y.Y. Fang, W. Dai, L. Chen, N Ma. Facile synthesis of ordered mesoporous carbon with finger citron residue as carbon precursor. Mater.Lett. 174 (2016) 246–248. DOI:10.1016/j.matlet.2016.02.086 |
| [3] | M. J. Xie, Y. F. Xia, J. Y. Liang, L. H. Chen, X. F. Guo, Ordered nitrogen doped mesoporous carbon assembled under aqueous acidic conditions and its electrochemical capacitive properties, Microporous Mesoporous Mater. 197 (2014)237-243. |
| [4] | M.X. Liu, X.X. Deng, D.Z. Zhu, et al., Magnetically separated and N, S co-doped mesoporous carbon microspheres for the removal of mercury ions. Chin. Chem.Lett. 27 (2016) 795–800. DOI:10.1016/j.cclet.2016.01.038 |
| [5] | H. Zhang, D.L. Liu, L.L. Zeng, M Li. β-Cyclodextrin assisted one-pot synthesis of mesoporous magnetic Fe3O4@C and their excellent performance for the removal of Cr (Ⅵ) from aqueous solutions. Chin.Chem.Lett. 24 (2013) 341–343. DOI:10.1016/j.cclet.2013.02.007 |
| [6] | X. J. Lu, H. Dou, X. G. Zhang, Mesoporous carbon nanospheres inserting into graphene sheets for flexible supercapacitor film electrode, Mater. Lett. 178 (2016)304-307. |
| [7] | M.J. Xie, K. Fang, Y. Shen, et al., Catalytic hydroxylation enables phenol to efficient assembly of ordered mesoporous carbon under highly acidic conditions. Microporous Mesoporous Mater. 223 (2016) 114–120. DOI:10.1016/j.micromeso.2015.10.042 |
| [8] | J.J. Cai, L.B. Kong, J. Zhang, Y.C. Luo, L Kang. A novel polyaniline/mesoporous carbon nano-composite electrode for asymmetric supercapacitor. Chin.Chem. Lett. 21 (2010) 1509–1512. DOI:10.1016/j.cclet.2010.07.003 |
| [9] | M.X. Liu, L.H. Gan, W. Xiong, et al., Partially graphitic micro-and mesoporous carbon microspheres for supercapacitors. Chin.Chem.Lett. 24 (2013) 1037–1040. DOI:10.1016/j.cclet.2013.07.013 |
| [10] | Z.H. Li, L.Q. Li, H.P. Zhu, H.Y. Liao, H.Y Zhang. Pore size control of porous carbons using novel silica-based copolymer template and their application in supercapacitor. Mater.Lett. 172 (2016) 179–183. DOI:10.1016/j.matlet.2016.02.109 |
| [11] | A.B. Chen, Y.F. Yu, Y.T. Li, Y.Q. Li, M.L Jia. Solid-state grinding synthesis of ordered mesoporous MgO/carbon spheres composites for CO2 capture. Mater. Lett. 164 (2016) 520–523. DOI:10.1016/j.matlet.2015.11.043 |
| [12] | ${referAuthorVo.mingEn} F.Rodríguez, M. Jaroniec, ${referAuthorVo.mingEn} B.L.López, N.P Wickramaratne. Aqueous synthesis of bimodal mesoporous carbons and carbon-silica mesostructures under basic conditions. Microporous Mesoporous Mater. 226 (2016) 299–308. DOI:10.1016/j.micromeso.2016.02.008 |
| [13] | X.Q. Dong, Y. Jiang, W.B. Shan, M.H Zhang. A novel highly ordered mesoporous carbon-based solid acid for synthesis of bisphenol-A. RSC Adv. 6 (2016) 17118–17124. DOI:10.1039/C5RA24966J |
| [14] | Y.R. Liu, J Zhang. Influence of pore symmetries on the supercapacitive performance of mesoporous carbons co-templated by F127 and PDMS-PEO. Microporous Mesoporous Mater. 206 (2015) 81–85. DOI:10.1016/j.micromeso.2014.12.020 |
| [15] | S.L. Ding, S.J. Zheng, M.J. Xie, et al., One-pot synthesis of boron-doped mesoporous carbon with boric acid as a multifunction reagent. Microporous Mesoporous Mater. 142 (2011) 609–613. DOI:10.1016/j.micromeso.2011.01.003 |
| [16] | A.L. Cazetta, A.C. Martins, O. Pezoti, et al., Synthesis and application of N-S-doped mesoporous carbon obtained from nanocasting method using bone char as heteroatom precursor and template. Chem.Eng.J. 300 (2016) 54–63. DOI:10.1016/j.cej.2016.04.124 |
| [17] | G. A. Ferrero, A. B. Fuertes, M. Sevilla, M. M. Titirici, Efficient metal-free N-doped mesoporous carbon catalysts for ORR by a template-free approach, Carbon 106 (2016)179-187. |
| [18] | J. Fan, S.W. Boettcher, G.D Stucky. Nanoparticle assembly of ordered multicomponent mesostructured metal oxides via a versatile sol-gel process. Chem.Mater. 18 (2006) 6391–6396. DOI:10.1021/cm062359d |
| [19] | C.W. Zhang, L.B. Xu, J.F Chen. High loading Pt nanoparticles on ordered mesoporous carbon sphere arrays for highly active methanol electro-oxidation. Chin.Chem.Lett. 27 (2016) 832–836. DOI:10.1016/j.cclet.2016.02.025 |
| [20] | M.J. Xie, J. Yang, J.Y. Liang, X.F. Guo, W.P Ding. In situ hydrothermal deposition as an efficient catalyst supporting method towards low-temperature graphitization of amorphous carbon. Carbon 77 (2014) 215–225. DOI:10.1016/j.carbon.2014.05.024 |
| [21] | X.F. Qian, Y. Wan, Y.L. Wen, et al., Synthesis of ordered mesoporous crystalline carbon-anatase composites with high titania contents. J.Colloid Interface Sci. 328 (2008) 367–373. DOI:10.1016/j.jcis.2008.08.067 |
| [22] | R. Xing, Y.M. Liu, Y. Wang, et al., Active solid acid catalysts prepared by sulfonation of carbonization-controlled mesoporous carbon materials. Microporous Mesoporous Mater. 105 (2007) 41–48. DOI:10.1016/j.micromeso.2007.06.043 |
| [23] | M.J. Xie, S.Y. Duan, Y. Shen, et al., In-situ-grown Mg (OH)2-derived hybrid a-Ni (OH)2 for highly stable supercapacitor. ACS Energy Lett. 1 (2016) 814–819. DOI:10.1021/acsenergylett.6b00258 |
 2017, Vol. 28
2017, Vol. 28