b Departments of Chemistry, Purdue University, West Lafayette, IN 47906, USA
DNA, a magic molecule, not only codes all mysteries of life on our planet, but also exhibits fantastic capability of molecular selfassembly based on its well-known Watson-Crick basepairing, which enables the syntheses of many DNA-based functional materials and devices [1 -3]. In DNA self-assembly, the construction of highly symmetric DNA nanocages, such as a cube and a truncated octahedron synthesized by Dr. Seeman and his colleagues in the early 1990s, is considered as a milestone in its history and indeed starts the era of DNA nanotechnology [4, 5]. Since then, the avenue of utilizing DNA as generic molecules to synthesize new materials with a designable fashion was opened and a large number of one-, two-, and three-dimensional (1D, 2D, and 3D) DNA nanostructures have been engineered through different assembly strategies [6 -11]. In particular, symmetric 3D nanocages with well-defined size and geometry stand as one of the most important categories of self-assembled DNA nanostructures owing to their unique structures and potential applications for multi-purposes [12]. For instance, taking advantage of the sequence specificity and spatial addressability inherited from DNA molecule, the resulting 3D nanostructures with tunable size and geometry have been widely used to direct heteroelements' arrangement, chemical reactions, biomolecular interactions, etc., generating a lot of useful materials and devices, including photonic metamaterials, biosensors with high sensitivity, novel drug delivery vehicles and so on [13 -17]. Meanwhile, to satisfy the increasing interests on their potential applications, it requires the scientists to synthesize more versatile nanostructures with DNA.Thus far, two advantageous and facile strategies: DNA origami and tile-based self-assembly, have been established and widely used to construct well-defined DNA nanocages in a one-pot process [18].Compared to DNA origami that develops rapidly and almost can build any desired architectures, the structure versatility derived from the latter approach is still low. In our previous work, DNA star motifs with varied branch arms have been employed to synthesize a series of highly symmetric DNA polyhedra through mimicking the formation of virus capsids which are assembled by protein units, resulting in the synthesis of DNA tetrahedron, cube, octahedron, icosahedron, etc. [19 -22]. It is worthy noting that these nanocages are typically assembled by a single type of star motif, limiting the structure versatility. Later, we once introduced two different types of sticky-ends into a three-point-star motif and then synthesized a series of DNA triangular prisms with controllable chirality [23]. In this case, all motifs are identical and it is still a single component assembly process. Following that, our group and researchers from other groups continued on putting efforts to increase the structural complexity using multiple types of DNAtiles. Forexample, byintroducing directing DNA star motifs, we can direct the co-existing assembly motifs to form more complicated nanocages which cannot be assembled otherwise [24]. Recently, Yan et al. also introduced flexibility into DNA tile assembly in order to tune the angles between motifs' arm and engineered their sticky-end interaction to control the selfassembly process, by which many complex wireframe nanostructures were successfullysynthesized [25]. Although significant progresses have been achieved, the DNA tile assembly is too young and simple comparedtothe naturallyexistedsystem, wheremultitypes of protein units are usually involved to form functional structures and their interactions are fine-tuned during the assembly [26]. Considering the huge versatility of protein-based structures, it could be expected to further expand the scope of DNA nanocages by engineering the interactions between DNA building blocks and bring prosperity of 3D structure synthesis via tile-based approach.
Toverify this assertion, herein a newbinary self-assembly using three pairs of DNA star motifs with different geometries was introduced. In the new approach, component motifs are no longer dividedintodirecting motifandassembling motif.Incontrast, allof them functionalize as the same and their sticky-ends are particularly designed to modulate motifs' interactions. As illustrated in Fig. 1, we modify our previously used DNA star motifs by changing the sequences of their sticky-ends located at the terminus of each branch, by which two types of DNA motifs were created and equipped with different sticky-ends (Fig. 1). In detail, we designed a three-point-star motif that contains two singlestranded overhangs with the sequence of 5'GGCG 3'/5' TAAC 3', which is refer to as A type sticky-end (represented by concaved rectangular box with light blue color). Meanwhile the other three kinds of star motifs (with 2-, 4-, and 5-fold rotational symmetry) are designed to contain B type of sticky-ends with sequence of 5' CGCC 3'/5' GTTA 3' (represented by brown triangular shape, see details in Supplementary data). Noted that the sequences of both A and B types of sticky-ends are not self-complementary but exactly complementary to each other. As such, three-point-star motif can only associate with other three types of star motifs in the binary assembly system, resulting in the formation of more complicated polyhedral nanostructures. Importantly, to design the new component motif for assembly, only trivial change on the stickyend sequences is needed and the overall structures of new building blocks are kept as the same as our previously used star motifs [19 -22]. Thus, each motif is typically assembled from three different types of strands: a long, repetitive, blue-red strand (Ln; n=2 -5, varied by the branch number of the motif, red represent the free segment in the motif to provide appropriate flexibility), multicopies (n equals to the numbers of branches) of medium green strands (M), and short, peripheral, black strands (S) (Fig. S1 in Supporting information). For example, the 3-point-star motif is composed by L3:M:S with a ratio of 1:3:3. To satisfy the sticky-end design, the medium strands and short strands of 2-, 4-, 5-pointstar motifs are slightly different from 3-point-star motif at the overhangsegments (sticky-ends).Therefore, M' and S' areused and denote to the corresponding medium and short strands used in 2-, 4-, 5-point-star motifs respectively. All sequences and motif structures can be found in Supplementary data.

|
Download:
|
| Figure 1. The schematic drawing of polyhedra assembled by binary DNA star motifs.The DNA point-star motifs used in the binary self-assembly were synthesized independently. 3-point-star motif is designed to contain A type sticky-ends, represented by blue concave rectangles; 2-, 4-, and 5-point-star motifs are designed to contain B type sticky-ends, represented by brown triangular shape. In the final assembled nanocages, 3-point-star motif is assigned to be light cyan color and the other three types of point-star motifs are assigned to be light brown color for a better visualization. | |
2. Results and discussion
We first investigate the simplest case of binary nanocage assembled with 2-and 3-point-star motifs. Although we have once realizedthis binaryassemblywhereextratails were designed on 2-point-star motif to capture gold nanoparticles and then fabricate molecular-like nanoparticle clusters [28], the assembled binary nanostructure itself has never been well characterized. As the 2-point-star motif is a linear structure, theoretically it will not change the overall geometry of the final product that the second motifs can form. Instead, its major function is to elongate the struts and produce an elongated DNA polyhedron. From the established designrules illustratedinprevious studies, a singletype of3-pointstar motif can be assembled into a series of DNA nanocages, such as tetrahedron, dodecahedron, and buckyball structures by changing the concentration and the flexibility of the motifs [19]. With a low concentration (less than 100 nmol/L) and high flexibility (5T hinge of the red segment), four of 3-point-star motifs can assemble into a closed structure and result in the simplest DNA nanocage, a tetrahedron (here refer to as single motif TET, or S-TET). When incorporated with a 2-point-star motif in each strut, it could be expected that the simplest structure assembled by 2-and 3-pointstar motifs is still an elongated TET (refer to as E-TET) with a doubled edge length compared to S-TET. Different from the single type motif self-assembly which is usually conducted in a one-pot process, the binary self-assembly is conducted in a two-step process. First individual 2-and 3-point-star motifs were annealed separately. Briefly, the corresponding DNA strands (2-point-star motifs: L2:M':S' = 1:2:2, and 3-point-star motifs: L3:M:S = 1:3:3) were mixed in a Mg2+-containing, neutral, aqueous buffer and slowly cooled down from 80 ℃ to 25 ℃ over 24 h. Then appropriate ratio of assembled 2-and 3-point-star motifs (6:4) were mixed together and brought the final three-point-star motifs as a concentration of 75 nmol/L. The binary assembly further was carried out by a mild annealing process, where the solution was heated up to 45 ℃ and then slowly cooled down to room temperature. The mild annealing process allowed the optimized recognition between 2-and 3-point-star motifs, resulting in a high yield of elongated DNA tetrahedron.
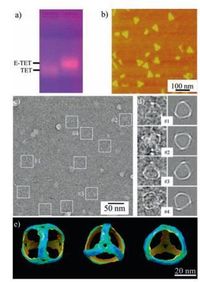
After the self-assembly, we first verify the E-TET formation by 1% agarose gel electrophoresis under a native condition (Fig. 2a).Clearly, a major sharp band with high yield (~ 90%, quantified by image processing software ImageJ) appears on the gel with a lower electrophoretic mobility than that of S-TET, indicating the increased size of the E-TET. Notice that the E-TET is almost double in size compared to S-TET based on the design. In each edge, it contains 84 base parings (bps) compared to 42 bps in S-TET.Therefore, the lower electrophoretic mobility is an indicative of successful formation of elongated TET. In addition, this increased size is also confirmed by DLS measurement where the hydrodynamic radius of E-TET is 36.4 ± 4.1 nm, which is much larger than that of S-TET (~20 nm) as we previously reported [19].
|
Download:
|
| Figure 2. The self-assembly and characterizations of elongated DNA tetrahedron using 2-and 3-point-star motifs. a) 1% agarose gel electrophoresis in the native condition; b) AFM images of the E-TETs, triangular shape particles were observed; c) Cryogenic TEM images of E-TETs, the particles were labeled with white square boxes; d) comparison between 2D projections from model and selected representative raw particles; e) different views of 3D model generated by single particle reconstruction. | |
To directly visualize the structural details of E-TET, different microscopy imaging techniques were applied to characterize its structure. Atomic force microscopy (AFM) reveals that E-TET nanocages are bigger than S-TET with a lateral size of ~39 nm by section analysis (Fig. S3 in Supporting information). Due to the dehydration and strong sample-substrate interaction, the E-TET collapse on the mica surface. However, most of them still kept their morphologies somehow and triangular shapes could be clearly observed in the AFM images (Fig. 2b). The obtained images also confirm that the E-TET particles are quite uniform in size. To elucidate the intrinsic 3D structure of E-TET, the products were further characterized by cryoTEM. Typically, a very thin layer of ETET solution is flash frozen, which would likely to keep the E-TET nanocages in its native conformation and allow visualization of the intact structure. In the raw cryoTEM image (Fig. 2c), the visible particles have tetrahedral shapes and expected size. The observed edges are ~28 nm long, matching very well with the designed model (27.6 nm), assuming that a DNA duplex has a pitch of 0.33 nm/base pair and a diameter of 2 nm. With experimentally observed particles, the structure of E-TET nanocage (Fig. 2e) at a resolution of 3.0 nm is revealed by single particle reconstruction, a technique that has been well-established in structural biology and DNA nanostructure determination [29, 30]. Moreover, 2D projections computed from this structural model match very well with the individual, raw particle images and the class averages generated by raw particles with similar orientations (Fig. 2d and Fig. S4 in Supporting information). As expected, the assembled nanocage has a real tetrahedral symmetry and the structure slightly deforms compared to the model shown in Fig. 1. On its 3D reconstructed map, the struts of E-TET are slightly curved instead of a straight line at present resolution, which is a little bit different from the S-TET. The curved feature of the edge even could be observed in the raw TEM image, where the projections of most of particles have arc fringes. DNA duplex is usually assumed to keep stiff within 50 bps. In the present study, each strut of E-TET has 84 bps. Therefore, it is not surprise that the struts can bend to facilitate the formation of closed structures and minimize the total energy of the E-TET.
The successful synthesis of E-TET demonstrates the sticky-end engineering could enable the construction of new 3D structures via multiple types of DNA motifs. However, the 2-point-star motifs only tune the size of the final structure rather than changing the overall geometry (as shown in E-TET, super vida). To synthesize novel nanostructures with new morphologies, we further investigate the binary assembly using 3-and 4-point-star motifs. When utilizing 3-and 4-point-star motif in the binary self-assembly, as shown in Fig. 1, theoretically rhombic dodecahedron can be synthesized. The R-DOD preparation is similar to the E-TET synthesis, in which both 3-and 4-point-star motif were individually annealed to form right building blocks first. Then 3-and 4-point-star motifs with a ratio of 8:6 were mixed together and annealed in a mild condition. After cooling down to room temperature, again the sample was characterized by gel, DLS, and further visualized by microscopy techniques. Fig. 3a shows a sharp band (with an assembly yield ~55%) appears above the DNA octahedronwhich is assembled by 6 units of four-point-star motifs, indicating the product is much larger than an octahedron. DLS reveals that the new structure has a hydrodynamic diameter of 44.4 nm (Fig. S5 in Supporting information), which is larger than a regular octahedron (27.2 nm in diameter) [22]. The DLS size is close to the theoretical calculation of the R-DOD (47.1 nm), which is 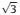

|
Download:
|
| Figure 3. Characterization of self-assembled binary nanocages composed by 3-and 4-point-star motifs. a) 1% agarose gel electrophoresis in the native condition; b) AFM images of the R-DODs; c) Cryogenic TEM images of R-DODs, the particles were labeled with white square boxes; d) comparison between 2D projections from model and selected representative raw particles; e) different views of 3D model generated by single particle reconstruction. | |
In principle, sticky-end engineering can be applied to any other types of DNA point-star motifs, by which more complicated DNA nanocages can be constructed by binary assembly. Regarding the formation of a closed nanocage, all sticky-ends in the point-star motifs will hybridize to each other, which can help to determine the ratio of motifs used during the assembly. When m-and n-point-star motifs with complementary sticky-ends are employed to form binary nanocages, the numbers of each used motif and their ratio can be simply calculated as x times of n:m or xn:xm. As in above 2 + 3 and 3 + 4 binary assemblies, x equals to 2 in both cases. Moreover, an important aspect of closed nanocages is that the assembled convex polyhedra should satisfy with Euler's polyhedron formula:
χ =V -E +F = 2
where V, E, F are the total numbers of vertices, edges, and faces respectively. For the DNA star motif assembly, each motif will form an individual vertice, and each arm contributes a half of the edge during the nanocage formation. In contrast, the formation of nanocage face is more complicated. In our present binary assembly, the simplest case of a face formation is constructing rhombic square by arranging the branches with A and B types of sticky-end in interval, as shown in Fig. 1. As such, each edge will be shared by two faces and four edges will form a closed rhombic square face, giving the F equals to half of E in terms of number. It also could be noticed that the overall symmetry of final product is usually determined by higher branched point-star motif. For instance, in 2 + 3 system, 3-point-star motif dominates the overall geometry and endows the final nanocage with a tetrahedral shape.Meanwhile the rhombic dodecahedron assembled by 3 + 4 point-star motifs contains an octahedral symmetry where 4-point-star motifs occupy octahedral-arranged six vertices. To validate these design principles and further expand the synthesis of more complicated nanocages, 3-and 5-point-star motifs are combined for binary assembly. To predict a final structure to be synthesized, x needs to be calculated to determine the total number of 3-and 5-point-star motifs composed in the cages. As mentioned above, the total vertices, edges and faces are calculated to be V = 8x, E = 15 x and F = 1/2E = 7.5 x respectively. According to the Euler's polyhedron formula, the x is calculated to be 4, which means the final nanocage will be consisted of 12 five-point-star motifs and 20 three-point-star motifs. With this ratio, a rhombic triacontahedron is obtained as shown in Fig. 1. Similarly, the gel electrophoresis reveals that the assembly yield of R-TRI is ~60% and its electrophoretic mobility on agarose gel is much lower than regular icosahedron, indicating significant large size of rhombic triacontahedron (Fig. S8a in Supporting information). AFM image shows the as-synthesized particles are larger than R-DOD, with a diameter ~60 nm in size (Fig. S8b, 8c). Cryo-TEM image (Fig. 4a) illustrates visible and round-shape particles with diameters ~60 nm, consistent with the size observed by AFM. Because of their relatively large size, deformed particles with irregular shapes in the image could be noticed. By selecting particles with good projections, the single particle reconstruction reveals a 3D model of rhombic triacontahedron structure. For R-TRI case, the resolution of reconstructed 3D model is 2.0 nm and its geometry matches our design exactly (Fig. 4c). As expected, its 2D projections and the class averages generated by raw particles with similar orientations are consistent with each other (Fig. 4b).

|
Download:
|
| Figure 4. Characterization of self-assembled binary nanocages composed by 3-and 5-point-star motifs. a) Cryogenic TEM images of rhombic triacontahedra b) comparison between 2D projections from model and typical classaverages from raw particles; c) different views of 3D model generated by single particle reconstruction. | |
3. Conclusion
In summary, we have expanded the DNA nanocage selfassembly towards more complicated structures via sticky-end engineering in a new binary assembly system. Based on the programmable feature of DNA hybridization, two types of pointstar motifs associate with each other upon deliberately designing the sequence at sticky-end, enabling the synthesis of new types of DNA polyhedral structures, including rhombic dodecahedron and rhombic triacontahedron, which have never been reported before.Following the same strategy, we believe that ternary and even quaternary nanocages are also feasible in future with specific sticky-end design. The DNA nanocages synthesized here fully demonstrate that the sticky-end engineering is a powerful way to design new structures in DNA nanotechnology, which paves the avenue to synthesize more complex and functional nanostructures, and realize the mimicry of sophisticated biomolecular machines. For instance, DNA nanocages assembled by binary DNA point-star motifs more closely resemble the real viral species which usually have a quasispherical capsid. Upon immobilizing essential functional groups on such artificial virus-like nanocages, their transfection behaviors may be investigated without the use of real fatal viruses.
4. ExperimentalDNA sequences were adapted from previous works, which were originally designed by a computer program "SEQUIN" [27]. All oligonucleotides were purchased from IDT, Inc. and purified by 10%-20% denaturing polyacrylamide gel electrophoresis (PAGE).
DNA sequences to form different point-star motifs: Central long DNA strand (Ln) sequences for 2-, 3-, 4-and 5-point-star motifs:
Strand L2: 5'-Agg CAC CAT CgT Agg TTT TTC TTg CCA ggC ACC ATC gTA ggT TTT TCT TgC C-3'.
Strand L3: 5'-Agg CAC CAT CgT Agg TTT TTC TTg CCA ggC ACC ATC gTA ggT TTT TCT TgC CAg gCA CCA TCg TAg gTT TTT CTT gCC -3'.
Strand L4: 5'-Agg CAC CAT CgT Agg TTT TTC TTg CCA ggC ACC ATC gTA ggT TTT TCT TgC CAg gCA CCA TCg TAg gTT TTT CTT gCC Agg CAC CAT CgT Agg TTT TTC TTg CC -3'.
Strand L5: 5'-Agg CAC CAT CgT Agg TTT TTC TTg CCA ggC ACC ATC gTA ggT TTT TCT TgC CAg gCA CCA TCg TAg gTT TTT CTT gCC Agg CAC CAT CgT Agg TTT TTC TTg CCA ggC ACC ATC gTA ggT TTT TCT TgC C -3'.
Medium green strands (M) to form 3-point-star motif with A type sticky-end:
Strand M: 5'-Tag CAA CCT gCC Tgg CAA gCC TAC gAT ggA CAC ggT AAT AAC-3'
Short, peripheral, black strand (S) to form 3-point-star motif with A type sticky-end:
Strand S: 5'-TTA CCg TgT ggT TgC Tag gCg-3'
Medium green strands (M') to form 2-, 4-and 5-point-star motif with B type sticky-end:
Strand M': 5'-TAg CAA CCT gCC Tgg CAA gCC TAC gAT ggA CAC ggT AAC gCC-3'
Short, peripheral, black strand (S') to form 2-, 4-, and 5-point -star motif with B type sticky-end:
Strand S': 5'-TTA CCg TgT ggT TgC TAg TTA -3'
The details of sample preparation and characterizations, including sticky-end design, gel electrophoresis, DLS analysis, AFM imaging, cryoTEM imaging, etc. are presented accordingly in the Supplementary data.
AcknowledgmentThis work was financially supported by the National Natural Science Foundation of China (Nos. 21504053, 21673139, and 91527304), the Program of Shanghai Medical Professionals Across Subject Funds (No. YG2016MS74), and the Recruitment Program of Global Experts (No. 15Z127060012). We also thank the NSF and Office of Naval Research for supporting this research. The CryoTEM images were taken at the Purdue Cryo-EM Facility and the Purdue Rosen Center for Advanced Computing (RCAC) provided the computational resource for the 3D reconstructions.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.01.012.
| [1] | N.C Seeman, Nanomaterials based on DNA. Annu.Rev.Biochem. 79 (2010) 65–87. DOI:10.1146/annurev-biochem-060308-102244 |
| [2] | A.V. Pinheiro, D. Han, W.M. Shih, H Yan, Challenges and opportunities for structural DNA nanotechnology. Nat.Nanotechnol. 6 (2011) 763–772. DOI:10.1038/nnano.2011.187 |
| [3] | O.I. Wilner, I Willner, Functionalized DNA nanostructures. Chem.Rev. 112 (2012) 2528–2556. DOI:10.1021/cr200104q |
| [4] | J. Chen, N.C Seeman, Synthesis from DNA of a molecule with the connectivity of a cube. Nature 350 (1991) 631–633. DOI:10.1038/350631a0 |
| [5] | Y.W. Zhang, N.C Seeman, Construction of a DNA-truncated octahedron. J.Am. Chem.Soc. 116 (1994) 1661–1669. DOI:10.1021/ja00084a006 |
| [6] | D.G. Liu, S.H. Park, J.H. Reif, T.H LaBean, DNA nanotubes self-assembled from triple-crossover tiles as templates for conductive nanowires. Proc.Natl.Acad. Sci.U.S.A. 101 (2004) 717–722. DOI:10.1073/pnas.0305860101 |
| [7] | E. Winfree, F.R. Liu, L.A. Wenzler, N.C Seeman, Design and self-assembly of two-dimensional DNA crystals. Nature 394 (1998) 539–544. DOI:10.1038/28998 |
| [8] | P.W.K Rothemund, Folding DNA to create nanoscale shapes and patterns. Nature 440 (2006) 297–302. DOI:10.1038/nature04586 |
| [9] | S.M. Douglas, H. Dietz, T. Liedl, Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 459 (2009) 414–418. DOI:10.1038/nature08016 |
| [10] | Y.G. Ke, L.L. Ong, W.M. Shih, P Yin, Three-dimensional structures self-assembled from DNA bricks. Science 338 (2012) 1177–1183. DOI:10.1126/science.1227268 |
| [11] | F.A. Aldaye, H.F Sleiman, Modular access to structurally switchable 3D discrete DNA assemblies. J.Am.Chem.Soc. 129 (2007) 13376–13377. DOI:10.1021/ja075966q |
| [12] | Y.G Ke, Designer three-dimensional DNA architectures. Curr.Opin.Struct.Biol. 27 (2014) 122–128. DOI:10.1016/j.sbi.2014.07.010 |
| [13] | W.Y. Liu, M. Tagawa, H.L. Xin, Diamond family of nanoparticle superlattices. Science 351 (2016) 582–586. DOI:10.1126/science.aad2080 |
| [14] | Y. Yang, J. Wang, H. Shigematsu, Self-assembly of size-controlled liposomes on DNA nanotemplates. Nat.Chem. 8 (2016) 476–483. DOI:10.1038/nchem.2472 |
| [15] | C. Zhang, C. Tian, F. Guo, DNA-directed three-dimensional protein organization. Angew.Chem.Int.Ed. 51 (2012) 3382–3385. DOI:10.1002/anie.v51.14 |
| [16] | H. Pei, N. Lu, Y.L. Wen, A DNA nanostructure-based biomolecular probe carrier platform for electrochemical biosensing. Adv.Mater. 22 (2010) 4754–4758. DOI:10.1002/adma.v22:42 |
| [17] | S.M. Douglas, I. Bachelet, G.M Church, A logic-gated nanorobot for targeted transport of molecular payloads. Science 335 (2012) 831–834. DOI:10.1126/science.1214081 |
| [18] | Y.G. Ke, P.F Wang, 3D DNA Nanostructure:Methods and Protocols, New York: Humana Press, 2017 . |
| [19] | Y. He, T. Ye, M. Su, Hierarchical self-assembly of DNA into symmetric supramolecular polyhedra. Nature 452 (2008) 198–201. DOI:10.1038/nature06597 |
| [20] | C. Zhang, S.H. Ko, M. Su, Symmetry controls the face geometry of DNA polyhedra. J.Am.Chem.Soc. 131 (2009) 1413–1415. DOI:10.1021/ja809666h |
| [21] | C. Zhang, M. Su, Y. He, Conformational fiexibility facilitates self-assembly of complex DNA nanostructures. Proc.Natl.Acad.Sci.U.S.A. 105 (2008) 10665–10669. DOI:10.1073/pnas.0803841105 |
| [22] | Y. He, M. Su, P.A. Fang, On the chirality of self-assembled DNA octahedra. Angew.Chem.Int.Ed. 49 (2010) 748–751. DOI:10.1002/anie.200904513 |
| [23] | C. Zhang, W.M. Wu, X. Li, Controlling the chirality of DNA nanocages. Angew.Chem.Int.Ed. 51 (2012) 7999–8002. DOI:10.1002/anie.v51.32 |
| [24] | C. Tian, X. Li, Z.Y. Liu, Directed self-assembly of DNA tiles into complex nanocages. Angew.Chem.Int.Ed. 126 (2014) 8179–8182. DOI:10.1002/ange.201400377 |
| [25] | F. Zhang, S.X. Jiang, S.Y. Wu, Complex wireframe DNA origami nanostructures with multi-arm junction vertices. Nat.Nanotechnol. 10 (2015) 779–784. DOI:10.1038/nnano.2015.162 |
| [26] | M.A. Krol, N.H. Olson, J. Tate, RNA-controlled polymorphism in the in vivo assembly of 180-subunit and 120-subunit virions from a single capsid protein. Proc.Natl.Acad.Sci.U.S.A. 96 (1999) 13650–13655. DOI:10.1073/pnas.96.24.13650 |
| [27] | N.C Seeman, De novo design of sequences for nucleic acid structural engineering. J.Biomol.Struct.Dyn. 8 (1990) 573–581. DOI:10.1080/07391102.1990.10507829 |
| [28] | Y.L. Li, Z.Y. Liu, G.M. Yu, W. Jiang, C.D Mao, Self-assembly of molecule-like nanoparticle clusters directed by DNA nanocages. J.Am.Chem.Soc. 137 (2015) 4320–4323. DOI:10.1021/jacs.5b01196 |
| [29] | S.J. Ludtke, P.R. Baldwin, W Chiu, EMAN:semiautomated software for high-resolution single-particle reconstructions. J.Struct.Biol. 128 (1999) 82–97. DOI:10.1006/jsbi.1999.4174 |
| [30] | T.D. Goddard, C.C. Huang, T.E Ferrin, Visualizing density maps with UCSF chimera. J.Struct.Biol. 157 (2007) 281–287. DOI:10.1016/j.jsb.2006.06.010 |
 2017, Vol. 28
2017, Vol. 28 


