b Chemistry Department, Polymer Lab.109, Faculty of Science, Assiut University, PB 71516, Assiut, Egypt
Specific interactions between proteins, DNA, peptides and carbohydrates, including hormone-receptor, antibody-antigen, or protease-inhibitor complexes, play a central function in all biological processes in living organisms, which are based on the molecular recognition [1]. Particularly, specific protein recognitions play an important role in cellular processes including cell-cell recognition, cellular signal transfer, gene expression, immune response, tumor metastasis and apoptosis [2]. Therefore, direct identification and regulation of proteins is an important component of disease diagnosis, bioseparation, and biosensing [3, 4]. Recently, smart biomimetic polymeric materials have attracted considerable interest for selective protein capture including molecularly imprinted polymers (MIPs) [5, 6], nanogels [7], surface materials [8, 9] and nanoparticles [10, 11]. Compared to these methods, utilizing individual smart polymer molecule may be a promising and effective way to recognize specific proteins because of its intelligent behavior and stable structures/morphologies. For example, Hoffman and Stayton have reported site-specific bioconjugates with poly(N-isopropylacrylamide) for phase separations of enzyme and control of the ligand-protein receptor recognition process [12]. However dendritic polymers may have more advantages over their liner counterparts because of their architectural characteristics. In addition, external stimuli including temperature, pH and/or light can regulate dendritic stimuli-responsive polymers in targeting specific proteins, which can control the activity of enzyme or protein and drug or gene delivery in a simple fashion. The dendritic smart polymer-based recognition systems for proteins may find applications in medicine and biotechnology [12].
It is important for polymers to be able to combine within their chains a ligand or an antibody to allow for the recognition and combination with target center or antigen. One popular example of such idea is combination of biotin and avidin. Avidin is a tetrameric glycoprotein enzyme found in egg whites and also its dimeric members can be found in some bacteria [13]. The capture of small molecules like biotin (vitamin B7, vitamin H) by avidin occurs with high degree of affinity and specifity, and this strong specific binding depends on the dissociation constant of avidin (KD ≈ 10-15 mol/L), which makes it one of the strongest known non-covalent bonds [14]. The bond formation between biotin and avidin is very rapid, and once formed, is normally unaffected by extremes of pH, temperature, organic solvents and other denaturing agents [14]. Among all the above mentioned, biotin-avidin system gets wide applications as fluorescence-activated cell sorting (FACS) [15], enzyme linked immunosorbent assay (ELISA) [16], cell-surface labeling [17], affinity purification [18] and electromobility shift assays (EMSA) [19].
Temperature response is one of the most attractive stimuli-responsive properties. Thus, thermoresponsive polymers have drawn significant interest and been utilized in biological/ biomedical science and biotechnology areas [20]. These smart polymers could change significantly their properties in response to temperature in aqueous solutions, which show lower critical solution temperatures (Tcps). Combining structure characteristics from cylindrical dendronized polymers and excellent thermoresponsive properties from dendritic oligoethylene glycols (OEGs), thermoresponsive dendronized polymers have been proved by our group to be promising candidates for bio-related applications.Recently, we have reported a series of dendronized polymethacrylates pendanted with OEG-based dendrons [21]. These polymers are promising candidates as thermoresponsive materials because of their unprecedented thermoresponsive properties: sharp phase transitions, small hysteresis, easily tunable phase transition temperatures (Tcps), as well as excellent biocompatibility. In addition, OEG-based dendron generations play a vital role on encapsulations of guest molecules during temperature-induced process due to the architecture effects [22]. It is well-known that polyethylene glycol (PEG) and oligo (ethylene glycol) (OEG) show excellent performance to reduce nonspecific protein adsorption on surfaces because of steric repulsion and excluded-volume effects [23]. Thus, OEG-based thermoresponsive dendronized polymers' analogues are supposed to show promising applications in biomaterial applications in tissue engineering and site-specific drug delivery [24]. Motivated by the unprecedented characteristics of these thermoresponsive dendronized copolymers, we here report on preparation of first (PG1) and second generation (PG2) of OEG-based dendronized copolymers carrying biotin moieties (Fig. 1), and investigation of their protein recognition through biotin-avidin interactions. The structural effects on tunable recognition ability to proteins between the biotinylated OEGbased copolymers PG1 and PG2 were compared by switching temperatures above or below their Tcps.

|
Download:
|
| Figure 1. Molecular structures of the ethoxyl-terminated dendronized copolymers PG1-Bio and PG2-Bio reported in the present work. Nomenclature: P represents copolymer; G1 and G2 represent the first (G1) and second generation (G2) of dendrons, respectively; Bio represents biotin. | |
2. Results and discussion 2.1. Synthesis of monomer and copolymers
Biotinylated monomer 6 was prepared in six steps as outlined in Scheme 1. First, the OEG amine 3 was prepared in three steps from triethylene glycol (TEG) according to procedures reported in literature [25]. By reaction of biotin with pentaflurophenol, biotin derivative 4 was obtained in a good yield, which was used directly to react with the amino compound 3 to yield biotin derivative 5. Reaction with methacrylic acid (MAA) in presence of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC·HCl) and N, N-dimethylaminopyridine (DMAP) afforded the novel biotin monomer 6 in a high yield of 85%. Ethoxyl-terminated OEG-dendrons were selected as their corresponding dendronized polymers show Tcps close to the physiological temperature. In order to take full advantage of the characteristic bulky structures, two new OEG-based thermoresponsive dendronized copolymers carrying biotin moieties, PG1-Bio and PG2-Bio, were prepared through free radical copolymerization of compound 6 with MG1 and MG2, respectively, with DMF as solvent and azobis (isobutyronitrile) (AIBN) as initiator. For both cases, the polymerization media became viscous after 45 min, which is an indication of fast polymerization kinetics. So, the polymerization processes were stopped after 4-5 h in order to avoid the possible gelation. Both PG1-Bio and PG2-Bio were purified by column chromatography using DCM as eluent in yields of 66% and 52%, respectively. All monomer and polymers were characterized using 1H NMR spectroscopy (see Supporting information). The molecular weights of these two copolymers were determined using gel permeation chromatography with THF as eluent, and the results are summarized in Table 1.
|
Download:
|
| Scheme1. Synthetic procedures for biotinylated monomer 6, as well as molecular structures of MG1 and MG2. Reagents and conditions: (a) TsCl, THF, H2O, 0 ℃-25 ℃, 1.5h (50%); (b) NaN3, DMF, 45 ℃, 12h (97%); (c) PPh3, THF, H2O, 45 ℃, 12h (93%); (d) Pfp-OH, EDC·HCl, DMF, 0 ℃-25 ℃, 12h (90%); (e) compound 3, DIEA, DMF, 0 ℃-25 ℃, 12h (94%); (f) MAA, EDC·HCl, DCM, DMAP, 0 ℃-25 ℃, 12h (85%). TsCl=4-toluene sulfonyl chloride, THF=tetrahydrofuran, DMF=dimethylformamide, PPh3=triphenylphosphine, Pfp-OH=pentafluorophenol, EDC·HCl=1-(3-dimethylaminopropyl)-3-ethylcarbodiimide, DIEA= N, N-diisopropylethylamine, MAA=methacrylic acid, DCM=dichloromethane, DMAP= N, N-dimethylaminopyridine. | |
|
|
Table 1 Conditions for and results from the copolymerization of MG1 and MG2 with monomer 6. |
2.2. Thermoresponsive properties
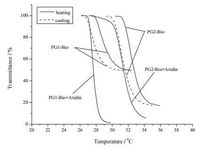
Both PG1-Bio and PG2-Bio are water-soluble at room temperature, but their aqueous solutions turned turbid at elevated temperature, which is a typical indication for their thermoresponsive properties. When combined with avidin, copolymer-protein conjugates also show thermoresponsiveness. In contrast, avidin itself is not thermoresponsive when heated up to 37 ℃.Therefore, thethermoresponsivebehaviorof thesecopolymers and polymer-protein conjugates was investigated by turbidity measurements using UV/vis spectroscopy, and the turbidity curves are shown in Fig. 2, and their corresponding Tcps are summarized in Table 1. These copolymers and copolymer-avidin conjugates show similar thermoresponsive behavior as their dendronized homopolymer counterparts, but their phase transitions and Tcps are slightly influenced by the protein interactions. After introducing biotin moieties into the dendronized polymers, thermally-induced phase transitions became less sharp (△ ~ 2 ℃) when comparing to their homopolymer counterparts (△ < 1 ℃) [21, 22]. At the same time, thermally-induced aggregation also became weak because the aggregate sizes became much smaller as indicated by the high transmittance at elevated temperatures. When conjugated with avidin, PG1-Bio+Avidin conjugate and PG2-Bio+Avidin conjugates show Tcps at 27.7 and 31.6 ℃, respectively, which are slightly lower than their dendronized counterparts PG1-Bio and PG2-Bio (Tcp=29.1 and 32.6 ℃, respectively). Interestingly, their corresponding phase transitions become much sharp as if avidin behave as a crosslinker, leading to decrease of the phase transition temperatures and sharper phase transition. Therefore, it can be concluded that involvement of avidin helps the collapse of OEG dendrons within the polymers. We assume this enhancement is mainly induced by the interaction between biotin moieties from the copolymers and avidin from the aqueous solution. Certainly, the possible interactions between OEG dendrons from copolymers and avidin may also facilitate the aggregation.
|
Download:
|
| Figure 2. Plots of transmittance vs. temperature for aqueous solutions of PG1-Bio, PG2-Bio, and their conjugates with avidin, respectively. Copolymer=0.25mg/mL, avidin=0.09mg/mL. Heating and cooling rate=0.2 ℃/min at 700nm. | |
2.3. Thickness effects of dendronized copolymers on protein recognition
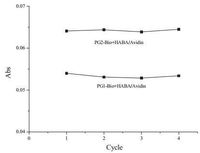
It is well-known that biotin-avidin conjugation is formed via non-covalent conjugation, which is often preferable than covalent conjugation and taking place quickly without any specific pH or temperature prerequisite. Addition of avidin to the biotinylated copolymers have been proven to show influence on thermoresponsive properties of these dendronized copolymers, indicating the strong interactions between the copolymers and avidin.Therefore, these copolymer-protein conjugations were investigated by UV/vis spectroscopy, and the spectra are shown in Fig. 3. For comparison, measurements with pure HABA/avidin complex were also included. The original avidin was modified by HABA dye molecules in four binding sites. After addition of avidin into biotinpendanted thermoresponsive polymer solutions, biotin molecule will compete with HABA dye molecule for biding sites to capture specific proteinsat roomtemperature. Owing tothe higher binding affinity for biotin complexation with avidin compared to complexation with HABA, HABA should be rapidly displaced by biotin from the copolymers, accompanied by absorbance decreasing at 500nm. So, by addition of either PG1-Bio or PG2-Bio solution into HABA/avidin complex, the color of HABA/avidin complex transformed gradually from original red into the yellowish complex, and the absorbance at 500nm decreases from 0.2015 to 0.0482 and 0.068, respectively. This demonstrates that biotin units from copolymers bound efficiently with avidin to successfully form polymer-protein conjugates. From the corresponding absorbance in Fig. 3, the recognition rates of PG1-Bio and PG2-Bio to avidin with the same concentration of biotin moieties are 76% and 66%, respectively, indicating the former showing more advantages for catching avidin than the latter. This suggests that the second generation OEG-based dendrons, which is larger in size, led to more significant steric hindrance, thus is not favorable for avidin recognition. Influence of thermoresponsiveness of the copolymers on avidin conjugation was further investigated. Solutions containing the thermoresponsive polymer-protein conjugates were incubated at 37 ℃ for 5min, then cooled to 20 ℃ for 5min, theirabsorbance were measured after each cycle as shown in Fig. 4. The absorbance of both PG1-Bio+Avidin complex and PG2-Bio+Avidin complex remain constant after each heating-cooling cycle, which indicates the dehydration and collapse of OEG units from the polymer main chain have no obvious effect on the binding between biotinylated polymers and avidin, irrespective of the OEG dendron generation.

|
Download:
|
| Figure 3. Plots of UV/vis absorbance of HABA/Avidin, and the HABA/avidin complex with PG1-Bio (0.20mg/mL), PG2-Bio (0.25mg/mL) solution at r.t. HABA/Avidin concentration: 0.09mg/mL. The concentration of biotin moieties from both copolymers are kept the same for comparison. Dot line is for eye guiding. | |
|
Download:
|
| Figure 4. UV/vis absorbance at 500nm of PG1-Bio (0.20mg/mL) and PG2-Bio (0.25mg/mL) conjugated with HABA/Avidin (0.09mg/mL) after each heating-cooling cycle, respectively. | |
The thickness effects of OEG-based dendronized polymers on recognition of avidin were further explored with dynamic light scattering (DLS) measurements below and above their Tcps, and the results are plotted in Fig. 5. The size (hydrodynamic radius Rh) of PG1-Bio in aqueous solution at 20 ℃ (below its Tcp) was mainly around 6nm, which should be corresponded to the size for individual copolymer chains, at the same time, small portion (less than 20%) of aggregates with Rh of 50nm coexisted. Once avidin is added, Rh is increased significantly into ~300nm, suggesting that interaction of avidin with the copolymer enhances greatly the intermolecular aggregation. This enhancement is a clear indication for the strong complexation between biotin from polymer and avidin from the solution. Upon increasing solution temperature to above its T cp, Rh of PG1-Bio is increased from 10nm to ~300nm in the absence of avidin, due to the entropy-driven intermolecular aggregations [21]. However, in the presence of avidin, Rh of PG1-Bio is increased from ~300nm to ~700nm, due to combination of the entropy-driven intermolecular aggregations and biotin-avidin conjugation. Therefore, avidin behaves as an efficient crosslinker for the G1 copolymer either below or above its Tcp. For comparison, further investigations were done for the second generation copolymer PG2-Bio to examine the thickness effect from dendronized polymers on the biotin-avidin interactions. Similar to that for PG1-Bio, Rh of PG2-Bio is increased from 6 nm into ~170nm when avidin is added at room temperature. While it changes from ~320 nm (in the absence of avidin) to ~400nm (in presence of avidin) above its Tcp. It should be pointed out that variation of the aggregate size taken place before and after avidin addition upon dehydration and aggregation is not so notable in PG2-Bio when compared to the case for PG1-Bio. This suggests that the tendency for PG1-Bio to avidin capture at temperature above its T cp (aggregation condition) is stronger than that for PG2-Bio. In case of PG1-Bio, the biotin moieties will be shield from the aggregation matrix onto peripheries due to the small size of G1-dendrons, providing chances for biotin moieties to shift onto the periphery of the aggregates. However, for the case of PG2-Bio, thick dendrons show higher ability to prevent transition of biotin moieties from interiors to peripheries of aggregates to be able to recognize avidin molecules. In this regard, we conclude that the attraction between PG1-Bio and avidin occurs through biotin-avidin capture (recognition) is much stronger than that between PG2-Bio and avidin.

|
Download:
|
| Figure 5. The size distribution of a) PG1-Bio, PG1-Bio conjugated with avidin, and b) PG2-Bio, PG2-Bio conjugated with avidin at different temperatures as probed by dynamic light scattering. Copolymer concentrations: 0.25mg/mL; HABA/Avidin concentration: 0.09mg/mL. | |
3. Conclusion
Biotinylated OEG-based dendronized copolymers were prepared efficiently via macromonomer route, which combine the cylindrical and thick structural characteristics from dendronized polymers, unprecedented thermoresponsiveness from dendritic OEGs, as well as unique recognition from biotin moieties. These copolymers exhibit typical thermoresponsive properties, but involvement of biotin deteriorates their phase transition processes to become broad. However, their phase transition processes can be improved much narrowly through conjugation of avidin, at the same time, their phase transition temperatures can be mediated.Furthermore, thermally-induced aggregation of these copolymers are also dependent on the complexation of biotin and avidin. All these results indicate that biotin moieties from the OEG-based dendronized copolymers show characteristic interaction with avidin, which mediate the thermoresponsive behavior of the copolymers, at the same time, suggest OEG dendrons show negligible influence on the complexation of biotin and avidin.When comparing the first generation dendronized copolymer to the second generation dendronized copolymer counterpart, thick dendrons were found to prevent biotin moieties from efficiently shifting onto peripheries of thermally-induced aggregates. This thickness effect is unique for dendronized polymers to mediate the protein recognitions just by variation of dendron generations. We thus believe these polymers are suitable candidates for promising applications as biomaterials in activity control of enzyme or proteins, and also for controlled drugs or genes delivery.
4. Experimental 4.1. MaterialsMonomers (MG1 and MG2) and compound 1 were synthesized according to our previous reports [26, 27]. Azobis(isobutyronitrile) (AIBN) was recrystallized twice from methanol. 4-Hydroxyazobenzene-2-carboxylic acid/avidin (HABA/avidin) reagent and biotin (>99%) were purchased from Sigma-Aldrich. Other reagents and solvents were purchased at reagent grade and used without further purification. Silica gel 60 M (Macherey-Nagel, 0.04-0.063 mm, 200-300 mesh) was used as the stationary phase for column chromatography.
4.2. Instrumentation and measurements1H NMR spectra were recorded on a Bruker AV 500 (500 MHz) spectrometer. Gel permeation chromatography (GPC) measurements were carried out on a Waters 1515 Isocratic HPLC Pump and Waters 2707 Auto-Samper instrument with 3 column set (Styragel HR3 +HR4 +HR5) equipped with refractive index detector (Waters 2414), and THF as eluent at 30 ℃. The calibration was performed with polystyrene standards in the range of Mp =1270-2 700 000 (SHOWA DENKO K.K.). UV/vis measurements were recorded on JASCO V-750 spectrophotometer equipped with a thermostatically regulated bath. The samples of polymer solutions were placed in the spectrophotometer (path length 1 cm). For turbidity measurements, the solutions were heated or cooled at a rate of 0.2 K/min, and the absorptions of the solution at λ= 700 nm were recorded every 5 s. The cloud point temperature (Tcp) is determined the one at which the transmittance at λ =700 nm reached 50% of its initial value. Dynamic light scattering (DLS) measurements were performed on DynaPro Nanostar.
4.3. Synthesis of compound 2NaN3 (4.20 g, 64.00 mmol) was added to a solution of compound 1 (7.90g, 26.00 mmol) in dry DMF (40 mL). The mixture was stirred overnight at 45 ℃. The crude product was concentrated under reduced pressure at 80 ℃ and the residue was dissolved with DCM, the organic layer washed with brine solution and dried over MgSO4. Purificationwith column chromatography on silica gel with DCM as eluent affords the product as yellow oil with a yield of 97% (4.40 g). 1H NMR (DMSO-d6) : δ 3.38 (t, 2H, CH2), 3.42 (t, 2H, CH2), 3.47-3.50 (m, 2H, CH2), 3.51-3.57 (m, 4H, CH2), 3.58 (t, 2H, CH2), 4.57 (t, 1H, OH).
4.4. Synthesis of compound 3Triphenylphosphine (9.89 g, 37.70mmol) was added to a solution of compound 2 (4.40g, 25.10 mmol) in THF (20 mL) and water (10 mL), and the mixture was stirred for 12 h at 45 ℃. The solvents were evaporated under reduced pressure at 40 ℃, and the residue was dissolved with slightly acidic water with pH 5-6 by using 10% KHSO4 aqueous solution. Water was removed under reduced pressure at 80 ℃. Faintly yellow oil was obtained with a yield of 93% (3.50g). The product was used in the next step without further purification. 1H NMR (D2O): δ 2.69 (s, 2H, NH2), 3.20 (t, 2H, CH2), 3.63 (t, 2H, CH2), 3.71-3.72 (m, 6H, CH2), 3.75(t, 2H, CH2), 5.43 (s, 1H, OH).
4.5. Synthesis of compound 4Biotin (2.95 g, 12.00 mmol) was dissolved in DMF (10 mL), and Pfp-OH (2.80g, 15.20mmol) was added to the biotin solution before EDC·HCl (5.70g, 29.70 mmol) in DMF (15 mL) were added to the mixture at 0 ℃ over 30min. The mixture stirred overnight at room temperature under nitrogen atmosphere. The product washed with DCM. White powder was obtained with a yield of 90% (4.50 g). The product was used in the next step without further purification. 1H NMR (D2O): δ 1.41-1.69 (m, 6H, CH2), 2.57 (d, 1H, CH2), 2.77-2.85 (m, 3H, CH2), 3.11-3.12 (m, 1H, CH), 4.14 (t, 1H, CH), 4.30 (t, 1H, CH), 6.37(d, 2H, NH).
4.6. Synthesis of compound 5A mixture of compound 3 (1.26 g, 8.45 mmol) and DIEA (2.70g, 20.89 mmol) in dry DMF (5mL) was added to a solution of compound 4 (2.90 g, 7.06 mmol) in dry DMF (20 mL) at 0 ℃. The mixture was stirred at 0 ℃ for 30min then stirred for 10 h at room temperature under nitrogen atmosphere. DMF was then removed under reduced pressure at 80 ℃, and the crude product was dissolved with DCM then the organic phase washed with brine solution and dried over MgSO4. Purification with column chromatography on silica gel with DCM/MeOH (15:1, v/v) as eluent affords the product as white powder with a yield of 94% (2.50g). 1H NMR (DMSO-d6) : δ 1.27-1.51 (m, 6H, CH2), 2.05 (t, 2H, CH2), 2.55 (d, 1H, CH2), 2.79-2.83 (m, 1H, CH2), 3.08 (t, 1H, CH), 3.09 (t, 2H, CH2), 3.15-3.51 (m, 10H, CH2), 4.10 (d, 1H, CH), 4.28 (d, 1H, CH), 4.60 (t, 1H, OH), 6.35 (s, 1H, NH), 6.41 (s, 1H, NH), 7.84 (s, 1H, NH).
4.7. Synthesis of compound 6Compound 5 (2.50g, 6.66mmol) was dissolved in dry DCM (20mL), and then MAA (0.86g, 9.99mmol), EDC·HCl (5.10g, 26.60mmol) and DMAP (40mg, 0.327mmol) were added at 0 ℃ over 30min. The mixture was stirred overnight at room temperature under nitrogen atmosphere, and the crude product was washed with DCM. Evaporation of solvent under vacuum affords the product as white powder with a yield of 85% (2.50g). The product was used in the next step without further purification. 1H NMR (DMSO-d6) : δ 1.27-1.51 (m, 6H, CH2), 1.87 (s, 3H, CH3), 2.05 (t, 2H, CH2), 2.55 (d, 1H, CH2), 2.79-2.83 (m, 1H, CH2), 3.06-3.10 (m, 1H, CH), 3.15-3.18 (m, 2H, CH2), 3.38 (t, 2H, CH2), 3.50 (t, 2H, CH2), 3.54 (t, 2H, CH2), 3.64 (t, 2H, CH2), 4.11 (t, 1H, CH), 4.20 (t, 2H, CH2), 4.29 (t, 1H, CH), 5.69-5.70 (d, 1H, CH2), 6.02 (s, 1H, CH2), 6.36-6.42 (d, 2H, NH), 7.83 (t, 1H, NH).
4.8. Synthesis of copolymer PG1-BiotinA mixture of MG1 (0.40g, 0.479mmol), compound 6 (0.018g, 0.0479mmol), and AIBN (1mg) were dissolved in dry DMF (0.1mL) in a Schlenk tube under nitrogen atmosphere. The solution was stirred at 70 ℃ for 4h, and then cooled to room temperature.Purification with column chromatography on silica gel (DCM as eluent) yielded the product as a colorless oil with a yield of 66%. 1H NMR (DMSO-d6) : δ 0.76 (br, CH3), 1.02-1.06 (t, CH3), 1.22 (br, CH2), 1.47 (br, CH2), 2.02 (br, CH2), 3.04-3.68 (m, CH2), 3.95-4.27 (m, CH2), 6.52 (br, Ar-H).
4.9. Synthesis of copolymer PG2-BiotinA mixture of MG2 (0.30g, 0.120mmol), compound 6 (0.004g, 0.009mmol), and AIBN (1mg) were dissolved in dry DMF (0.1mL) in a Schlenk tube under nitrogen atmosphere. The solution was stirred at 70 ℃ for 5h, and then cooled to room temperature.Purification with column chromatography on silica gel (DCM as eluent) yielded the product as a colorless oil with a yield of 52%. 1H NMR (DMSO-d6) : δ 0.87 (br, CH3), 1.06 (s, CH3), 1.28-1.63 (m, CH2), 2.06 (br, CH2), 3.43-3.82 (m, CH2), 4.03-4.28 (d, CH2), 6.53 (br, Ar-H).
4.10. HABA/avidin assay40-Hydroxyazobenzene-2-carboxylic acid/avidin (HABA/avidin) reagent (from Sigma Aldrich) is used for the spectrophotometric measurement referring to biotinylated dendronized copolymers. After the powdered HABA/avidin reagent was reconstituted with 10mL of deionized water, HABA/avidinsolution(0.4mL)waspoured into a 2mL cuvette with added deionized water (1.6mL). The absorbance was recorded from 450nm to 700nm by UV/vis spectrophometer, and the absorbance at λmax=500 nm was marked as AHABA/Avidin 500. With the same way, HABA/avidin solution (0.4mL) accompanied deionized water (1.1mL) was poured into a 2mL cuvette with addition of copolymer solution (0.5mL). The absorbance was also recorded from 450nm to 700nm, and the absorbance at λmax=500nm was marked as AHABA/Avidin+sample 500.The recognition rate was calculated by (△A500/AHABA/Avidin 500) ×100%, where △A500 =AHABA/Avidin 500 -AHABA/Avidin+sample 500 [28].
AcknowledgmentsWe sincerely thank Dr. Hongmei Deng from the Instrumental Analysis and Research Center of Shanghai University for her assistance with NMR measurements. Financial supports from the National Natural Science Foundation of China (Nos. 21374058, 21474060 and 21574078), the Ph.D. Programs Foundation of Ministry of Education of China (No 201331081100166) and the Shanghai Rising-Star Program (No. 16QA1401800) are acknowledged.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.11.016.
| [1] | W.E Stites, Protein-protein interactions:interface structure, binding thermodynamics, and mutational analysis. Chem.Rev. 97 (1997) 1233–1250. DOI:10.1021/cr960387h |
| [2] | M.W. Peczuh, A.D Hamilton, Peptide and protein recognition by designed molecules. Chem.Rev. 100 (2000) 2479–2494. DOI:10.1021/cr9900026 |
| [3] | C.S. Mahon, D.A Fulton, Mimicking nature with synthetic macromolecules capable of recognition. Nat.Chem. 6 (2014) 665–672. DOI:10.1038/nchem.1994 |
| [4] | R Schirhagl, Bioapplications for molecularly imprinted polymers. Anal.Chem. 86 (2014) 250–261. DOI:10.1021/ac401251j |
| [5] | L. Perez, Y.J. Ghang, P.B. Williams, Cell and protein recognition at a supported bilayer interface 97via in situ cavitand-mediated functional polymer growth. Langmuir 31 (2015) 11152–11157. DOI:10.1021/acs.langmuir.5b03124 |
| [6] | N. Jayasuriya, S. Bosak, S.L Regen, Supramolecular surfactants:polymerized bolaphiles exhibiting extraordinarily high membrane-disrupting activity. J. Am.Chem.Soc. 112 (1990) 5851–5854. DOI:10.1021/ja00171a027 |
| [7] |
(a)T. Nochi, Y. Yuki, H. Takahashi, et al. , Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines, Nat. Mater. 9(2010)572-578; (b)T. Vermonden, R. Censi, W. E. Hennink, Hydrogels for protein delivery, Chem. Rev. 112(2012)2853-2888. |
| [8] | ${referAuthorVo.mingEn} M.Calderón, M.A. Quadir, S.K. Sharma, R Haag, Dendritic polyglycerols for biomedical applications. Adv.Mater. 22 (2010) 190–218. DOI:10.1002/adma.v22:2 |
| [9] | E. Tziampazis, J. Kohn, P.V Moghe, PEG-variant biomaterials as selectively adhesive protein templates:model surfaces for controlled cell adhesion and migration. Biomaterials 21 (2000) 511–520. DOI:10.1016/S0142-9612(99)00212-4 |
| [10] | V. Wagner, A. Dullaart, A.K. Bock, A Zweck, The emerging nanomedicine landscape. Nat.Biotechnol. 24 (2006) 1211–1217. DOI:10.1038/nbt1006-1211 |
| [11] | O.C. Farokhzad, R Langer, Nanomedicine:developing smarter therapeutic and diagnostic modalities. Adv.Drug Deliv.Rev. 58 (2006) 1456–1459. DOI:10.1016/j.addr.2006.09.011 |
| [12] | A.S. Hoffman, P.S Stayton, Bioconjugates of smart polymers and proteins: synthesis and applications. Macromol.Symp. 207 (2004) 139–152. DOI:10.1002/(ISSN)1521-3900 |
| [13] | Y. Ma, G.Q. Pan, Y. Zhang, X.Z. Guo, H.Q Zhang, Narrowly dispersed hydrophilic molecularly imprinted polymer nanoparticles for efficient molecular recognition in real aqueous samples including river water, milk, and bovine serum. Angew.Chem.Int.Ed. 52 (2013) 1511–1514. DOI:10.1002/anie.201206514 |
| [14] | N. M. Green, Avidin. 1. The use of(14-C)biotin for kinetic studies and for assay, Biochem. J. 89(1963)585-591. |
| [15] | A. Sardo, T. Wohlschlager, C. Lo, Burkavidin:a novel secreted biotin-binding protein from the human pathogen Burkholderia pseudomallei. Protein Expr.Purif. 77 (2011) 131–139. DOI:10.1016/j.pep.2011.01.003 |
| [16] | M. Zhu, X. Gong, Y.H. Hu, Streptavidin-biotin-based directional double nanobody sandwich ELISA for clinical rapid and sensitive detection of influenza H5N1. J.Trans.Med. 12 (2014) 352. DOI:10.1186/s12967-014-0352-5 |
| [17] | S. Fornera, T.E. Balmer, B. Zhang, ${referAuthorVo.mingEn} A.D.Schlüter, P Walde, Immobilization of peroxidase on SiO2 surfaces with the help of a dendronized polymer and the avidin-biotin system. Macromol.Biosci. 11 (2011) 1052–1067. DOI:10.1002/mabi.v11.8 |
| [18] | A.T. Marttila, O.H. Laitinen, K.J. Airenne, Recombinant NeutralLite avidin: a non-glycosylated, acidic mutant of chicken avidin that exhibits high affinity for biotin and low non-specific binding properties. FEBS Lett. 467 (2000) 31–36. DOI:10.1016/S0014-5793(00)01119-4 |
| [19] | L.M. Hellman, M.G Fried, Electrophoretic mobility shift assay(EMSA)for detecting protein-nucleic acid interactions. Nat.Protoc. 2 (2007) 1849–1861. DOI:10.1038/nprot.2007.249 |
| [20] |
(a)L. E. Bromberg, E. S. Ron, Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery, Adv. Drug Deliv. Rev. 31 (1998)197-221; (b)E. S. Gil, S. M. Hudson, Stimuli-responsive polymers and their bioconjugates, Prog. Polym. Sci. 29(2004)1173-1222. |
| [21] | W. Li, A. Zhang, K. Feldman, P. Walde, A. D. Schlüter, Thermoresponsive dendronized polymers, Macromolecules 41(2008)3659-3667. |
| [22] | L.X. Liu, W. Li, J.T. Yan, A Zhang, Thermoresponsive dendronized polymeric sensors. J.Polym.Sci.A Polym.Chem. 52 (2014) 1706–1713. DOI:10.1002/pola.v52.12 |
| [23] |
(a)C. M. Yam, J. M. Lopez-Romero, J. Gu, C. Cai, Protein-resistant monolayers prepared by hydrosilylation of alpha-oligo(ethylene glycol)-omega-alkenes on hydrogen-terminated silicon(111) surfaces, Chem. Commun. (Camb. ) (2004)2510-2511; (b)X. Y. Zhu, Y. Jun, D. R. Staarup, et al. , Grafting of high-density poly(ethylene glycol)monolayers on Si(111), Langmuir 17(2001)7798-7803. |
| [24] |
(a)R. Langer, J. P. Vacanti, Tissue engineering, Science 260(1993)920-926; (b)A. G. A. Coombes, S. Tasker, M. Lindbald, et al. , Biodegradable polymeric microparticles for drug delivery and vaccine formulation: the surface attachment of hydrophilic species using the concept of poly(ethylene glycol) anchoring segments, Biomaterials 18(1997)1153-1161. |
| [25] | E.M. Ericsson, K. Enander, L. Bui, Site-specific and covalent attachment of his-tagged proteins by chelation assisted photoimmobilization:a strategy for microarraying of protein ligands. Langmuir 29 (2013) 11687–11694. DOI:10.1021/la4011778 |
| [26] | W. Li, A. Zhang, Y. Chen, et al. , Low toxic, thermoresponsive dendrimers based on oligoethylene glycols with sharp and fully reversible phase transitions, Chem. Commun. (Camb. )(2008)5948-5950. |
| [27] | M.J.N. Junk, W. Li, A.D. Schlüter, EPR spectroscopic characterization of local nanoscopic heterogeneities during the thermal collapse of thermoresponsive dendronized polymers. Angew.Chem.Int.Ed. 49 (2010) 5683–5687. DOI:10.1002/anie.v49:33 |
| [28] | N.M Green, Spectrophotometric determination of avidin and biotin. Methods Enzymol. 18 (1970) 418–424. DOI:10.1016/0076-6879(71)18342-5 |
 2017, Vol. 28
2017, Vol. 28 



