Artificial cellular scaffolds have attracted great attentions in the field of tissue engineering from both academic research and applied standpoints, especially in the areas of pluripotent maintenance of stem cells, host-implant responses and cell navigation in wound healing [1 -4]. Cells are subjected to both chemical and physical factors in their environments, and respond to culture conditions through a complex signalling cascade in order to maintain physical integrity and stabilize physiological functions [5, 6]. Profound knowledge of the interactions between cells and their substrates can assist the development of materials with specific desirable functions that are suitable as artificial scaffolds.In previous studies, substrates with differences in chemical composition, charge, wettability, roughness, and rigidity have been used to control cellular morphology, spreading, migration, proliferation and differentiation [7 -9]. The surficial topology can modulate the distribution of the cytoskeleton, and the extracellular matrix (ECM), which is a complex, three-dimensional dynamic protein network made from fibronectin, forms the substrate for many cells [10 -12]. Cells sense their substrates and organize the ECM though integrins, integrins transmit signals to the cytoskeleton, and transmitted signals guide cell fate. Therefore, integrins act as a'transducer' and participate in the formation of focal adhesion complexes [13]. The cytoskeleton is then reorganized to balance the compression and tension caused by alterations to spatial position, substrate morphology, intercellular interactions, and other factors.
Although cells respond to wide range of different input signals from the environment through this complex signal transduction cascade, our understanding of the effects of surficial curvature on cell behavior is incomplete, as highlighted recently in the field of biointerface research [8, 14, 15]. Pioneering research on cell curvature involved guiding cells onto a convex cylindrical fiber with a radius of 100 μm [16], and the morphology of cells was polarized along the long axis of the fiber. In an attempt to understand this phenomenon, Sanz-Herrera et al. proposed a model that simulated the distribution of the cytoskeleton in cells on curved surfaces to explain the effect of substrate curvature on cell mechanics [17]. The model indicated that contractile force was inhibited in cells on curved surfaces, and theoretical calculations based on the model were consistent with the observed behavior of cells along the fiber [18]. These results can be applied to guide nerve cells outgrowths. However, in this model, the radius of curvature was more than 50 μm, which is larger than entire cell spreading area, and the curvature of substrates was not investigated at the subcellular scale. An understanding of the subcellular morphology, including the relationship between surface curvature and cell behavior, is important for designing artificial tissues.
Over the past few decades, soft lithography has been widely used to fabricate subcellular patterns using micro-contact printing, photolithography, microfluidic modelling and replica modelling [19 -21]. However, these techniques are restrained by harsh experimental conditions, expensive machinery or complex preparation procedures, which rendered them unaffordable and unsuitable for large-scale applications [22 -24]. Meanwhile, 'bottom-up' self-assembly strategies are becoming more versatile for fabricating multilevel spatial architectures and morphologies ranging at the nanometre and micrometer scale, based on the principle of minimum energy [25]. Compared with disordered cell supports, hexagonally patterned substrates exhibit closer-packing features that act as a distinct platform for homogeneous cell distribution, and provide a suitable medium for investigating cell-matrix and cell-cell interactions [26]. Periodic colloidal crystal array (PCCA) and honeycomb array membrane (HCM) structuresaretypical hexagonalsurfaces and both can facilitatethe proper development of tissue [27, 28]. These structures are similar in topography, but completely opposite (inverse) in terms of curvature. The morphology and function of hepatocytes, cardiac myocytes, and endothelial cells grown on honeycomb surfaces have been systematically manipulated and analysed [29 -31], and colloidal crystal arrays are considered promising building blocks for biological scaffolds [32]. However, exactly how the curvature of hexagonal structures influences cell behavior remains unknown.The aim of the present work was to gain insight into how the curvature of hexagonal structures influences cell behaviors. To this end, the effects on cell morphology, cellular adhesive force and the cytoskeleton were investigated, and mechanical analysis was performed in combination with the tensegrity model. The results provide a platform for the design of artificial scaffolds in biomedical applications.
2. Results and discussion 2.1. Preparation of concave and convex surficial curvatureScheme 1 presents the procedures used to prepare the two different substrate types. HCM is a hexagonally periodic porous array obtained using solvent evaporation-induced water condensations self-assembly approach known as the breath figure method. The evaporation of organic solvent cools down the air-solution interface and leads to the condensation of water in humid air and the formation of water droplets. Due to the barrier from the polystyrene solution and the driving force-thermal convection, the spherical water droplets self-organize into a hexagonal array [33]. After the evaporation of organic solvent and water droplets, a honeycomb film with spherical holes is achieved [34, 35]. Colloidal crystallization is an alternative self-assembly method for forming hexagonal sphere arrays. The structural periodicity of a PCCA can be attributed to the thermo-dynamics of solvent evaporation-induced confined area self-assembly (Scheme 1b) [36]. Eventually, both PCCA and HCM can be obtained that share a hexagonal topography but display inverted substrate curvature.

|
Download:
|
| Scheme 1. Scheme showing the fabrication of surfaces with inverted curvature via a self-assembly approach: (a) Breath figure method for the fabrication of a honeycomb pore array (HCM); (b) solvent evaporation-induced confined area assembly method for the preparation of a periodic colloidal crystal array (PCCA). | |
HCM structural parameters can be modulated by manipulating the preparation conditions such as the polymer concentration, humidity and solvent (Fig. S1 in Supporting information). It is worthy to mention that the diameters of honeycomb holes and colloidal array also play important roles on cell behaviors which had been individually demonstrated in the previous works [36, 37].
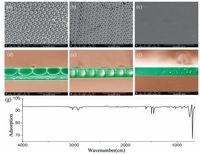
As mentioned above, the curvature of substrates has not been investigated at the subcellular scale which is quite important to investigate the cell-matrix interactions. Thus, the pore size would be focused on the subcellular size (above 2 μm) in this work. For the purpose of the investigating cell-matrix interactions, HCM with a subcellular size of ~2-3 μm was prepared, and scanning electron microscopy (SEM) was utilized to characterize the morphology. As shown in Fig. 1a, the diameter of honeycomb holes was 2 ±0.3 μm, and a corresponding cross-sectional SEM photograph of the honeycomb structure (Fig. 1d) confirmed the array of ordered holes and the spherical concave curvature. Subsequently, PCCA from commercial mono-disperse polystyrene colloidal particles with a similar 2 μm diameter (Fig. 1b) confirmed that the PCCA sphere array had a convex hexagonal surface.
|
Download:
|
| Figure 1. Scanning electron microscopy (SEM) photographs of substrates. (a) HCM. (b) PCCA. (c) PS plane. The corresponding cross-sectional SEM photographs are shown in d-f.(g) FT-IR of the surfaces confirming the identity of the chemical components. | |
Cells respond to both the chemistry and topography of substrates. Thus, in order to eliminate chemical influences, polystyrene (PS) was used as the main chemical component (Fig. 1g), because PS is the most widely used polymer in the biomedical field [38]. Subsequent studies focused directly on the effects of convex and concave curvature on cell behaviors.
2.2. Mathematical modelling of substratesBefore attempting to interpret the effects of surface curvature on cell fate, it was necessary to assess the morphological features of the surfaces. Herein, the curved surfaces prepared via selfassembly methods were mathematically modelled using Matlab [39]. As shown in Fig. 2, hexagonal arrays are presented in the top panel, in which red represents the convex surface and blue represents the concave domain. The profile figures revealed additional detail, and pictures show the potential movement paths for cell pseudopodia. When the cell pseudopodium was less than the radius (Z2), cells could fully spread on the convex surface. However, as the pseudopodium was increased (Z1>radius), cells subsequently spread onto adjacent spheres due to spatial hindrance. When on the HCM surface, the pseudopodium is unable to achieve mechanical balance at a length of Z2 due to the lack of support, until the pseudopodium is extended to the inner hole Z1. Generally, only the top half of the convex PCCA surface was available, whereas the whole surface of the cavity was potentially available for attachment on the concave HCM surface. The roughness of the models was subsequently calculated. As illustrated in Fig. 2, zero horizontal lines were set on the apexes of convex spheres and concave cavities, the cellular pseudopodium sensing length was set as Z, and the roughness of the resulting surface was calculated according to the following equation:

|
Download:
|
| Figure 2. Mathematical models of substrates. (a) PCCA, a hexagonal convex sphere array. (b) HCM, a hexagonal concave pore array. The profile pictures show possible movement paths for cell pseudopodia. Typically, the available route on the convex PCCA was restricted to the top half, whereas cells on the concave HCM can potentially attach to the entire surface of the cavity. | |

|
where Ra is the cellular roughnesssensing capacity, Zi is the cellular pseudopodium sensing length; Zd is the maximum morphological length (the depth of holes in HCM and the diameter of holes in PCCA). The results indicated significant differences in the ability of cells on concave and convex surfaces to sense roughness (Fig. S2 in Supporting information). On the PCCA surface, roughness sensing increased with increasing cellular pseudopodium sensing length up to the point at which the sensing length Zi reached the radius of the particles, after which roughness sensing remained stable. This phenomenon can be easily explained by the close packing of the particles, which makes it difficult for cells to attach to the bottom surface of the particles. However, in the case of HCM, when Zi was less than the particle radius, cells failed to anchor to the substrate until the pseudopodium extended to the bottom. As a consequence, the HCM surface was a rougher structure for cells. This difference may play a critical role in controlling cell behaviors.
2.3. Cell morphologyTo understand how the concave and convex surface affects cell function, HeLa cells were cultured onto the different surfaces for 12 h, and onto a flat surface as a control. Cells on the flat surface adopted a classic spindle shape with affluent filopodia, indicating a normal attachment (Fig. 3a, d). In contrast, cells on HCM displayed an elongated spindle structure, with smaller thread-like filopodia substituted by larger filopodia, especially those over the HCM cavity (Fig. 3b). As shown in Fig. 3e, these coarse filopodia strode over the holes and failed to touch the inner surfaces of the honeycomb structure. As shown in the above calculations, HCM provided a rough surface for cells. In order to reach the inner surface of honeycomb holes, cells must stretch their pseudopodia significantly, which makes it difficult for them to balance the external mechanical force [40, 41]. Therefore, cells preferred to remain on the HCM surface rather than enter the holes.

|
Download:
|
| Figure 3. SEM images of cell morphology on different substrates. (a, d) Flat surface. (b, e) Concave HCM surface. (c, f) Convex PCCA surface. HeLa cells were cultured for 12 h and subsequently fixed by glutaraldehyde anddehydrated using an ethanol gradient. Photographs of whole cell morphology are shown in row 1, and a detailed view of pseudopodiais shown in row 2. | |
Meanwhile, cells on the PCCA surface were larger and spread further, and an obvious increase in attachment area was apparent (Fig. 3c). The pseudopodia of cells on the PCCA surface remained in the lamellate stage (Fig. 3f), indicating more extensive spreading.Therefore, cells on the HCM surface were elongated, while cells on the PCCA surface underwent more extensive spreading, compared with control cells on the flat surface. These phenomena indicated that the shape and pseudopodia of cells was correlated with balancing cellular stress force on different substrates.
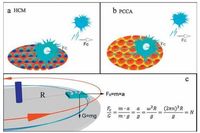
2.4. Cellular adhesive forceIt is well known that adhesion between cells and substrates is strongly influenced by the topology of the substrate, and the adhesive force is related to the number of integrin-associated bonds and their distribution in the ECM, the cell-substrate contact area and shape, and focal adhesion kinases (FAK) [42, 43]. In this work, the adhesive force of cells was evaluated using a centrifugation approach as shown in Scheme 2 [44]. A glass container in which cells were attached to substrates inculture solution was fixed at a rotation plate. The mean radius (R) between cells and the axle-center was ~1 cm, and the rotation speed was defined as n. In order to simplify calculation, the multiple of gravity (G) was employed to measure the adhesive force between cells and substrates. When cells are removed by centrifugation at a particular rotation speed, the adhesive force can be calculated according to the equation in Scheme 2 c, and a value can be calculated based on the weight of the cell, which was estimated to be 0.655 μN (calculated in Supporting information). Taking into account proper contrast, n was optimized to be 4000 rpm in this experiment, based on a centrifugal force of ~160 times gravity. HeLa cells were then incubated on the different substrates at the same density and the residual cell number, average cell spreading area and aspect ratio of cells before and after centrifugation were compared (Fig. 4).
|
Download:
|
| Scheme2. Measurement of the adhesive force between cells and substrates using a centrifugation approach. Cells attached to substrates were fixed on a rotation plate at a distance of 1 cm from cell to axis. Due to the centrifugal force, unstably attached cells are pulled away from the surface. Centrifugal force was calculated as a multiple of gravity. | |

|
Download:
|
| Figure 4. Statistical analysis of cell number (a), cell spreading area (b), and aspect area (c) before and after centrifugation, following AO/EB double staining. Fluorescence images of cells on flat PS (d), PCCA (e) and HCM (f) surfaces following staining with AO/EB. Immunofluorescence LCSM images of cells on flat PS (g), PCCA (h) and HCM (i) surfaces. Blue domains indicate the location of nuclei, red domains reveal the distribution of tubulin, and green fibres show the arrangement of microfilaments. | |
Significant differences were observed between the three surface types. Contributing to the topological roughness, the cell exhibited larger spreading area [45]. The cellular spreading area on PCCA was ~150% higher than the area on the flat surface, consistent with the SEM results. Meanwhile, cells on the HCM surface were elongated, and had an aspect ratio of 2.52, which was notably larger than in cells on flat (1.54) and PCCA (1.41) surfaces. After centrifugation, more than 70% of cells became detached from the flat surface. However, ~65% of cells were retained on PCCA, presumably due to increased spreading area, as well as larger anchoring FAK proteins, as confirmed in subsequent experiments.Cells on the HCM honeycomb structure exhibited the highest retention following centrifugation (~85%), and therefore adhered most strongly, despite occupying a smaller spreading area that was observed on the PCCA surface. The strong adhesive force on the honeycomb surface could be because cells covered most of the honeycomb holes (Fig. 3e). Due to the cover layer on the honeycomb holes, several sealed spaces were formed, and these helped cells resist detachment by centrifugation by generating negative pressure [46]. Thus, the convex PCCA surface appeared to promote cell spreading and hence more surface adhesion receptors, while the concave HCM surface appeared to generate negative pressure that anchored cells firmly to the substrate.Control cells on the flat surface did not benefit from these phenomena and were therefore more easily detached.
To further investigate the cytoskeleton, immunofluorescence staining was performed using laser confocal scanning microscopy (LCSM; Fig. 4g -i). Cells on flat surfaces displayed a typical spindle morphology, with numerous microfilaments at the edge of the cell (Fig. 4g, green), which was also observed as major filopodia (Fig. 3d). Meanwhile, tubulins were mainly aggregated around the nucleus (Fig. 4g, red). The elongated cells present on the HCM surface exhibited the largest and brightest red areas, indicating more extensive tubulin, especially indomains spanning holes (Fig. 4h). Microtubules were enriched in cells above honeycomb holes, possibly to provide enhanced mechanical support to balance the increased tension and compression forces [47]. Compared with cells on the flat surface, microfilaments in cells on PCCA were aggregated around the cell boundary (Fig. 4i, green), where they combined with each other into a lamellate structure, consistent with the SEM images (Fig. 3f). The formation of a lamellate structure indicated a decrease in compressive force and effective cell spreading [44, 48].
2.5. Mechanism of the effect of surface curvature on cell mechanical balanceFrom both cell morphology and adhesion force measurements, we observed significant differences in the behavior of cells grown on substrates with different curvature. Balancing mechanical forces may be the most important factor regulating cell behavior. Based on the tensegrity framework, cells are considered to be composed of molecular struts (microtubules), ropes (intermediate filaments) and cables (microfilaments) [49 -51]. Pressure, compression or tension caused by geodesic structures can be reflected by the distribution of these three structural elements. Thus, the tensegrity model was adopted in this work to study the cellular mechanical behavior and cell morphology [42]. Cells assemble and adopt various shapes when adapting to and balancing forces associated with recognizing their spatial position, substrate morphology, and intercellular interactions.
According to the tensegrity model, living cells are constantly subjected to mechanical stimulation from both the external environment and internal physiological conditions. This stimulation interferes with the original balance, resulting in the generation of stresses and strains. To rebalance, cells remodel their morphology through pulling forces exerted by microfilaments, and supporting forces from the rebuilding of microtubules.On the concave HCM surface, cells spanned over the honeycomb holes, and the compression caused by gravity would be sensed and balanced by microtubules [52, 53], as shown in Fig. 5. Thus, strong microtubules formed in cells spanning honeycomb holes by continuous stacking of repeating protein units within filopodia, resulting in strong groups of tubules that could adequately span across cavities. As shown in Fig. 3e, filopodia in cells on concave surfaces were much coarser and larger than those in cells on flat or convex surfaces. In addition, cells on concave surfaces were elongated due to growth of microtubules in a single direction, essentially caused by the anisotropic force distribution.

|
Download:
|
| Figure 5. Schematic illustration of a cell on the concave HCM surface. (a) SEM of cellular filopodia over HCM, (b) schematic cell on the HCM surface, (c) microtubules help to resist the compression force caused by microgravity, while microfilaments support tension from substrates (based on the tensegrity model). | |
Compared to the HCM surface, cells on the convex PCCA surface underwent more extensive spreading and displayed a higher number of pseudopodia, indicatinga greater number of binding sites (FAK) that may explain the high adhesive force. The convex surface helped to supported cells against microgravity, which presumably alleviated some of the compression force. As illustrated in Fig. 6, a new dynamic balance can be achieved by breaking and rebuilding microtubules, as well as the formation of microfilaments. Without limitations due to compression from gravity, microtubules can freely spread across the surface.Therefore, cells on the convex spherical surface tended to spread out isotropically, and round spreading cells were observed on the PCCA surface.

|
Download:
|
| Figure 6. Schematic illustrationof cells on the convex PCCA surface. (a) SEM of cellular filopodia over PCCA, (b) schematic cell on the PCCA surface, (c) Microtubules resist compression forces caused by microgravity, while microfilaments support tension from substrates (based on the tensegrity model). | |
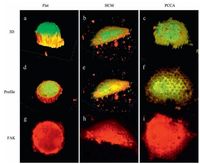
As a proof of concept, 3D immunofluorescence staining was applied to further demonstrate the validity of the tensegrity model on typical subcellular curved surfaces. Fig. 7a shows that cells on flat surfaces exhibited were rounder and formed thicker spherical structures that cells on curved surfaces. The distribution of FAK in cells on flat surfaces suggests adhesive sites are mainly situated around the nucleus (Fig. 7g). In cells on the HCM surface, a greater number of microtubules supported against compression due to gravity (Fig. 7e, yellow). The discontinuity of domains stained red indicates that FAK protein binding sites were not present in cells above cavities on the HCM surface (Fig. 7h). By contrast, cells on PCCA exhibited the largest FAK area, which resulted in a greater spreading and adhesive force (Fig. 7i). Herein, 3D immunofluorescence staining clearly revealed the distribution of the cytoskeleton, which further proved that surface curvature influences mechanical balance in cells, and affects cell morphology and adhesion.
|
Download:
|
| Figure 7. Three dimensional fluorescence photographs of cells on different surfaces. Blue domains indicate the location of nuclei, yellow reveal the distribution of tubulin, green fibres represent microfilaments, and red domains depict the distribution of FAK. Profiles show surfaces at cell-substrate interface. The distribution of FAK is displayed more clearly in g-i. | |
3. Conclusion
In conclusion, we fabricated hexagonal structured surfaces using a self-assembly approach to investigation the effects of subcellular curvature on cell behavior. On the prepared highly porous concave honeycomb film, cells failed to enter the honeycomb holes, and instead strode across them, presumably due to the high surficial roughness, which promoted cells to adopt an elongated shape. In contrast, cells on the convex PCCA surface underwent a high degree of spreading, and the cellular adhesive force on this honeycomb structure was mechanically enhanced due to the generation of negative pressure. The mechanism underlying the effect of surface curvature on cell behavior was subsequently explored using the tensegrity model. Under compression caused by microgravity, microtubule polymerisation caused cell elongation, which allowed cells to span over the honeycomb holes. On the PCCA surface, compression from microgravity was released, which caused cells to spread more isotropically. We conclude that systematic analysis of cell behavior on convex and concave hexagonal surfaces can offer new insight into cellular scaffolds for tissue engineering.
4. Experimental 4.1. MaterialsPolystyrene (PS) was chosen as the substrate for cell culture dishes. An aqueous suspension of PS spheres (2.5%, 2.0 μm) was purchased from Alfa-Aladdin. PS (mw = 250, 000 Da) was obtained from Acros Reagents (Shanghai). The solvents toluene and ethanol were from Sinopharm Chemical Reagent Co., Ltd. (Tianjin). HeLa cells were purchased from the Cell Resource Centre of Life Sciences in Shanghai. Cell culture medium, fetal bovine serum (FBS) and Dulbecco's Modified Eagle's Medium (DMEM) were obtained from Gibco BRL (USA). Other reagents used for cell culture were purchased from Beyotime Institute of Biotechnology (Suzhou).Water was purified using a Hitech System to achieve a resistivity of >18.2 MΩ cm.
4.2. Fabrication of a concave hexagonal array honeycomb membrane (HCM)Linear PS was dissolved in toluene at a concentration of 2 mg/ mL and 40 mL of this solution was coated onto pre-treated glass in a chamber constructed in-house. A flow of humid air (approximately 60% R.H., 4 L/min) was blown over the surface to induce condensation of water droplets. Due to hydrothermal convection, water droplets self-arranged into a highly ordered hexagonal array. All processes were performed at room temperature. After completion of the breath figure process, films were heated at 100 ℃ for 1 h to ensure attachment to the glass substrate.
For comparison, a flat PS membrane (control) was also prepared by solvent self-evaporation in a dry chamber.
4.3. Fabrication of a convex hexagonal polymer colloidal crystal array (PCCA)A dilute suspension of 0.25 wt.% PS spheres was dropped onto the substrate and placed in a chamber and subjected to moderate air flow (1 mL/min) to induce water evaporation. The temperature of the chamber was maintained at 30 ℃. Due to capillary and gravitational forces; particles resisted interference from Brownian motion and ordered themselves into a close-packed arrangement.After evaporation of the solution, the highly ordered colloidal crystal array was subsequently heated at 80 ℃ to ensure attachment to the glass substrate.
4.4. Surface characterizationThe morphology of substrates was examined by scanning electron microscopy (SEM) on an FEI Nova NanoSEM (FEI, USA).Fourier-transform infrared spectroscopy (FTIR) was performed using a Spectrum 100 apparatus (PerkinElmer, USA) to investigate the chemical nature of the surfaces. In order to avoid interference from the glass slide, all membranes were peeled from glass slides and attached onto a KBr tablet prior to FTIR spectroscopy measurements.
4.5. Cell cultureHeLa cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% FBS and 1% penicillin-streptomycin and suspended in solution prior to seeding onto the prepared substrate membranes that were sterilized via medicinal alcohol rinsing and UV light exposure. Samples were then placed in a 24-well plate and rinsed with PBS (2 ×1 mL). HeLa cells were seeded at a density of 1 ×105 cells per well, and incubated at 37 ℃ in a humid atmosphere containing 5% CO2. Membrane-adhered cells were then rinsed twice with PBS to remove unattached cells. Fluorescence double staining was carried out using live/dead acridine orange/ethidium bromide (AO/EB) dye that stains live cells with AO, which causes them to emit green fluorescence. An inverted fluorescence microscope (IX 71, Olympus, USA) equipped with a CCD camera was used for cell imaging, and cells were counted and images overlayed using Image J software. Cells for SEM were fixed with 2.5% glutaraldehyde solution at room temperature for 20 min and dehydrated with graded ethanol solutions (30%, 50%, 70%, 90%, 95%, and 100%). Samples were sputtered with Pt and examined using an FEI Nova NanoSEM 450 instrument (FEI, USA).
4.6. Cell adhesive force measurementA rotation test was applied to measure the force required to detach cells from the surface. Films with adhered cells were fixed in a Petri plate immersed in DMEM and submitted to a spin-coater.After rotation, samples were stained with AO/EB to quantify the proportion of retained cells. Multiple variables can affect the detachment of cells, including culture time, rotation speed and centrifugal radius. In this study, these parameters were optimized and a centrifugal radius of 1 cm, a rotation speed of 4000 rpm, and a culture time of 6 h were employed.
4.7. Cell immunofluorescence stainingCells were fixed with 2.5% glutaraldehyde solution for 20 min.
After removing excess glutaraldehyde, samples were permeabilized using Triton-100 containing PBS (0.1%). Samples were subsequently blocked using PBS/BSA solution (1%) for 30 min to minimize non-specific binding. Cells were then treated with FITClabelled phalloidin (Actin-Tracker Green, Beyotime) for 1 h at 37 ℃, followed by Alexa Fluor 555-labeled α-tubulin antibody (TubulinTracker Red, Beyotime) for 1 h at 37 ℃. After staining, cells were treated with DAPI solution for 10 min at 37 ℃, and fluorescence images were recorded using a TCS SP8 STED 3X confocal microscope (Leica, GER).
4.8. Statistical analysisExperiments were performed at least three times unless otherwise stated. Values are presented as means ±SD and were subjected to the one-tailed heteroscedastic T test. Differences were considered statistically significant at p < 0.005.
AcknowledgmentsThis work was supported by the Major Program of Chinese National Programs for Fundamental Research and Development (973 Project, No. 2012CB933803) and the National Science Foundation of China (No. 21574081).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.10.039.
| [1] | T. Yeung, P.C. Georges, L.A. Flanagan, Effects of substrate stiffness on cell morphology cytoskeletal structure, and adhesion. Cell Motil.Cytoskeleton 60 (2005) 24–34. DOI:10.1002/(ISSN)1097-0169 |
| [2] | C.A. Mullen, T.J. Vaughan, M.C. Voisin, Cell morphology and focal adhesion location alters internal cell stress. J.R.Soc.Interface 11 (2014) 20140885. DOI:10.1098/rsif.2014.0885 |
| [3] | B.G. Keselowsky, D.M. Collard, A.J. García, Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J.Biomed.Mater.Res.A 66A (2003) 247–259. DOI:10.1002/jbm.a.v66a:2 |
| [4] | L.J. Gibson, M.F Ashby, The mechanics of three-dimensional cellular materials. Proc.R.Soc.A 382 (1982) 43–59. DOI:10.1098/rspa.1982.0088 |
| [5] | J. Chen, J. Irianto, S. Inamdar, Cell mechanics structure, and function are regulated by the stiffness of the three-dimensional microenvironment. Biophys.J. 103 (2012) 1188–1197. DOI:10.1016/j.bpj.2012.07.054 |
| [6] | G. Bao, S Suresh, Cell and molecular mechanics of biological materials. Nat. Mater. 2 (2003) 715–725. DOI:10.1038/nmat1001 |
| [7] | J. Tan, W.M Saltzman, Biomaterials with hierarchically defined micro-and nanoscale structure. Biomaterials 25 (2004) 3593–3601. DOI:10.1016/j.biomaterials.2003.10.034 |
| [8] | A. Ranella, M. Barberoglou, S. Bakogianni, C. Fotakis, E Stratakis, Tuning cell adhesion by controlling the roughness and wettability of 3D micro/nano silicon structures. Acta Biomater. 6 (2010) 2711–2720. DOI:10.1016/j.actbio.2010.01.016 |
| [9] | L.Z. Zhao, S.L. Mei, P.K. Chu, Y.M. Zhang, Z.F Wu, The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions. Biomaterials 31 (2010) 5072–5082. DOI:10.1016/j.biomaterials.2010.03.014 |
| [10] | C.S. Chen, M. Mrksich, S. Huang, G.M. Whitesides, D.E Ingber, Geometric control of cell life and death. Science 276 (1997) 1425–1428. DOI:10.1126/science.276.5317.1425 |
| [11] | A. Newhart, S.M Janicki, Seeing is believing:visualizing transcriptional dynamics in single cells. J.Cell.Physiol. 229 (2014) 259–265. DOI:10.1002/jcp.24445 |
| [12] | G.P. Dubey, S. Ben-Yehuda, Intercellular nanotubes mediate bacterial communication. Cell 144 (2011) 590–600. DOI:10.1016/j.cell.2011.01.015 |
| [13] | D.A. Fletcher, D Mullins, Cell mechanics and the cytoskeleton. Nature 463 (2010) 485–492. DOI:10.1038/nature08908 |
| [14] | K. Yamaki, I. Harada, M. Goto, C.S. Cho, T Akaike, Regulation of cellular morphology using temperature-responsive hydrogel for integrin-mediated mechanical force stimulation. Biomaterials 30 (2009) 1421–1427. DOI:10.1016/j.biomaterials.2008.11.036 |
| [15] | L. Chen, X.L. Liu, B. Su, Aptamer-mediated efficient capture and release of T lymphocytes on nanostructured surfaces. Adv.Mater. 23 (2011) 4376–4380. DOI:10.1002/adma.201102435 |
| [16] | M.D. Frame, I.H Sarelius, Flow-induced cytoskeletal changes in endothelial cells growing on curved surfaces. Microcirculation 7 (2000) 419–427. DOI:10.1111/micc.2000.7.issue-6 |
| [17] | J. A. Sanz-Herrera, P. Moreo, J. M. García-Aznar, M. Doblaré, On the effect of substrate curvature on cell mechanics, Biomaterials 30(2009)6674-6686. |
| [18] | J. James, E.D. Goluch, H. Hu, C. Liu, M Mrksich, Subcellular curvature at the perimeter of micropatterned cells influences lamellipodial distribution and cell polarity. Cell Motil.Cytoskeleton 65 (2008) 841–852. DOI:10.1002/cm.v65:11 |
| [19] | M Tamura, Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280 (1998) 1614–1617. DOI:10.1126/science.280.5369.1614 |
| [20] | S.V. Kim, W.Z. Mehal, X.M. Dong, Modulation of cell adhesion and motility in the immune system by Myo1f. Science 314 (2006) 136–139. DOI:10.1126/science.1131920 |
| [21] | E. Cukierman, R. Pankov, D.R. Stevens, K.M Yamada, Taking cell-matrix adhesions to the third dimension. Science 294 (2001) 1708–1712. DOI:10.1126/science.1064829 |
| [22] | A. Raic, L. Rödling, H. Kalbacher, C. Lee-Thedieck, Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells. Biomaterials 35 (2014) 929–940. DOI:10.1016/j.biomaterials.2013.10.038 |
| [23] | J. Park, J.E Babensee, Differential functional effects of biomaterials on dendritic cell maturation. Acta Biomater. 8 (2012) 3606–3617. DOI:10.1016/j.actbio.2012.06.006 |
| [24] | A. J. García, Get a grip: integrins in cell-biomaterial interactions, Biomaterials 26(2005)7525-7529. |
| [25] | G.M. Whitesides, B Grzybowski, Self-assembly at all scales. Science 295 (2002) 2418–2421. DOI:10.1126/science.1070821 |
| [26] | Y.X. Xu, K.X. Sheng, C. Li, G.Q Shi, Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 4 (2010) 4324–4330. DOI:10.1021/nn101187z |
| [27] | S.Y. Zhang, M.D. Regulacio, M.Y Han, Self-assembly of colloidal one-dimensional nanocrystals. Chem.Soc.Rev. 43 (2014) 2301–2323. DOI:10.1039/c3cs60397k |
| [28] | H. Yamazaki, S. Gotou, K. Ito, Micropatterned culture of HepG2 spheroids using microwell chip with honeycomb-patterned polymer film. J.Biosci. Bioeng. 118 (2014) 455–460. DOI:10.1016/j.jbiosc.2014.03.006 |
| [29] | León A.S.de, ${referAuthorVo.mingEn} J.Rodríguez-Hernández, A.L Cortajarena, Honeycomb patterned surfaces functionalized with polypeptide sequences for recognition and selective bacterial adhesion. Biomaterials 34 (2013) 1453–1460. DOI:10.1016/j.biomaterials.2012.10.074 |
| [30] | Y.D. Zhu, R.L. Sheng, T. Luo, Honeycomb-structured films by multifunctional amphiphilic biodegradable copolymers:surface morphology control and biomedical application as scaffolds for cell growth. ACS Appl.Mater.Interfaces 3 (2011) 2487–2495. DOI:10.1021/am200371c |
| [31] | X.H. Wu, S.F Wang, Regulating MC3T3-E1 cells on deformable poly (e-caprolactone)honeycomb films prepared using a surfactant-free breath figure method in a water-miscible solvent. ACS Appl.Mater.Interfaces 4 (2012) 4966–4975. DOI:10.1021/am301334s |
| [32] | F.L. Yap, Y Zhang, Assembly of polystyrene microspheres and its application in cell micropatterning. Biomaterials 28 (2007) 2328–2338. DOI:10.1016/j.biomaterials.2007.01.034 |
| [33] | ${referAuthorVo.mingEn} M.Hernández-Guerrero, M.H Stenzel, Honeycomb structured polymer films via breath figures. Polym.Chem. 3 (2012) 563–577. DOI:10.1039/C1PY00219H |
| [34] | S.S. Chen, X.M. Lu, D.D. Zhu, Q.H Lu, Targeted grafting of thermoresponsive polymers from a penetrative honeycomb structure for cell sheet engineering. Soft Matter 11 (2015) 7420–7427. DOI:10.1039/C5SM01769F |
| [35] | S.S. Chen, X.M. Lu, Y. Hu, Q.H Lu, Biomimetic honeycomb-patterned surface as the tunable cell adhesion scaffold. Biomater.Sci. 3 (2015) 85–93. DOI:10.1039/C4BM00233D |
| [36] | Y.D. Yin, A.P Alivisatos, Colloidal nanocrystal synthesis and the organic-inorganic interface. Nature 437 (2005) 664–670. DOI:10.1038/nature04165 |
| [37] | T. Kawano, M. Sato, H. Yabu, M Shimomura, Honeycomb-shaped surface topography induces differentiation of human mesenchymal stem cells (hMSCs):uniform porous polymer scaffolds prepared by the breath figure technique. Biomater.Sci. 2 (2014) 52–56. DOI:10.1039/C3BM60195A |
| [38] | E. Biazar, M.T. Khorasani, M.D Joupari, Cell adhesion and surface properties of polystyrene surfaces grafted with poly(N-isopropylacrylamide). Chin.J.Polym. Sci. 31 (2013) 1509–1518. DOI:10.1007/s10118-013-1335-3 |
| [39] | The software was provided on the website: http://cn.mathworks.com/index.html?s_tid=gn_logo. |
| [40] | X. Yao, R. Peng, J.D Ding, Cell-material interactions revealed via material techniques of surface patterning. Adv.Mater. 25 (2013) 5257–5286. DOI:10.1002/adma.201301762 |
| [41] | H. Jeon, Jr. C.G.Simon, G Kim, A mini-review:cell response to microscale, nanoscale, and hierarchical patterning of surface structure. J.Biomed.Mater. Res.Part B Appl.Biomater. 102 (2014) 1580–1594. |
| [42] | D.M. Mosser, J.P Edwards, Exploring the full spectrum of macrophage activation. Nat.Rev.Immunol. 8 (2008) 958–969. DOI:10.1038/nri2448 |
| [43] | M.J. Dalby, N. Gadegaard, R.O.C Oreffo, Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat.Mater. 13 (2014) 558–569. DOI:10.1038/nmat3980 |
| [44] | S.H. Juan, J.M.M Tur, Tensegrity frameworks:static analysis review. Mech. Mach.Theory 43 (2008) 859–881. DOI:10.1016/j.mechmachtheory.2007.06.010 |
| [45] | G.H. Zhang, R.X. Hou, D.X. Zhan, Fabrication of hollow porous PLGA microspheres for controlled protein release and promotion of cell compatibility. Chin.Chem.Lett. 24 (2013) 710–714. DOI:10.1016/j.cclet.2013.05.011 |
| [46] | L.P. Heng, X.F. Meng, B. Wang, L Jiang, Bioinspired design of honeycomb structure interfaces with controllable water adhesion. Langmuir 29 (2013) 9491–9498. DOI:10.1021/la401991n |
| [47] | M. Dembo, Y.L Wang, Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys.J. 76 (1999) 2307–2316. DOI:10.1016/S0006-3495(99)77386-8 |
| [48] | D.E Ingber, Cellular tensegrity:defining new rules of biological design that govern the cytoskeleton. J.Cell Sci. 104 (1993) 613–627. |
| [49] | D.E. Ingber, I Tensegrity, Cell structure and hierarchical systems biology. J.Cell Sci. 116 (2003) 1157–1173. DOI:10.1242/jcs.00359 |
| [50] | D.E. Ingber, I.I Tensegrity, How structural networks influence cellular information processing networks. J.Cell Sci. 116 (2003) 1397–1408. DOI:10.1242/jcs.00360 |
| [51] | D.E Ingber, Tensegrity:the architectural basis of cellular mechanotransduction. Annu.Rev.Physiol. 59 (1997) 575–599. DOI:10.1146/annurev.physiol.59.1.575 |
| [52] | S.J. Crawford-Young, Effects of microgravity on cell cytoskeleton and embryogenesis. Int. J. Dev.Biol. 50 (2006) 183–191. DOI:10.1387/ijdb.052077sc |
| [53] | A. Cogoli, A. Tschopp, P. Fuchs-Bislin, Cell sensitivity to gravity. Science 225 (1984) 228–230. DOI:10.1126/science.6729481 |
 2017, Vol. 28
2017, Vol. 28 


