Gram-negative bacteria are a set of notorious bacteria that lead to a variety of diseases, including pneumonia, meningitis, gonorrhea, bacterial dysentery, cholera, gastritis, etc. [1]. Thus, an understanding of their adhesive behaviors on material surfaces is very necessary to have control over them, which is normally determined by surface properties of materials. Among these, wettability is one of most important properties to affect their adhesion [2, 3]. Moderate wettable surfaces prefer to attract bacteria [4 -6] and superwettable surfaces often inhibit their adhesion [7, 8]. However, one of critical issues is how wettability regulates gram-negative bacteria adhesion if surface wettability varies from superhydrophilic to superhydrophobic, as well as what is the contributing role of the lipopolysaccharide (LPS) layer (the main components of outer layer of the gram-negative shell) during this procedure [9]. The existence of such issues should be closely related to the constant variations of surface topography when surface wettability is adjusted over a broad range. This can lead to unexpected influential elements on adhesion behaviors [10], leading to the complex interaction mechanism between gramnegative bacteria and substrates and is becoming a roadblock to the systematic study of wettability regulated adhesion in a broad scale wettability.
The bacterial adhesion stage is largely influenced by the topographical features on the surface and the influence of topography on bacteria adhesion has been frequently investigated [11, 12]. Such topographies are usually created by employing different pattern technologies, including topographical patterning [13], photolithography [14], nanoimprint lithography [15], plasma treatment [16] etc. Surface chemistry and functional groups on the surface also influence bacterial adhesion [17] thus, wettable and superwettable surfaces have been developed for controlling bacterial adhesion [18]. In addition, bacterial adhesion can be directly affected by mechanical cues which in some cases can impact even to a greater degree compared to that induced through chemical signals [19]. However, the adhesion of gram-negative bacteria is a complex process which is affected, not only by nanoscale topographical, but also by micro-topographical features, that is, by hierarchical multi-scale levels [20, 21]. Hence, creating hierarchical substrates with both micro-and nano-structures will be certainly necessary in order to investigate the interaction mechanism between wettable surfaces and gram-negative bacteria under biomimetic environments.
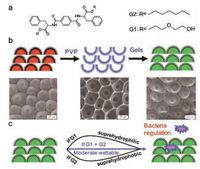
Herein, to mimic hierarchical structures, rose petal structures are successfully duplicated onto supramolecular gelators based films. The duplication not only enables the biomimetic structures with constant topographies (Fig. 1), but also can circumvent the unexpected influence from the varied topographies on the investigation of bacteria adhesion [22]. The surface wettability of the hierarchical structures can be adjusted from superhydrophilic to superhydrophobic by varying the interfacial chemical composition of the films self-assembled from supramolecular gelators [23]. Here, the inherent amplification character of rose petal structures guarantees the formation of superwettable surfaces [22]. The study of gram-negative bacteria adhesion on the hierarchical structures reveals that the bacteria have completely different interaction mechanisms with different wettable surfaces (Fig. 1c) and this procedure is found to be directly mediated by LPS. This study provides a methodology having good control over bacteria adhesion by properly designing wettable surfaces of bioinspired structures.
|
Download:
|
| Figure 1. (a) The chemical structures of supramolecular gelators G1 and G2, respectively. (b) Up: firstly, the inverted rose structures are copied onto poly(vinyl alcohol) (PVP) films; secondly, using the inverted PVP structures as template, the mimic rose structures are duplicated onto xerogel films. Below: corresponding SEM images of rose structures, inverted rose structures, duplicated rose structures. (c) The surface wettability can be tuned by using different self-assembled precursors, which may further regulate gram-negative bacteria adhesion. | |
2. Experimental 2.1. Materials
All chemicals were purchased from Aladdin (Shanghai, China) and used without further purification. G1 and G2 were synthesized with high yields through conventional liquid phase reaction in three steps according to Scheme S1 in Supporting information and Ref. [20]. For G1: (bis(2-(2-hydroxyethoxy)ethyl)2, 2'-(terephthaloylbis(azanediyl))bis(3-phenyl-propanoate); G2: dihexyl 2, 2'-(terephthaloylbis(azanediyl))-bis(3-phenylpropanoate).
2.2. Preparation of gel filmG1, G2, and the G1 and G2 mixture were firstly added to xylene (2 mg/mL) and heated until gelators were dissolved. The gel could be formed after cooling. The gel films (the height is about 5 mm) were placed on the substrates (glasses or silicon slices) and dried in the air at room temperature for 6 h.
2.3. Transmission electron microscope (TEM)TEM images were taken with an Analytical Transmission Electron Microscope (JEM-2010/INCA OXFORD, working voltage of 200 kV). One drop of sample (0.1 wt% gelators, ) was placed onto a copper grid, and a thin film was produced by blotting off the redundant liquid with filter paper.
2.4. Scanning electron microscopy (SEM)The dilute gel solution (0.2 wt%) was placed on silicon slices and dried in the air at room temperature and further visualized with an FEI QUANTA 250 (an accelerating voltage of 10 kV and working distance of 10 mm).
2.5. Duplicating hierarchical structuresPoly(vinyl alcohol) (PVA, MW = 22, 000 g/mol) water solution (10 wt%) was poured onto the fresh surface of a red rose petal and exposed to air at r.t. After evaporation, the PVA film was peeled off, which should have mirror image of the petal structure. PVA film was used as a"stamp" and pressed onto G1, G2, or a mixture of G1 and G2 xylene gel films. After air drying and peeling off the PVA film, the petal structure is duplicated onto G1, G2, or a mixture of G1 and G2 gel films. The roughness of the surface was tested by the Carl Zeiss Confocal Laser Scanning Microscope (LSM 700).
2.6. Static contact angle measurementsThe static contact angle measurements were done using a JC2000D Optical Contact Angle Meter (Zhongchen, Shanghai). The water droplet used in each experiment was 2 μL. The reading of the angle was done after 30 s when the droplet is steady. The measurements were performed for at least three trials at different areas of the sample surface.
2.7. Adsorption of LPSLPS was purchased from Shanghai Xinyu Biotechnology Co. Ltd.
Dropping 100 ml solution of LPS (20 ng/mL) on the glass (diameter: 1 cm) coated G1 and G2 sample (0.02 mg) at r.t. The adsorbed LPS on different films was tested by LPS Elisa Kit. The absorbance of LPS was measured with a Tecan Infinite 200 Pro Spectrometer using λ = 450 nm and correlating these intensity values to a calibration curve.
2.8. Bacterial adhesionThe selected bacteria were Escherichia coli (E. coli Top 10 and E. coli OH5α.) and were gifts from Shuangjun Lin group, School of Biomedical Engineering, Shanghai Jiaotong University. A single isolated bacterium colony was inoculated in 5 mL Tryptic Soy Broth (TSB) overnight at 35 ℃. The bacterial culture was centrifuged at 3000 rpm for 10 min, and the bacteria pellet was resuspended in TSB. The optical density of the suspension was adjusted to 0.5 at 600 nm. All samples were individually placed in a 24 well-plate (Corning). Subsequently, 100 μL of bacterial culture was added in each well, and the plate was then statically incubated at 37 ℃ for 2 h. The samples were removed from the bacterial culture, washed with PBS and deionized water twice (to remove the detached or un-adhered bacteria), and stained with 100 μL DAPI dye solution (10 μg/mL) for 20 min. After washing with PBS twice, the samples were imaged using Olympus IX73 Inverted Fluorescent Microscope under 400× objective. The amount of attached bacterial cells was expressed as the mean of bacteria ± standard deviation of three images. Statistical analysis was done using Software Image J. All the chemicals used in the bacterial adhesion tests, such as TSB, DAPI, PBS, etc., were purchased from Shanghai YESEN Biotechnology Co., Ltd.
3. Results and discussionThe 1, 4-benyldicarbonxamide-phenylalanine based supramolecular gelators were respectively coupled with hydrophilic diethylene glycol (G1) and hydrophobic n-hexyl (G2) (Scheme S1). The hydrophobic character of appended groups can provide hydrophobic interactions to facilitate self-assembly between gelators [24]. The self-assembly is reinforced by the formation of intermolecular hydrogen-bonds between the respective amide moieties. The combination of hydrophobic interactions and hydrogen-bonds can further stabilize the secondary structures of the nano-fibrous gels as characterized by scanning electronic microscopy (SEM) (Fig. S1a and b in Supporting information).
Using rose petals as templates, hierarchical structures were copied onto gel films through solvent-evaporation-driven imprint pattern transfer process (Fig. 1b and S1c). The micro/nano hierarchical structures were perfectly duplicated onto the dried gel surfaces (Fig. 1b). In addition, such duplication process could be applied to different precursors (mass ratio of G1:G2 from 1:3, 1:1 to 3:1), which was not restricted by chemical components or interface properties of the gel films (Fig. 2b -f). Similar to periodic microstructures of the original rose petals, all the copied hierarchical structures have the closely packed arrays with approximately hemispherical micropapillae as those in rose petals. Typically, these micropapillae exhibit cuticular folds in the nanometer scale on the top, which keep the remarkable hierarchical topography of rose petals. As those of rose petal (6.3 μm), the roughness of the duplicated surfaces are also in the range from 5 μm to 7 μm (Fig. 2).

|
Download:
|
| Figure 2. SEM images of (a) red rose petal and duplicated films: (b) G1, (c) G1:G2 = 3:1, (d) G1:G2 = 1:1, (e) G1:G2 = 1:3, (f) G2. Scale bar: 150 μm. | |
Surface wettability of the films was characterized by static water contact angles (CA). For unduplicated G1 and G2 films, CA values were 42 ± 1° and 89 ± 2°, respectively (Fig. S2 in Supporting information). After duplicating rose petal hierarchical structures onto the films, the duplicated G1 surfaces became superhydrophilic (CA = 7 ± 3°) and duplicated G2 surfaces became superhydrophobic with CA values of 151 ± 2°. According to the Wenzel model [25] and the Cassie-Baxter model [26], this could ascribe to the magnification effect from surface roughness in the hierarchical structures [27, 28]. The CA should greatly decrease with the increased surface roughness if the surface was originally hydrophilic, while CA will largely increase for originally hydrophobic surfaces. This could be further proved by the decrease of CA from 48 ± 2° to 34 ± 2° if the hydrophilic films (G1:G2 = 3:1) were duplicated with rose petal structures, from 68 ± 3° to 58 ± 3° for the hydrophilic (G1:G2 = 1:1), and the increase from 84 ± 3° to 125 ± 3° for the hydrophobic films (G1:G2 = 1:3) (Fig. S2).
For the superhydrophobic G2 films, a high contact angle hysteresis was proved by the sphere shape of water droplets when the films were upside down (Fig. S3 in Supporting information). This should be a Cassie-Baxter impregnating wetting state [22, 29] and water droplets could enter into micro-scale grooves of the surface but not into nano-scale ones in this state. With a more hydrophilic component in the mixed films, water molecules preferred to interact with the nano-scale structures for achieving the minimum energy state. In turn, it may induce the transition of the droplet from a metastable Cassie-Baxter impregnating wetting state to a stable Wenzel state, which was proved by the unchanged contact area of a water droplet during evaporation (Fig. S4 in Supporting information) [30].

|
Download:
|
| Figure 3. (a) Schematic diagram of bacteria adhesion on wettable surfaces. Fluorescent images of E. coli Top 10 on various wettable surfaces after 2 h culture: (b) CA = 7°; (c) CA = 34°; (d) CA = 58°; (e) CA = 125°; (f) CA = 151°. Scale bar: 20 μm. | |

|
Download:
|
| Figure 4. (a) LPS adsorption on different surfaces within 180 min. (b) Pseudo-firstorder kinetic analysis of LPS adsorption: the LFO plots of ln(1 -qt/qe) vs. time. The slope of line represents -k1 (green curve: G1, CA = 7°; blue curve: G1:G2 = 3:1, CA = 34°; red curve: G1:G2 = 1:1, CA = 58°; black curve: G1:G2 = 1:3, CA = 125°; pink curve: G2, CA = 151°). (c) The curves of LPS adsorption and gram-negative bacteria E. coli Top 10 and (d) E. coli OH5α adhesion on various wettable films. | |
The bacterial adhesion on the structured surface was subsequently tested by two kinds of gram-negative bacteria (E. coli Top 10 and E. coli OH5α). Since surface wettability could alter the physicochemical interactions between bacteria and substrate in the initial phase [31], bacterial adhesion behaviors were investigated within 2 h after staining with DNA-binding dye 4', 6-diamidino-2-phenylindole (DAPI), the adsorbed E. coli Top 10 were observed on all the wettable surfaces by fluorescent images (Fig. 3b -f). Moderate wettable surfaces preferred to enhance bacterial adhesion. However, significant decrease of E. coli Top 10 adhesion was observed on both superwettable G1 (CA = 7°) and G2 (CA = 151°) surfaces. The adhered amount of E. coli Top 10 was decreased by 78% on the duplicated G1 and by 83% on G2 films compared to that on moderate wettable surfaces. Similar adhesion phenomenon was also achieved for E. coli OH5α.
Since the outer membrane of a gram-negative cell wall is a LPS layer toward the outer side (Fig. S5 in Supporting information), thus, the interaction between the LPS layer and surfaces should be the first interaction for gram-negative bacteria adhesion. To prove the critical role of LPS on bacterial adhesion, the interaction between LPS and different wettable surfaces were investigated in detail. Fig. 4 shows time-dependent curves of the adsorbed amount of LPS on the films. After 60 min, LPS adsorption reached an equilibrium state for all substrates with the adsorbed amounts on the film from superhydrophilic to superhydrophobic calculated as 0.36, 1.03, 1.96, 2.42, 0.26 ng/cm 2, respectively. Maximum LPS adsorption occurred on hydrophobic surfaces with CA 1258 (2.4 ng/cm 2), while only a lesser amount of LPS was adsorbed on both superhydrophilic G1 (CA = 78, 0.36 ng/cm 2) and superhydrophobic G2 (CA = 151°, 0.25 ng/cm 2) surfaces. The LPS adsorption rate constants (k1) were further determined by using Lagergren's pseudo-first order (LFO) equation (for calculation details, see supporting information Part 6) [32]. The linear fit relationship of the amount of LPS vs. time was plotted within 90 min (Fig. 4b) and the slope represented association constants of LPS. The calculated k1 of LPS adsorption on the superwettable G1 and G2 surfaces were both extremely low, 0.0164 min-1 and 0.0178 min-1, respectively.

|
Download:
|
| Figure 5. Schematic depiction of LPS mediated gram-positive bacteria adhesion on (a) superhydrophilic surface, (b) moderate wettable surface, and (c) superhydrophobic surface. | |
While, the k1 on the moderate wettable surfaces (G1:G2 = 1:3, 1:1, 3:1) were 0.0459 min-1, 0.0785 min-1, 0.0932 min-1, respectively.This suggested a fast adsorption rate of LPS on the moderate wettable surfaces, which might be expected to have strong interaction between LPS and the moderate wettable surfaces.Interestingly, the varying trend of adsorbed amount of LPS was basically consistent with that of the adhered E. coli Top 10 (Fig. 4c).Similar results were also obtained for E. coli OH5α (Fig. 4d). Thus, it can be reasonably speculated that wettable structures are recognized by gram-negative bacteria through the direct interaction between substrate and LPS, which may release different signals to bacteria and result in different bacteria/substrate interaction.
For a superhydrophilic surface, a tightly bound water layer adjacent to G1 interface should be formed, which obviously brought repulsive forces between LPS and G1 films due to the hydrophobic character of LPS [33]. From superhydrophilicity to moderate wettability, the interaction between LPS and films could become stronger as proven by increased amounts of LPS adsorbed on the films (Fig. 5). The resistance to LPS on superhydrophobic surfaces was attributed to their specific hierarchical structures, where air could be trapped into the nano-scale interstices, leading to a minimal hydrophobic interaction area between LPS and substrate [34, 35]. Although the determination of the exact mechanism of bacterial adhesion is beyond the scope of this report, it offers a new perspective to have good control over gramnegative bacterial adhesion on different stage wettable surfaces through precise design of materials structures.
4. ConclusionIn conclusion, good control over gram-negative bacteria adhesion is achieved on different wettable surfaces with a constant hierarchical structure. By avoiding the variation of topographies, it proves that different wettable surfaces regulate the bacteria adhesion through different interaction mechanisms, typically, this regulation is closely related with LPS layer. The study not only makes it possible to understand wettability regulated grambacteria adhesion under biomimetic environment, but also provides a methodology to have good control over bacteria cell adhesion by properly designing wettable surface structures. Such design ideas may be helpful in developing new generations of biomaterials in order to gain control over a variety of diseases induced by gram-negative bacteria, which still continues to be very important and necessary in the fields of biomedicine.
AcknowledgmentsWe thank the NSFC (Nos. 51273111, 51173105, 51573092), the National Basic Research Program of China (973 Program, No.2012CB933803), SJTU-UM Collaborative Research Program, the Program for Professor of Special Appointment (Eastern Scholar) at the Shanghai Institutions of Higher Learning.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.08.002.
| [1] | W.R.J.D. Galloway, J.T. Hodgkinson, S.D. Bowden, M. Welch, D.R Spring, Quorum sensing in gram-negative bacteria:small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem.Rev. 111 (2011) 28–67. DOI:10.1021/cr100109t |
| [2] | K.S. Liu, X. Yao, L Jiang, Recent developments in bio-inspired special wettability. Chem.Soc.Rev. 39 (2010) 3240–3255. DOI:10.1039/b917112f |
| [3] | A. Saadeddin, ${referAuthorVo.mingEn} A.Rodrigo-Navarro, V. Monedro, Functional living biointer-phases. Adv.Healthc.Mater. 2 (2013) 1213–1218. DOI:10.1002/adhm.v2.9 |
| [4] | A. Okada, T. Nikaido, M. Ikeda, et al. , Inhibition of biofilm formation using newly developed coating materials with self-cleaning properties, Dent. Mater. J. 27 (2008)565-572. |
| [5] | Y. Arima, H Iwata, Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled mono-layers. Biomaterials 28 (2007) 3074–3082. DOI:10.1016/j.biomaterials.2007.03.013 |
| [6] | S. Gon, K.N. Kumar, ${referAuthorVo.mingEn} K.Nüsslein, M.M Santore, How bacteria adhere to brushy PEG surfaces:clinging to flaws and compressing the brush. Macromolecules 45 (2012) 8373–8381. DOI:10.1021/ma300981r |
| [7] | R.B. Pernites, C.M. Santos, M. Maldonado, Tunable protein and bacterial cell adsorption on colloidally templated superhydrophobic polythiophene films. Chem.Mater. 24 (2012) 870–880. DOI:10.1021/cm2007044 |
| [8] | Y.N. Nie, C. Kalapos, X.Y. Nie, Superhydrophilicity and antibacterial property of a Cu-dotted oxide coating surface. Ann.Clin.Microbiol.Antimicrob. 9 (2010) 25. DOI:10.1186/1476-0711-9-25 |
| [9] | K. Hori, S Matsumoto, Bacterial adhesion:from mechanism to control. Biochem. Eng.J. 48 (2010) 424–434. DOI:10.1016/j.bej.2009.11.014 |
| [10] | C.P. Stallard, K.A. McDonnell, O.D. Onayemi, ${referAuthorVo.mingEn} J.P.O'Gara, D.P Dowling, Evaluation of protein adsorption on atmospheric cues plasma deposited coatings exhibiting superhydrophilic to superhydrophobic properties. Biointerphases 7 (2012) 1–12. DOI:10.1007/s13758-011-0001-y |
| [11] | H.G. Craighead, C.D. James, A.M.P Turner, Chemical and topographical patterning for directed cell attachment. Curr.Opin.Solid State Mater.Sci. 5 (2001) 177–184. DOI:10.1016/S1359-0286(01)00005-5 |
| [12] | K.M. Wiencek, M Fletcher, Effects of substratum wettability and molecular topography on the initial adhesion of bacteria to chemically defined substrata. Biofouling 11 (1997) 293–311. DOI:10.1080/08927019709378338 |
| [13] | M. Ramalingama, A Tiwari, Spatially controlled cell growth using patterned biomaterials. Adv.Mater.Lett. 1 (2010) 179–187. DOI:10.5185/amlett |
| [14] | K. Baranes, N. Chejanovsky, N. Alon, A. Sharoni, O Shefi, Topographic cues of nano-scale height direct neuronal growth pattern. Biotechnol.Bioeng. 109 (2012) 1791–1797. DOI:10.1002/bit.v109.7 |
| [15] | X. G. Liang, S. J. Wi, Transport characteristics of multichannel transistors made from densely aligned sub-10 nm half-pitch graphene nanoribbons, ACS Nano 6 (2012)9700-9710. |
| [16] | D.G. Waugh, C. Toccaceli, A.R. Gillett, Surface treatments to modulate bioadhesion:a critical review. Rev.Adhes.Adhes. 4 (2016) 69–103. DOI:10.7569/RAA.2016.097304 |
| [17] | J. Yang, Y.Q. Wan, C.F. Tu, Enhancing the cell affinity of macroporous poly (L-lactide)cell scaffold by a convenient surface modification method. Polym.Int. 52 (2003) 1892–1899. DOI:10.1002/(ISSN)1097-0126 |
| [18] | L.A. Goetz, B. Jalvo, R. Rosal, A.P Mathew, Superhydrophilic anti-fouling electro-spun cellulose acetate membranes coated with chitin nanocrystals for water filtration. J.Membr.Sci. 510 (2016) 238–248. DOI:10.1016/j.memsci.2016.02.069 |
| [19] | A.K. Epstein, A.I. Hochbaum, P. Kim, J Aizenberg, Control of bacterial biofilm growth on surfaces by nanostructural mechanics and geometry. Nanotechnology 22 (2011) 494007. DOI:10.1088/0957-4484/22/49/494007 |
| [20] | M. Arnold, M. Schwieder, ${referAuthorVo.mingEn} J.Blümmel, Cell interactions with hierarchically structured nano-patterned adhesive surfaces. Soft Matter 5 (2009) 72–77. DOI:10.1039/B815634D |
| [21] | F. X. Zhang, H. Z. Li, X. Wang, H. Y. Low, X. Li, Hierarchically imprinted polymer substrates for enhanced attachment of Escherichia coli, J. Colloid Interface Sci. 343 (2010)109-114. |
| [22] | L. Feng, Y.N. Zhang, J.M. Xi, Petal effect:a superhydrophobic state with high adhesive force. Langmuir 24 (2008) 4114–4119. DOI:10.1021/la703821h |
| [23] | G.F. Liu, D. Zhang, C.L Feng, Control of three-dimensional cell adhesion by the chirality of nanofibers in hydrogels. Angew.Chem.Int.Ed. 53 (2014) 7789–7793. DOI:10.1002/anie.201403249 |
| [24] | J.D. Hartgerink, E. Beniash, S.I Stupp, Self-assembly and mineralization of pep-tide-amphiphile nanofibers. Science 294 (2001) 1684–1688. DOI:10.1126/science.1063187 |
| [25] | R.N Wenzel, Resistance of solid surfaces to wetting by water. Ind.Eng.Chem. 28 (1936) 988–994. DOI:10.1021/ie50320a024 |
| [26] | A.B.D. Cassie, S Baxter, Wettability of porous surfaces. Trans.Faraday Soc. 40 (1944) 546–551. DOI:10.1039/tf9444000546 |
| [27] | K.S. Liu, L Jiang, Bio-inspired design of multiscale structures for function inte-gration. Nanotoday 6 (2011) 155–175. DOI:10.1016/j.nantod.2011.02.002 |
| [28] | R.B. Pernites, R.R. Ponnapati, R.C Advincula, Superhydrophobic-superoleophilic polythiophene films with tunable wetting and electrochromism. Adv.Mater. 23 (2011) 3207–3213. DOI:10.1002/adma.v23.28 |
| [29] | P. G. de Gennes, F. Brochard-Wyart, D. Que're', Capillarity and Wetting Phenomena -Drops, Bubbles, Pearls, Waves, 129, Springer, New York, 2004. |
| [30] | Z.J. Han, B. Tay, C. Tan, M. Shakerzadeh, K Ostrikov, Electrowetting control of Cassie-to-Wenzel transitions in superhydrophobic carbon nanotube-based nano-composites. ACS Nano 3 (2009) 3031–3036. DOI:10.1021/nn900846p |
| [31] | D.J. Balazs, K. Triandafillu, Y. Chevolot, Surface modification of PVC endo-tracheal tubes by oxygen glow discharge to reduce bacterial adhesion. Surf. Interface Anal. 35 (2003) 301–309. DOI:10.1002/(ISSN)1096-9918 |
| [32] | S Lagergren, Kungliga svenska vetenskapsakademiens. Handlingar 24 (1898) 1–39. |
| [33] | V. Williams, M Fletcher, Pseudomonas fluorescens adhesion and transport through porous media are affected by lipopolysaccharide composition. Appl.Environ. Microbiol. 62 (1996) 100–104. |
| [34] | J.Y. Shiu, P.L Chen, Addressable protein patterning via switchable superhydro-phobic microarrays. Adv.Funct.Mater. 17 (2007) 2680–2686. DOI:10.1002/(ISSN)1616-3028 |
| [35] | P. Roach, D. Farrar, C.C Perry, Surface tailoring for controlled protein adsorption: effect of topography at the nanometer scale and chemistry. J.Am.Chem.Soc. 128 (2006) 3939–3945. DOI:10.1021/ja056278e |
 2017, Vol. 28
2017, Vol. 28 


