Lung cancer is the first leading cause of cancer death worldwide. More than 1.8 million new patients are annually diagnosed and approximately 1.6 million cancer deaths occurred each year, which are about 20% of total cancer deaths [1]. The 5-year survival rate of lung cancer is extremely low, as it is very difficult to diagnose in early-stage and the lung cancer is easy to metastasize distally [2, 3]. However, many efforts are to focus on the researches of the prevention and treatment for lung cancer.
The combination therapy of multiple drugs or genes has been proved to be an effective strategy for cancer treatment, which can not only suppress and reduce multidrug resistance, but also decrease drug side effects [4, 5]. Lv et al. designed an amphiphilic triblock copolymer (mPEG-b-PLG-b-PLL/DOCA) for co-delivery doxorubicin and paclitaxel. They found that DOX/PTX co-delivered nanoparticles exhibited significant antitumor efficiency for lung cancer compared to single drug-loaded nanoparticles [6]. Li et al.synthesized the cisplatin crosslinked polysaccharide nanoparticles for delivery of doxorubicin using succinic acid decorated dextran (Dex-SA), and the results showed that these nanoparticles could enhance therapeutic efficacy in tumor-bearing mice compared to single drugs [7]. However, conventional approaches by systemic administration of drugs (especially for the combination of free drugs) have some limitations, such as low tumor accumulation, serious side adverse effects and unsatisfactory tumor inhibition effects [8].
Pulmonary administration, as one of the localized cancer treatment, has several advantages compared with systemic administration [9]. The drugs or genes can be delivered into lungs directly and most of drugs or genes can accumulate in the lungs. Pulmonary administration may facilitate therapy effects due to the prolonged residence time of drugs or genes in the lungs, and the side effects on the normal tissues are much lower with decreasing dose of drugs or genes compared with intravenous administration [10, 11]. In recent years, various delivery systems via the pulmonary administration for drugs or genes delivery have been reported, such as cationic liposomes [12], porous microparticles [13, 14], and cationic polymers [15, 16]. In this article, polyethyleneimine (PEI 25 K) which is regarded as the "gold standard" for non-viral gene delivery with high transfection efficiency [17] is used to carry therapeutic gene (Bcl2 siRNA) by electrostatic complexes.PEI/Bcl2 siRNA and DOX are combined for synergistic anti-tumor (Scheme 1). The intracellular uptake, gene silencing ability, cytotoxicity, cell apoptotic effects of PEI/Bcl2 siRNA and DOX were evaluated in in vitro studies. Furthermore, the biodistribution of siRNA and DOX in B16F10 mice with metastatic lung cancer via pulmonary administration was investigated.

|
Download:
|
| Scheme 1. Combined delivery of DOX and siRNA by pulmonary administration. | |
2. Results and discussion 2.1. Cellular uptake
The cellular uptake of free DOX, free siRNA, PEI/siRNA, PEI/DOX and PEI/siRNA/DOX in B16F10 cells was measured using FAM labeled siRNA. The efficiency of cellular uptake was assessed quantitatively via flow cytometry after incubating for 24 h (Fig. 1). From Fig. 1, cells incubated with free DOX and PEI/siRNA/DOX had high DOX fluorescent intensity with PEI or without PEI, which showed that DOX existed in the solution of PEI/siRNA/DOX with free forms and entered to the cells by diffusion [18]. Low FAM siRNA fluorescent intensity was detected in cells treated with free siRNA, which illustrated that free siRNA was difficult to enter into cells. However, the cells treated with PEI/FAM siRNA showed high cell uptake efficiency, and most of the siRNA entered into cells. This data implied that PEI could enhance the cellular uptake of siRNA, which is helpful for high gene transfection [19, 20].

|
Download:
|
| Figure 1. Cellular uptake of PEI/siRNA/DOX in B16F10 cells by FCM. | |
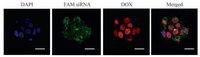
The intracellular location of siRNA and DOX inside B16F10 cells was observed using CLSM. As shown in Fig. 2, the green fluorescence (FAM-siRNA) was observed in the cytoplasm of the cells and the DOX molecules (red) were mainly located in the nuclei after 24h incubation, suggesting that siRNA and DOX could be internalized successfully by tumor cells. Furthermore, the zeta potential of PEI/siRNA nanoparticles was positive charged (+21.2 ±2.3mV) and particle size of PEI/siRNA nanoparticles was about 113 ±5.7nm, which could enhance the cellular internalization and tumor accumulation [21, 22].
|
Download:
|
| Figure 2. CLSM images of B16F10 cells incubated with PEI/siRNA/DOX for 24 h. Scale bars = 20 μm. | |
2.2. The expression of Bcl2 mRNA
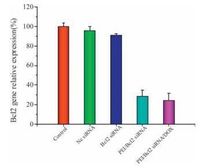
The down-regulation of Bcl2 mRNA mediated by PEI/ Bcl2 siRNA was measured by qRT-PCR. The Bcl2 mRNA levels were determined in B16F10 cells after 48h. As shown in Fig. 3, cells incubated with the naked Nc siRNA and Bcl2 siRNA showed no significant difference for Bcl2 mRNA expression compared with control group. However, about 70% of Bcl2 mRNA was knocked down by PEI/Bcl2 siRNA. These results indicated that combined administration of DOX and PEI/Bcl2 siRNA exhibited high gene silencing efficiency to the targeted Bcl2 mRNAs, and the Bcl2 gene relative expression was not affected after introducing DOX.
|
Download:
|
| Figure 3. The expression of Bcl2 mRNA detected by real-time PCR method in B16F10 cells. Data were shown as mean ±SD (n=3). | |
2.3. In vitro cytotoxicity studies
The in vitro cytotoxicity of combined DOX and Bcl2 siRNA was evaluated using MTT assay in B16F10 cells. Cells were treated with PEI/Nc siRNA, PEI/Bcl2 siRNA, PEI/Nc siRNA/DOX and PEI/ Bcl2 siRNA/DOX at different DOX concentrations for 24h (Fig. 4A) and48h(Fig. 4B), respectively.Asshownin Fig. 4, the cell viability of PEI/Bcl2 siRNA was 78.2% at 24h and 70.2% at 48h, which was lower than cells treated with PEI/Nc siRNA. The cells treated with PEI/Nc siRNA/DOX and PEI/Bcl2 siRNA/DOX showed dose-dependent cell proliferation inhibition behavior and the combination of DOX and siRNA leaded to enhanced cell proliferation inhibition. The above results illustrated that the Bcl2 siRNA and DOX could effectively enhance cytotoxicity against B16F10 cells, which may induce high tumor inhibition capability in vivo.

|
Download:
|
| Figure 4. In vitro cell viability of B16F10 cells following treated with PEI/Nc siRNA, PEI/Bcl2 siRNA, PEI/Nc siRNA/DOX and PEI/Bcl2 siRNA/DOX for 24h (A) and 48h (B), respectively. Values were showed as mean ± SD (n=6). | |
2.4. Apoptosis assay
The percentage of cell apoptosis treated with combined DOX and Bcl2 siRNA was determined by flow cytometer. As shown in Fig. 5, the percentage of apoptosis of PEI/Nc siRNA treated cells was 17.9% after 48 h incubation. However, cells treated with PEI/ Bcl2 siRNA displayed 28.3% of cell apoptosis. These results suggested that Bcl2 related to the mitochondrial pathway of apoptosis [23] could be down-regulated by PEI/Bcl2 siRNA. There were about 50.08% apoptosis cells treated with PEI/Nc siRNA/DOX. As expected, for Bcl2 siRNA and DOX co-treatment, the apoptosis percentage reached up to 67.3%, much higher about 17% and 39% than that of PEI/Nc siRNA/DOX and PEI/Bcl2 siRNA. These results revealed that combined DOX and Bcl2 siRNA could significantly enhance the cell apoptosis, which was consistent with the in vitro cytotoxicity studies.

|
Download:
|
| Figure 5. The apoptosis of B16F10 cells treated with PEI/Nc siRNA, PEI/Bcl2 siRNA, PEI/Nc siRNA/DOX and PEI/Bcl2 siRNA/DOX for 48h, respectively. | |
2.5. Biodistribution in vivo
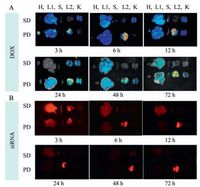
The biodistribution was observed after administration of PEI/ siRNA/DOX to lung tumor bearing mice by pulmonary delivery (PD) and systemic delivery (SD), the siRNA was labeled by Cy5. Heart, liver, spleen, lung and kidney were excised at defined time intervals. Fig. 6A showed the DOX fluorescence and Fig. 6B showed the siRNA fluorescence. Both in Fig. 6A and B, the distribution of DOX and siRNA in each tissue was observed after pulmonary delivery and systemic delivery. After pulmonary administration, the fluorescence intensity of DOX and siRNA in the tissues was much higher than that treated with systemic administration, especially in the lungs. And the fluorescence intensity of DOX and siRNA in the lungs could last for 3 days, which suggested that DOX and siRNA could be effectively accumulated in the lungs by pulmonary administration. These results indicated that combined delivery of DOX and PEI/siRNA for metastatic lung cancer by pulmonary delivery may have high potential ability to enhance the tumor inhibition effects.
|
Download:
|
| Figure 6. The bioistribution of DOX (A) and siRNA (B) in the organs (H, heart; L1, liver; S, spleen; L2, lung; K, kidney) after 3 h, 6 h, 12 h, 24 h, 48 h and 72 h via the systemic delivery (SD) and the pulmonary delivery (PD). | |
3. Conclusion
In this study, PEI was used to complex Bcl2 siRNA, and DOX combined with PEI/Bcl2 siRNA was employed for anti-tumor treatment by pulmonary delivery. The cellular uptake efficiency, Bcl2 mRNA silencing ability, cytotoxicity and cell apoptotic effects of combined DOX and siRNA showed that DOX and Bcl2 siRNA could be efficiently delivered into the tumor cells, possessed high gene silencing of Bcl2 mRNA, and induced high cytotoxicity and cell apoptosis in B16F10 cells. Notably, compared with the systemic delivery, most of DOX and siRNA by the pulmonary delivery could accumulate in the lungs and showed a long-term retention in the lungs. These results showed that the combination of DOX and PEI/ Bcl2 siRNA is an effective method with high anti-tumor effects against B16F10 cells and has great potential for treating metastatic lung cancer by the pulmonary delivery.
4. Experimental 4.1. MaterialsDoxorubicin hydrochloride (DOX·HCl) was obtained from Beijing Huafeng United Technology Co., Ltd. (Beijing, China). The mixture of Bcl2-1 siRNA and Bcl2-2 siRNA in equal molar ratio which was named Bcl2 siRNA were purchased from Genepharma (Suzhou, China) and the sequences were as follows: Bcl2-1 siRNA (sense) 50-UGU GGA UGA CUG AGU ACC UGAdTdT-30, (anti-sense) 50-UCA GGU ACU CAG UCA UCC ACAdTdT-30; Bcl2-2 siRNA (sense) 50-GUA CAU CCA UUA UAA GCU GUCdTdT-30, (anti-sense) 50-GAC AGC UUA UAA UGG AUG UACdTdT-30. The FAM labeled siRNA and Cy5 labeled siRNA were obtained from RiboBio (Guangzhou, China). Branched PEI (25, 000 Da) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Other reagents were purchased from Beijing Chemical Works (Beijing, China).
4.2. Preparation of PEI/siRNA complex nanoparticlesPEI and siRNA were dissolved in RNase-free water respectively, the solution of PEI and siRNA was mixed with a ratio of 5:1 (wt/ wt). After the solution was gently vortexed and incubated at room temperature for 30 min, the PEI/siRNA complex nanoparticles were obtained. The particle size and the zeta potentials of PEI/ siRNA complex nanoparticles were measured using a zeta potential/BI-90Plus particle size analyzer (Brookhaven, USA).
4.3. Cell uptake studiesThe B16F10 cells were maintained in DMEM supplemented with 10% fetal bovine serum. The cell uptake studies were conducted when cells were 70%-80% confluent. B16F10 cells were plated in 6-well plates at the density of 2 ×105 cells per well. The cells were treated with PEI/FAM siRNA and DOX for 24 h at a siRNA amount of 2 μg of siRNA per well and 2 μg of DOX per well. After incubation, the cells were washed twice with phosphate-buffered saline (PBS). The intracellular uptake of siRNA and DOX in B16F10 cells was monitored by flow cytometry (FCM, BD, USA), and the localization of siRNA and DOX in cells was observed by confocal laser scanning microscopy (CLSM, ZEISS LSM 780, Germany).
4.4. Quantification of Bcl2 gene expressionBcl2 gene expression in the B16F10 cells was confirmed by quantitative real-time PCR. About 1 ×105 cells per well was seeded in 6-well plates. The cells were treated with Nc siRNA, Bcl2 siRNA, PEI/Bcl2 siRNA (5/1, wt/wt) and PEI/Bcl2 siRNA/DOX for 48 h, respectively. The total RNA of B16F10 cells was isolated using the Trizol reagent (Invitrogen) according to the manufacturer's protocol. The RNA was converted to a complimentary DNA (cDNA) by using reverse transcription kit from Takara Biotechnology Co., Ltd. (Dalian, China). Quantitative real-time PCR was performed by Mxpro 3005P Real-Time PCR Detection system (Stratagene, USA) using PrimeScriptTM RT Master Mix and SYB® Premix Ex TaqTM (Dalian, China). The primers for Bcl2 were as follows: Forward, 5'-AGG AGC AGG TGC CTA CAA GA-3'; Reverse, 5'-GCA TTT TCC CAC CAC TGT CT-3'. For β-actin: Forward, 5'-TGT TAC CAA CTG GGA CGA CA-3'; Reverse, 5'-GGG G TG TTG AAG GTC TCA AA-3'.
4.5. Cytotoxicity assayThe B16F10 cells were seeded into 96-well plates at a density of 1 ×104 cells/well and then cultured overnight at 37 ℃ in 5% CO2 incubator. PEI/Nc siRNA (5/1, wt/wt), PEI/Bcl2 siRNA (5/1, wt/wt), PEI/Nc siRNA/DOX (5/1/(0.1, 0.25, 0.5, 1, 2.5), wt/wt/wt) and PEI/ Bcl2 siRNA/DOX (5/1/(0.1, 0.25, 0.5, 1, 2.5)) were incubated for 24 h and 48 h, respectively. The cytotoxicity was determined by MTT assay. The absorbance of the solution at 492 nm was measured using a Bio-Rad 680 Microplate Reader. The data were expressed as the percentages of cell viability compared to control group.
4.6. Cell apoptosis assayThe apoptotic cell distribution was determined by Annexic VFITC/PI Apoptosis Detection Kit. The B16F10 cells were placed in a 6-well plates at a density of 2 ×105 cells/well. After 24 h incubation, the medium was replaced with fresh medium, and PEI/Nc siRNA (5/1, wt/wt), PEI/Bcl2 siRNA (5/1, wt/wt), DOX, PEI/Nc siRNA/DOX (5/1/0.5, wt/wt/wt), and PEI/Bcl2 siRNA/DOX (5/1/0.5, wt/wt/wt) were added to the cells and incubated at 37 ℃ for 48 h, respectively. The cells were trypsinized and harvested, and then cells were suspended in 200 mL binding buffer and stained with 5 mL Annexin V-FITC and 10 mL propidium iodide at the room temperature in the dark for 15 min. The apoptosis cells were analyzed using a flow cytometer.
4.7. Biodistribution in vivoThe C57BL/6 mice were handled in the guidelines of Northeast Normal University (China). To generate metastatic lung cancer model, B16F10 cells (1 ×104 cells per mouse) were intravenously injected into C57BL/6 mice. After 16 days, the metastatic lung cancer was established. Mice bearing tumors were randomly divided into two groups. One group was treated with PEI/Cy5 siRNA/DOX (Cy5 labeled siRNA 20μg, DOX 10 μg) through pulmonary administration using a liquid aerosol device (MicroSprayer1 Aerosolizer, Penn-Century, Philadelphia, PA). The other group was treated PEI/Cy5 siRNA/DOX through intravenous administration as a control. After injection, mice were sacrificed at fixed time intervals.The majororgans (heart, liver, spleen, lung and kidney) were excised for imaging with a Maestro in vivo Imaging System (Cambridge Research & Instrumentation, Inc., USA).
AcknowledgmentsThe authors are thankful to the National Natural Science Foundationof China (Nos. 51503200, 21474104, 5123300451520105004 and 51390484), Jilin Province Science and Technology Development Program (No. 20160204032GX), and the National Program for Support of Top-notch Young Professionals for financial support.
| [1] | A. Aggarwal, G. Lewison, S. Idir, The state state of lung cancer research:a global analysis. J.Thorac.Oncol. 11 (2016) 1040–1050. DOI:10.1016/j.jtho.2016.03.010 |
| [2] | A.S. Tsao, G.V. Scagliotti, Jr. P.A.Bunn, Scientific advances in lung cancer 2015. J.Thorac.Oncol. 11 (2016) 613–638. DOI:10.1016/j.jtho.2016.03.012 |
| [3] | Y.J. Xu, Y. Du, Y Fan, Long noncoding RNAs in lung cancer:what we know in 2015. Clin.Transl.Oncol. 18 (2016) 660–665. DOI:10.1007/s12094-015-1448-y |
| [4] | ${referAuthorVo.mingEn} C.Núñez, J.L. Capelo, G. Igrejas, An overview of the effective combination therapies for the treatment of breast cancer. Biomaterials 97 (2016) 34–50. DOI:10.1016/j.biomaterials.2016.04.027 |
| [5] | P.Y. Teo, W. Cheng, J.L. Hedrick, Y.Y Yang, Co-delivery of drugs and plasmid DNA for cancer therapy. Adv.Drug Deliv.Rev. 98 (2016) 41–63. DOI:10.1016/j.addr.2015.10.014 |
| [6] | S.X. Lv, Z.H. Tang, M.Q. Li, Co-delivery of doxorubicin and paclitaxel by PEG-polypeptide nanovehicle for the treatment of non-small cell lung cancer. Biomaterials 35 (2014) 6118–6129. DOI:10.1016/j.biomaterials.2014.04.034 |
| [7] | M.Q. Li, Z.H. Tang, S.X. Lv, Cisplatin crosslinked pH-sensitive nanoparticles for efficient delivery of doxorubicin. Biomaterials 35 (2014) 3851–3864. DOI:10.1016/j.biomaterials.2014.01.018 |
| [8] | Y.F. Wang, J.H. Zhou, L.H. Qiu, Cisplatin-alginate conjugate liposomes for targeted delivery to EGFR-positive ovarian cancer cells. Biomaterials 35 (2014) 4297–4309. DOI:10.1016/j.biomaterials.2014.01.035 |
| [9] | C.N. Xu, H.Y. Tian, X.S Chen, Pulmonary drugs and genes delivery systems for lung disease treatment. Chin.J.Chem. 32 (2014) 13–21. DOI:10.1002/cjoc.201300741 |
| [10] | M. Bivas-Benita, R. Zwier, H. E. Junginger, G. Borchard, Non-invasive pulmonary aerosol delivery in mice by the endotracheal route, Eur. J. Pharm. Biopharm. 61 (2005)214-218. |
| [11] | J.K.W. Lam, W.L. Liang, H.K Chan, Pulmonary delivery of therapeutic siRNA. Adv.Drug Deliv.Rev. 64 (2012) 1–15. |
| [12] | O.B. Garbuzenko, M. Saad, S. Betigeri, Intratracheal versus intravenous liposomal delivery of siRNA, antisense oligonucleotides and anticancer drug. Pharm.Res. 26 (2009) 382–394. DOI:10.1007/s11095-008-9755-4 |
| [13] | T.S. Feng, H.Y. Tian, C.N. Xu, Doxorubicin-loaded PLGA microparticles with internal pores for long-acting release in pulmonary tumor inhalation treatment. Chin.J.Polym.Sci. 33 (2015) 947–954. DOI:10.1007/s10118-015-1642-y |
| [14] | H. Meng, M. Liong, T. Xia, Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano 4 (2010) 4539–4550. DOI:10.1021/nn100690m |
| [15] | C.N. Xu, H.Y. Tian, H. Sun, A pH sensitive co-delivery system of siRNA and doxorubicin for pulmonary administration to B16F10 metastatic lung cancer. RSC Adv. 5 (2015) 103380–103385. DOI:10.1039/C5RA21934E |
| [16] | C.N. Xu, P. Wang, J.P. Zhang, Pulmonary codelivery of doxorubicin and siRNA by pH-sensitive nanoparticles for therapy of metastatic lung cancer. Small 11 (2015) 4321–4333. DOI:10.1002/smll.v11.34 |
| [17] | J. Chen, Z.X. Jiao, L. Lin, Polylysine-modified polyethylenimines as siRNA carriers for effective tumor treatment. Chin.J.Polym.Sci. 33 (2015) 830–837. DOI:10.1007/s10118-015-1632-0 |
| [18] | Y.J. Pu, S. Chang, H. Yuan, The anti-tumor efficiency of poly(L-glutamic acid)dendrimers with polyhedral oligomeric silsesquioxane cores. Biomaterials 34 (2013) 3658–3666. DOI:10.1016/j.biomaterials.2013.01.082 |
| [19] | H.Y. Tian, J. Chen, X.S Chen, Nanoparticles for gene delivery. Small 9 (2013) 2034–2044. DOI:10.1002/smll.v9.12 |
| [20] | A. Masotti, F. Moretti, F. Mancini, Physicochemical and biological study of selected hydrophobic polyethylenimine-based polycationic liposomes and their complexes with DNA. Bioorg.Med.Chem. 15 (2007) 1504–1515. DOI:10.1016/j.bmc.2006.10.066 |
| [21] | M. A. Mintzer, E. E. Simanek, Nonviral vectors for gene delivery, Chem. Rev. 109 (2009)259-302. |
| [22] | S. Dufort, L. Sancey, J.L Coll, Physico-chemical parameters that govern nanoparticles fate also dictate rules for their molecular evolution. Adv.Drug Deliv.Rev. 64 (2012) 179–189. DOI:10.1016/j.addr.2011.09.009 |
| [23] | A. J. García-Sáez, The secrets of the Bcl-2 family, Cell Death Differ. 19(2012) 1733-1740. |
 2017, Vol. 28
2017, Vol. 28 


