b School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China;
c Institute for Natural & Synthetic Organic Chemistry, Changzhou University, Changzhou 213164, China
Supramolecular polymers have attracted considerable attentions in the past decades, and among them, non-covalent interactions were always employed to hold small-molecularweight building blocks together[1]. The higher binding affinity of non-covalent interactions was necessary in order to obtain supramolecular polymers with the higher molecular weight[2]. Among the non-covalent interactions, the highly directional quadruple hydrogen bonding unit 2-ureido-4[1H]-pyrimidinone (UPy) reported by Meijer and co-workers was one of the mostly used units in fabricating supramolecular polymers due to its high association constant (Kdim > 107 L/mol in CHCl3) and synthetic accessibility[3]. In 2012, our group fabricated a linear supramolecular polypseudorotaxane based on two orthogonal noncovalent recognition motifs: the self-complementary UPy unit and the pillar[5]arene-alkyl diamine complex unit respectively[4].In 2013, Huang's group constructed a series of dendronized organoplatinum (Ⅱ) metallacyclic polymers by combining the hierarchical coordination-driven self-assembly and intermolecular UPy H-bonding together[5].
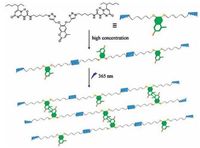
The unique "dynamic" nature of non-covalent interactions endowed supramolecular polymers with fascinating properties and functions, such as stimuli-responsiveness[6]. Among the different kinds of stimuli, light as a trigger was particularly attractive for the reason that the light could be remote-controlled with high spatial resolution, and it could be focused into specific areas easily without producing any by-products as well[7]. Novel tailorable and smart supramolecular polymers could be used in material science for nanotechnology, and biomedical fields for the main reason that the molecular weight, structure, and shape could be reversibly transformed upon external stimulus. Up to now, lots of photo-responsive quadruple hydrogen bonded supramolecular polymers based on photochromic moieties have been efficiently constructed[8]. However, to the best of our knowledge, the strategy of modulating the properties of quadruple-hydrogen-bonded supramolecular polymers via photo-cross-linking has been limited yet. Coumarin and its derivatives have been widely used in functional materials owing to the[2 + 2] photodimerization upon UV irradiation[9]. Inspired by the properties of coumarin, we designed and synthesized a novel coumarin group bridged bifunctional UPy derivative 1, and 1 could self-assemble into the linear supramolecular polymers which were driven by quadruple hydrogen bonding interactions at high concentrations. More importantly, the linear supramolecular polymers could form the large three-dimensional polymer networks upon UV light irradiation, which provides a viable and alternative procedure to modulate the properties of supramolecular polymers (Scheme 1).
|
Download:
|
| Scheme 1. Schematic illustration of the photo-cross-linkable hydrogen bonded supramolecular polymers. | |
2. Results and discussion
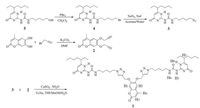
Compound 1 was synthesized using 2 and 3 as starting materials (Scheme 2) in good yield according to the reported methods[10]. The widely used CuAAC "click" reaction was chosen owing to its functional group tolerance and high yield. The chemical structures of compounds were completely confirmed by 1H NMR, 13C NMR, and LR-ESI-MS.
|
Download:
|
| Scheme 2. The synthetic route of 1. | |
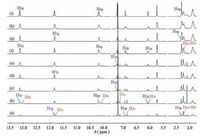
The concentration-dependent 1H NMR spectroscopy was a powerful tool in investigating the self-assembly behavior. As shown in Fig. 1, 1H NMR spectra of 1 were performed at concentrationsin the range of 2-100 mmol/L. The UPy N-H signals showed a large downfield shifts (between 10 and 13.5 ppm), giving the direct evidence for the UPy unit dimerization. At the low concentrations, the signals of protons H2, H3 and H8 were all obviously split into two sets of peaks assigned to the cyclic oligomers (H2c, H3c, and H8c) and the linear assemblies (H2p, H3p, and H8p), respectively. As the concentration was increased, the relative intensity of linear assemblies increased gradually, whereas the intensity of the cyclic oligomers decreased, suggesting that linear polymers were predominant at high concentration. Meanwhile, we found that the protons H1, H4, H6 and H7 were also concentration-dependent.
|
Download:
|
| Figure 1. Partial 1H NMR spectra (400 MHz, CDCl3, 298 K) of 1 at different concentrations: (a) 2, (b) 5, (c) 10, (d) 15, (e) 20, (f) 30, (g) 50, (h) 80, and (i) 100 mmol/L. Signals originating from cyclic oligomers are labeled "c", those from polymeric aggregates are labeled "p". | |
The relatively high solution viscosity at high concentration was a characteristic property and direct index for supramolecular polymers. Thus viscosity measurements were carried out in CHCl3 solution using a micro-Ubbelohde viscometer. The linear supramolecular polymers assembled from the monomer exhibited a viscosity transition, and was characterized by a change in slope in the double logarithmicplotofspecific viscosity versus concentration (Fig. 2a). The slope of the curve was 1.09 in the low-concentration region, demonstrating a linear relationship between specific viscosity and concentration, which is characteristic for noninteracting assemblies of constant size, indicating the predominance of cyclic oligomers in dilute solutions. When the concentration increased above the critical polymerization concentration (CPC; approximately 9 mmol/L), a sharp increase in the viscosity was observed (slope = 2.31), indicating a transition from cyclic oligomers to linear supramolecular polymers with increased size.

|
Download:
|
| Figure 2. (a) Specific viscosity (ηsp) of 1 (CHCl3, 298 K) versus the monomer concentration; (b) SEM micrograph of a fiber drawn from a very concentrated solution of 1 in CHCl3. | |
Further direct physical evidence for the formation of supramolecular polymers was obtained by SEM (Fig. 2b). A long thin rodlike fiber with a regular diameter of about 26 mm was drawn from a highly concentrated solution of 1, reflecting a significant chain extension to generate the desired high-molecular-weight polymeric aggregates.
As well known, coumarin derivatives were apt to undergo[2 + 2] photodimerization[11]. Benefitting from the unique property of coumarin motif, the properties of supramolecular polymers could be efficiently tuned by UV-light irradiation. The bifunctional UPy derivative solution was filled into a quartz cell and photo-cross-linked by 365 nm UV light irradiation from a 500 W mercury lamp, the distance between the quartz wall and the spot light was about 10 cm. As shown in Fig. 3a, upon irradiating with UV light at room temperature, the absorption peak of coumarin motif around 330 nm decreased continuously with the increase of irradiation time, indicating a growing degree of dimerization. Furthermore, the fluorescence spectra also showed the corresponding change of 1 in response to UV light (Fig. 3b). The fluorescent emission intensity decreased significantly after UV light irradiation, suggesting the photodimerization of the coumarin moieties. These results indicated that the linear supramolecular polymers could be photo-cross-linked to form a large threedimensional network structure.

|
Download:
|
| Figure 3. (a) UV-vis spectra (2 ×10-5mol/L) and (b) fluorescence spectra (1 ×10-7mol/L) of 1 in CHCl3 irradiated by 365nm UV light. | |
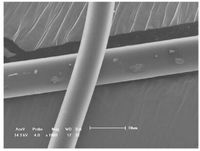
Moreover, photoconversion of liner supramolecular polymers into their corresponding three-dimensional polymer networks was confirmed using DLS measurements. The aggregate of 1 at a concentration of 50 mmol/L in chloroform showed a hydrodynamic diameter value of 245 nm, indicating the formation of large sized supramolecular polymers. Whereas the hydrodynamic diameter value increased to 1120 nm after 365 nm UV light irradiation for 2 days, indicating the formation of bigger aggregates (Fig. 4). Before irradiation, the diffusion coefficient of the supramolecular polymers (15 mmol/L) was 2.57 ×10-10 m2/s. After irradiation, the diffusion coefficient decreased to 2.04 ×10-10m2/s, reflecting the formation of photo-cross-linking supramolecular polymers. Furthermore, long thin rod-like fibers with regular diameter of about 10 mm were drawn from the highly concentrated solution of 1 after being irradiated by 365nm UV light (Fig. 5). All these phenomenajointly indicatedthat the properties of supramolecular polymers could be efficiently modulated by photo-cross-linking methodology via the photodimerization of the coumarin motif.

|
Download:
|
| Figure 4. DLS plots of 1 (50mmol/L) in CHCl3 before and after irradiated by 365nm UV light for 2 days. | |
|
Download:
|
| Figure 5. SEM micrograph of fibers drawn from a very concentrated solution of 1 after being irradiated by 365nm UV light. | |
3. Conclusion
In conclusion, we synthesized a bifunctional UPy derivative 1 with coumarin-bridging unit, and 1 could form linear formed linear supramolecular polymers at relatively high concentrations, as demonstrated by 1H NMR, DLS, viscosity measurements, and SEM. More importantly, the properties of supramolecular polymers could be efficiently tuned by UV light irradiation at 365nm due to the photodimerization ability of the coumarin moiety. The present study provides a novel and simple method for the construction of smart supramolecular materials with tailored properties. Our future work will focus on developing more controllable and complicated supramolecular polymers by noncovalent interactions.
4. ExperimentalAll reactions were performed in atmosphere unless noted. The commercially available reagents and solvents were either employed as purchased or dried according to procedures described in the literature. Compound 5 was prepared bypublished literature procedures[12]. NMR spectra were recorded on a Bruker DPX 300MHz or 400MHz spectrometer and the chemical shifts (δ) were expressed in ppm and J values were given in Hz. Lowresolution electrospray ionization mass spectra (LR-ESI-MS) were obtained on Finnigan MatTSQ 7000 instruments.
Synthesis of compound 4: To a solution of 5 (0.20g, 0.59mmol) in CH2Cl2 (50mL) at 0 ℃ was added PBr3 (0.17mL, 1.77mmol) and the mixture was stirred at 0 ℃ for 1h and at 40 ℃ for 30h. The reaction mixture was then poured into ice water (40mL) and extracted with CH2Cl2 (2 ×30mL). The combined organic phase was washed with water (2 ×30mL), brine (30mL), and dried over anhydrous Na2SO4. After the solvent was removed with an evaporator under reduced pressure, the resulting residue was subjected to column chromatography (CHCl3) to give 4 (0.10g, 41.7%) as a white solid. 1H NMR (300MHz, CDCl3, 298K): δ 13.23 (s, 1H), 11.93 (s, 1H), 10.25 (s, 1H), 5.82 (s, 1H), 3.41 (t, 2H, J=6.8Hz), 3.29-3.25 (m, 2H), 2.36--2.26 (m, 1H), 1.96-1.87 (m, 2H), 1.69-1.49 (m, 8H), 1.33-1.21 (m, 4H), 0.92-0.85 (m, 6H). 13C NMR (75MHz, CDCl3, 298K): δ 173.2, 156.8, 155.5, 154.9, 106.3, 45.4, 39.8, 33.7, 33.0, 32.5, 29.4, 28.6, 26.7, 25.6, 22.6, 14.0, 11.8. LR-ESI-MS: m/z calcd. for [M+H]+ 401.16, found 401.15, [M+Na]+ 423.14, found 423.10.
Synthesis of compound 3: Sodium azide (0.16g, 2.50mmol), NaI (0.19g, 1.25mmol) and 4 (0.50g, 1.25mmol) were dissolved in 30mL of acetone and 6mL of H2O. After heating at reflux for 24h, water was added to quench the reaction. After removal of acetone, the solution was extracted with CH2Cl2 (3 ×30mL). The combined organic phase was washed with brine (30mL), and dried over anhydrous Na2SO4, and concentrated in vacuo toyield white solid 3 (0.43g, 95.6%). 1H NMR (300MHz, CDCl3, 298K): δ 13.23 (s, 1H), 11.92 (s, 1H), 10.26 (s, 1H), 5.82 (s, 1H), 3.31-3.25 (m, 4H), 2.36-2.23 (m, 1H), 1.70-1.40 (m, 10H), 1.35-1.21 (m, 4H), 0.92-0.85 (m, 6H).13C NMR (75MHz, CDCl3, 298K): δ 173.3, 156.9, 155.7, 155.0, 106.4, 51.5, 45.5, 39.9, 33.0, 29.5, 29.1, 28.7, 26.8, 24.2, 22.6, 14.0, 11.9. LRESI-MS: m/z calcd. for[M+H]+ 364.25, found 364.20, [M+Na]+ 386.23, found 386.20.
Synthesis of compound 2: To a stirred solution of 6, 7-dihydroxy-4-methylcoumarin (0.70g, 3.64mmol) and 3-bromo-1-propyne (1.25mL, 14.56mmol) in DMF (20mL), potassium carbonate (2.01g, 14.56mmol) was added at room temperature.
The mixture was then stirred at 80 ℃ for 24h under N2 atmosphere. Upon cooling to room temperature, the solution was pouredintowater (150mL), extracted with CH2Cl2 (3 ×30mL).
The combined organic phase was washed with brine (40mL), and dried over anhydrous Na2SO4. After the solvent was removed with an evaporator under reduced pressure, the resulting residue was subjected to column chromatography (CH2Cl2/n-hexane, 2:1, v/v) to give 2 (0.76g, 78.4%) as a white solid. 1H NMR (300MHz, CDCl3, 298K): δ 7.23 (s, 1H), 7.05 (s, 1H), 6.21 (s, 1H), 4.84 (d, 2H, J=2.4Hz), 4.82 (d, 2H, J=2.4Hz), 2.59-2.56 (m, 2H), 2.41 (s, 3H). 13C NMR (75MHz, CDCl3, 298K): δ 161.3, 152.3, 151.3, 149.9, 144.1, 113.5, 113.1, 110.4, 102.6, 78.1, 76.7, 57.9, 57.0, 19.0. LR-ESI-MS: m/z calcd.for[M+H]+ 269.08, found 269.05.
Synthesis of compound 1: 2 (0.43g, 1.61mmol), 3 (1.35g, 3.71mmol), CuSO4·5H2O (0.80g, 3.22mmol), and sodium ascorbate (1.27g, 6.44mmol) were dissolved in a mixture of THF (40mL), MeOH (8mL), and H2O (8mL). The reaction mixture was stirred at 60 ℃ for 48h. The solvents were removed in vacuo. The residue was poured into water (70mL), extracted with CH2Cl2 (2 ×30mL). The combined organic phase was washed with brine (50mL), and dried over Na2SO4. After the solvent was removed with an evaporator under reduced pressure, the resulting residue was subjected to column chromatography (CH2Cl2/CH3OH, 40:1, v/v) to give 1 (0.65g, 40.6%) as a white solid. 1H NMR (300MHz, CDCl3, 298K): δ 13.21 (s, 2H), 11.91 (s, 2H), 10.26 (s, 2H), 7.73 (s, 1H), 7.70 (s, 1H), 7.31 (s, 1H), 7.00 (s, 1H), 6.15 (s, 1H), 5.81 (s, 2H), 5.30 (s, 4H), 4.40-4.33 (m, 4H), 3.29-3.21 (m, 4H), 2.38 (s, 3H), 2.33-2.28 (m, 2H), 2.00-1.93 (m, 4H), 1.68-1.39 (m, 16H), 1.32-1.22 (m, 8H), 0.91-0.84 (m, 12H). 13C NMR (75MHz, CDCl3, 298K): δ 173.2, 161.3, 156.7, 155.6, 154.8, 152.5, 152.0, 149.6, 144.8, 143.6, 142.6, 123.2, 123.1, 113.3, 112.6, 110.6, 106.2, 102.2, 64.0, 63.0, 50.4, 45.3, 39.6, 32.9, 29.9, 29.3, 28.7, 26.6, 23.9, 22.5, 18.9, 13.9, 11.7. LR-ESI-MS: m/z calcd. for[M+H]+ 995.56, found 995.55, [M+Na]+ 1017.54, found 1017.50.
AcknowledgmentsThis work was sponsored by National Natural Science Foundation of China (Nos. 21602112 and 21472088) and supported by Scientific Starting Fund from Nanjing University of Posts and Telecommunications (NUPTSF) (No. NY215057).
| [1] |
(a)C. Schmuck, W. Wienand, Self-complementary quadruple hydrogen-bonding motifs as a functional principle: from dimeric supramolecules to supramolecular polymers, Angew. Chem. Int. Ed. 40(2001)4363-4369; (b)S. L. Li, T. Xiao, C. Lin, L. Wang, Advanced supramolecular polymers constructed by orthogonal self-assembly, Chem. Soc. Rev. 41(2012)5950-5968; (c)T. Aida, E. W. Meijer, S. I. Stupp, Functional supramolecular polymers, Science 335(2012)813-817; (d)Q. Wang, Y. Chen, Y. Liu, Supramolecular ternary polymer mediated by cucurbituril and cyclodextrin, Polym. Chem. 4(2013)4192-4198; (e)X. Y. Hu, T. Xiao, C. Lin, F. Huang, L. Wang, Dynamic supramolecular complexes constructed by orthogonal self-assembly, Acc. Chem. Res. 47(2014) 2041-2051; (f)X. Ma, H. Tian, Stimuli-responsive supramolecular polymers in aqueous solution, Acc. Chem. Res. 47(2014)1971-1981; (g)L. Yang, X. Tan, Z. Wang, X. Zhang, Supramolecular polymers: historical development, preparation, characterization, and functions, Chem. Rev. 115 (2015)7196-7239; (h)P. Wei, X. Yan, F. Huang, Supramolecular polymers constructed by orthogonal self-assembly based on host-guest and metal-ligand interactions, Chem. Soc. Rev. 44(2015)815-832; (i)H. Q. Peng, C. L. Sun, L. Y. Niu, et al. , Supramolecular polymeric fluorescent nanoparticles based on quadruple hydrogen bonds, Adv. Funct. Mater. 26 (2016)5483-5489. |
| [2] |
(a)T. F. A. D. Greef, M. M. J. Smulders, M. Wolffs, et al. , Supramolecular polymerization, Chem. Rev. 109(2009)5687-5754; (b)B. Zheng, F. Wang, S. Dong, F. Huang, Supramolecular polymers constructed by crown ether-based molecular recognition, Chem. Soc. Rev. 41(2012)1621-1636; (c)Y. Liu, Z. Huang, X. Tan, Z. Wang, X. Zhang, Cucurbit[8] uril-based supramolecular polymers: promoting supramolecular polymerization by metal-coordination, Chem. Commun. 49(2013)5766-5768; (d)C. Li, Pillararene-based supramolecular polymers: from molecular recognition to polymeric aggregates, Chem. Commun. 50(2014)12420-12433. |
| [3] | R.P. Sijbesma, F.H. Beijer, L. Brunsveld, Reversible polymers formed from self-complementary monomers using quadruple hydrogen bonding. Science 278 (1997) 1601–1604. DOI:10.1126/science.278.5343.1601 |
| [4] | X.Y. Hu, P. Zhang, X. Wu, Pillar[5] arene-based supramolecular polypseudorotaxanes constructed from quadruple hydrogen bonding. Polym.Chem. 3 (2012) 3060–3063. DOI:10.1039/c2py20285a |
| [5] | X. Yan, B. Jiang, T.R. Cook, Dendronized organoplatinum(Ⅱ)metallacyclic polymers constructed by hierarchical coordination-driven self-assembly and hydrogen-bonding interfaces. J.Am.Chem.Soc. 135 (2013) 16813–16816. DOI:10.1021/ja4092193 |
| [6] |
(a)R. J. Wojtecki, M. A. Meador, S. J. Rowan, Using the dynamic bond to access macroscopically responsive structurally dynamic polymers, Nat. Mater. 10 (2011)14-27; (b)Y. C. Zhang, L. Chen, H. Wang, et al. , Pleated polymeric foldamers driven by donor-acceptor interaction and conjugated radical cation dimerization, Chin. Chem. Lett. 27(2016)817-821; (c)J. F. Xu, Y. Z. Chen, L. Z. Wu, C. H. Tung, Q. Z. Yang, Dynamic covalent bond based on reversible photo[4+4] cycloaddition of anthracene for construction of double-dynamic polymers, Org. Lett. 15(2013)6148-6151; (d)F. Wang, C. Han, C. He, et al. , Self-sorting organization of two heteroditopic monomers to supramolecular alternating copolymers, J. Am. Chem. Soc. 130 (2008)11254-11255; (e)X. Ji, Y. Yao, J. Li, X. Yan, F. Huang, A supramolecular cross-linked conjugated polymer network for multiple fluorescent sensing, J. Am. Chem. Soc. 135(2013) 74-77; (f)X. Yan, D. Xu, X. Chi, et al. , A multiresponsive, shape-persistent, and elastic supramolecular polymer network gel constructed by orthogonal self-assembly, Adv. Mater. 24(2012)362-369. |
| [7] |
(a)M. M. Russew, S. Hecht, Photoswitches: from molecules to materials, Adv. Mater. 22(2010)3348-3360; (b)F. Ercole, T. P. Davis, R. A. Evans, Photo-responsive systems and biomaterials: photochromic polymers, light-triggered self-assembly, surface modification, fluorescence modulation and beyond, Polym. Chem. 1(2010)37-54. |
| [8] |
(a)M. Takeshita, M. Hayashi, S. Kadota, K. H. Mohammed, T. Yamato, Photoreversible supramolecular polymer formation, Chem. Commun. (2005)761-763; (b)S. L. Li, T. Xiao, W. Xia, et al. , New light on the ring-chain equilibrium of a hydrogen-bonded supramolecular polymer based on a photochromic dithienylethene unit and its energy-transfer properties as a storage material, Chem. Eur. J. 17(2011)10716-10723; (c)J. F. Xu, Y. Z. Chen, D. Wu, et al. , Photoresponsive hydrogen-bonded supramolecular polymers based on a stiff stilbene unit, Angew. Chem. Int. Ed. 52(2013)9738-9742; (d)X. Liu, J. F. Xu, Z. Wang, X. Zhang, Photo-responsive supramolecular polymers synthesized by olefin metathesis polymerization from supramonomers, Polym. Chem. 7(2016)2333-2336. |
| [9] |
(a)N. K. Mal, M. Fujiwara, Y. Tanaka, Photocontrolled reversible release of guest molecules from coumarin-modified mesoporous silica, Nature 421(2003) 350-353; (b)Q. Zhang, D. H. Qu, X. Ma, H. Tian, Sol-gel conversion based on photoswitching between noncovalently and covalently linked netlike supramolecular polymers, Chem. Commun. 49(2013)9800-9802; (c)M. Cheng, C. Yao, Y. Cao, et al. , 4-Methylcoumarin-bridged fluorescent responsive cryptand: from[2+2] photodimerization to supramolecular polymer, Chem. Commun. 52(2016)8715-8718. |
| [10] |
(a) H. Wang, Z.J. Zhang, H.Y. Zhang, Y. Liu, Synthesis of a bistable [3]rotaxane and its pH-controlled intramolecular charge-transfer behavior, Chin. Chem.Lett. 24 (2013) 563-567; (b) S.J. Zhang, Q. Wang, M. Cheng, et al., A switchable bistable [2]rotaxane based on phosphine oxide functional group, Chin. Chem. Lett. 26 (2015) 885-888. |
| [11] | Q. Zhang, D.H. Qu, J. Wu, A dual-modality photoswitchable supramolecular polymer. Langmuir 29 (2013) 5345–5350. DOI:10.1021/la4012444 |
| [12] | A.D. Celiz, O.A Scherman, Controlled ring-opening polymerization initiated via self-complementary hydrogen-bonding units. Macromolecules 41 (2008) 4115–4119. DOI:10.1021/ma702699t |
 2017, Vol. 28
2017, Vol. 28 


