b Collaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin 300071, China
Molecular recognition at self-assembled interfaces is gaining more and more attention benefiting from the unique physicochemical characteristics of interfaces, which offers distinguished binding affinities and selectivities from bulk solutions[1-4]. One of the most pronounced characteristics is the lower dielectric constant in the critical recognition zone close to the interface due to the dramatic change in polarity from the aqueous phase to the interface. Consequently, two kinds of typically noncovalent interactions, electrostatic and hydrogen bond, act much more efficiently at microscopic and macroscopic interfaces than in bulk water, which has been well demonstrated by Kunitake's group in their works on interfacial molecular recognition[3, 5-7]. With the same design principle, Prof. Schrader made a great contribution on building the interfacial sensing ensembles. By embedding artificial receptors in self-assembled mono-or bilayers they achieved the selective detection of noradrenaline and catecholamines[8, 9]. Moreover, interfaces are elegant platforms affording multipoint recognition, which enables efficient sensing of analytes with multiple sites, such as proteins, lections, glycans and beyond [10-15]. The Reinhoudt and Huskens's group has developed selfassembled monolayers of β-cyclodextrin receptors on gold or glass, which were called molecular printboards[14, 16, 17]. Multivalent host-guest interactions at interfaces were exploited to tune the binding strength and dynamics of the interaction of guest molecules with the printboard. However, the molecular recognition properties of the cyclodextrin cavities with small monovalent guests were surprisingly unaltered by the surface immobilization, which indicates that the microenvironment of a cyclodextrin cavity in the monolayer is comparable to that of a cavity in solution[18].
We have focused on molecular recognition of macrocyclic receptors in aqueous media for a long time, including cyclodextrin, sulfonatocalixarene and cucurbituril[19-21]. Very recently, our special interest on molecular recognition moves from bulk water to interface, which was employed to fabricate various functionalized self-assembled materials[22-24]. In this work, we are aiming to investigate the interfacial molecular recognition of macrocyclic receptors and their thermodynamic origins, to demonstrate the rationale behind it. Among kinds of macrocycles, p-sulfonatocalix[4]arene (SC4A) was screened because electrostatic, hydrogen bond and π-stacking interactions are generally involved in the host-guest association[25-31]. All these interactions are polaritydependent, and therefore, it is envisaged that the interfacial recognition varies drastically from that in aqueous phase. Despite not being extensively investigated, the interfacial recognition of SC4A owns at least the following three advantages: (1) The recognition and sensing of SC4A in aqueous solution has been well demonstrated[32-37], and embedding it in the self-assembled interface may create de novo interfacial sensing systems with different binding preferences owing to the unique physicochemical characteristics of interfaces; (2) Molecular recognition in biological systems usually proceeds at microscopic interfaces such as cell surface and protein surface, and such an artificial interfacial recognition provides better mimic models of biological systems in comparison with bulk solution; (3) One intriguing application of calixarene derivatives is activating bio-related molecules[38, 39], and the activity of calixarene as an artificial transmembrane transporter depends dominantly on its recognition at the lipid interface but not in bulk water.
To get more deep insight on the interfacial recognition, we systematically compared the binding of SC4A in aqueous solution and at the self-assembled interface by means of isothermal titration calorimetry (ITC). ITC is a powerful tool for determining the host-guest interactions because it not only gives the binding affinities (Ka) but also yields their thermodynamic parameters (enthalpy and entropy changes △H°, △S°). The combined data of Ka as well as △H° and △S° are envisioned to help us understanding the similarities and differences of recognition between in aqueous solution and at interface more clearly.
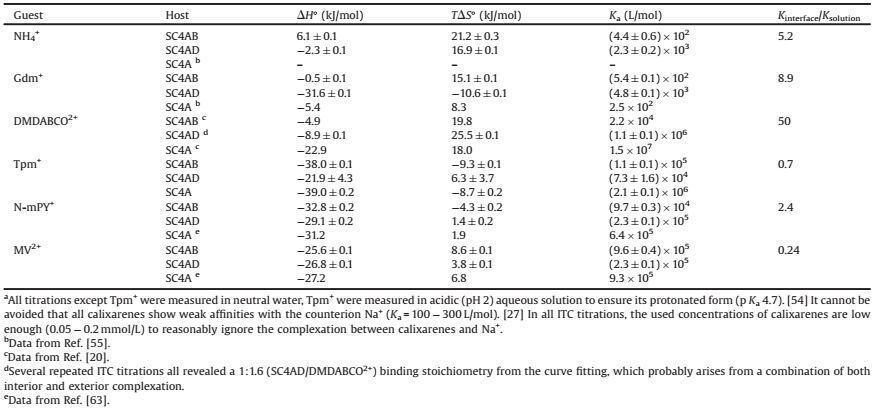
2. Results and discussionTwo lower-rim alkyl-appended derivatives of SC4A, p-sulfonatocalix[4]arene tetrabutyl ether (SC4AB) and p-sulfonatocalix[4] arene tetradodecyl ether (SC4AD), were employed as receptors (Scheme 1). SC4AB, with relatively higher critical micelle concentration (CMC, 3.20 mmol/L)[40], was engaged in the recognition in aqueous solution, while SC4AD (CMC = 0.02 mmol/ L) was engaged in the recognition at interface[41]. It should be noticed that these amphiphilic SC4A derivatives form micellar aggregates[42, 43], and after binding with guests, the hydrophiliclipophilic balance was destroyed, leading to further aggregation[44-46]. Generally, the complexes form larger supra-amphiphilic aggregates with lower CMC (complexation-induced aggregation)[20, 44]. Moreover, as demonstrated by Prof. Garcia-Rio more recently[47], both interior (host cavity) and exterior (micellar pseudophase) complexation may occur in an amphiphilic SC4A micellar system. All these factors make the interfacial recognition with formidable complexity. To address this issue, we embedded SC4AD into the N-[2-(2-hydroxyethoxy)ethyl]pentacosa-10, 12-diynamide (HEEPCDA) vesicle. HEEPCDA was reported to form pretty tight vesicular aggregation in water[48-50], which can serve as a hard media to investigate the interfacial recognition. Moreover, HEEPCDA is one kind of nonionic amphiphiles, which would affect the interfacial recognition of SC4AD to limited extent[51].
|
Download:
|
| Scheme 1. (a) Chemical structures of SC4AD, SC4AB, HEEPCDA and SDBS. (b) Schematic illustration of molecular recognition of SC4AB in bulk water and SC4AD at self-assembled interface. | |
Mixing SC4AD with HEEPCDA at a molar ratio of 1:4 generates a vesicular aggregation with the hydrodynamic diameter of 100 nm and Zeta potential of -38 mV (Figs. 1a and 1d). The remarkably negative Zeta potential indicates the substantial incorporation of SC4AD into the HEEPCDA vesicle, in good accordance with our previous result of incorporating amphiphilic calixarene into lipid[52]. The SC4AD/HEEPCDA co-assembly is stable enough to achieve a shelf life of several months without agglomeration and precipitation. More importantly, upon adding guests (Gdm+ and MV2+) into the SC4AD/HEEPCDA co-assembly, no appreciable change of diameter was observed (Figs. 1b and 1c), which rules out the complexation-induced aggregation (causing interferential heat effect in ITC measurements). On the other hand, in the SC4AD/ HEEPCDA co-assembly, SC4AD was surrounded and isolated by HEEPCDA molecules, and therefore, the exterior complexation is reasonably eliminated.

|
Download:
|
| Figure 1. Dynamic light scattering data of the SC4AD/HEEPCDA vesicle (a) upon complexation with Gdm+ (b) and MV2+ (c). Zeta potential of the SC4AD/HEEPCDA vesicle (d). | |
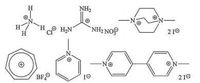
Taking into account the main driving forces for SC4A accommodating guests into their cavities, including hydrogen bond, electrostatic and π-stacking interactions[31], a total of six cationic molecules were screened as model guests (Fig. 2) to investigate comparably the molecular recognition between in aqueous media and at interface[53]. The obtained binding affinities, entropy and enthalpy changes were listed in Table 1, i.e., SC4AB in aqueous phase and SC4AD at self-assembled interface. The corresponding data of SC4A were presented as a reference. The effect of lower-rim modification on the recognition of SC4A has been demonstrated by us before[20, 36].
|
Download:
|
| Figure 2. Structures of the employed guests: ammonium (NH4+), guanidinium (Gdm+), N, N'-dimethyl-1, 4-diazabicyclo[2.2.2]octane (DMDABCO2+), tropylium (Tpm+), Nmethyl pyridinium (N-mPY+), methyl viologen (MV2+). | |
|
|
Table 1 The association constants (Ka), enthalpy (△H°) and entropy (T△S°) changes for the complexation of SC4A in aqueous phase, SC4AB in aqueous phase and SC4AD at selfassembled interface with guests at 298.15 K.a. |
At a glance of the data, it is intuitively found that the binding with DMDABCO2+, Gdm+ and NH4+ was noticeably strengthened to 1 -2 orders of magnitude from in aqueous phase to at interface, whereas the binding with MV2+, N-mPY+, Tpm+ increase slightly or even decrease to some extent. The binding enhancement arises from the lower dielectric constant due to the dramatic change in polarity from the aqueous phase to the interface, which gives rise to stronger electrostatic and hydrogen bond interactions. Furthermore, the contribution of either electrostatic or hydrogen bond could be dissected from the enthalpy and entropy terms. The complexation of DMDABCO2+ and NH4+ is consistently entropydriven both in aqueous phase and at interface, and then, the dominant driving force is assumed as electrostatic interaction. It has been well demonstrated in the cases of SC4A recognition, the extensive electrostatic-directed desolvation of negatively charged host and positively charged guest contributes to the entropy term[28]. The increasing factor (Kinterface/Ksolution) of DMDABCO2+ is almost 10 times larger than that of NH4+, probably due to the divalent electrostatic interaction. To further validate the contribution of electrostatic interaction, we employed inorganic cation Gd3+ as an alternative guest. The complexation of SC4A with divalent and trivalent metal ions is definitely governed by entropy gain, accompanied with unfavorable enthalpy change, resulting from the electrostatic-directed desolvation[27, 28]. We tried several times to measure the interfacial complexation of SC4AD with Gd3+ by ITC, but all failed because of the precipitation during the course of titration. Alternatively, the binding affinity was obtained by competitive fluorescence titration (see the Supporting Information), in which much more dilute solution was engaged. As expected, the Ka value is up to 1.4 × 106 L/mol due to the interfacereinforced electrostatic interaction, while the reported Ka value between SC4A and Gd3+ in bulk water is on the magnitude of almost 104 L/mol[28].
The similar enhancement of binding affinity was also observed in the case of Gdm+. However, differing from DMDABCO2+ and NH4+, the enthalpy and entropy terms of Gdm+ varied extraordinarily when the recognition process moved from bulk water to interface. The complexation of Gdm+ in bulk water is absolutely governed by the entropy term (T△S° = 15.1 kJ/mol) with a neglectable enthalpy change (△H° = -0.5 kJ/mol), while that at interface is dominantly governed by the enthalpy term (△H° = -31.6 kJ/mol) accompanied with an unfavorable entropy change (T△S° = -10.6 kJ/mol). Such an inversion between enthalpic and entropic contribution originates from the hydrogen bond interaction, which is drastically strengthened at the interface due to the lower dielectric constant.
It is interesting to compare the NH4+ and Gdm+ species. The cation radius of Gdm+ (0.21 nm) is larger than NH4+ (0.15 nm), which leads to the lower charge density of Gdm+[56]. Comparing with NH4+, Gdm+ is more weakly hydrated[57]. In other words, Gdm+ is more chaotropic than NH4+. Upon complexation with calixarenes, Gdm+ affords weaker electrostatic interaction and the corresponding less electrostatic-directed desolvation than NH4+ (in the case of SC4AB, T△△S°(Gdm+ -NH4+) = -6.1 kJ/mol). On the other hand, Gdm+ is a stronger hydrogen bond donor than NH4+, which can form the resonance-assisted hydrogen bond[58]. All these differences on physicochemical characteristics synergistically make the dramatically distinct thermodynamic origins upon complexation with Gdm+ and NH4+ at the interface (△△H°(Gdm+ -NH4+) = -29.3 kJ/mol; T△△S°(Gdm+ -NH4+) = -27.5 kJ/mol) although with similar binding affinities (△△G° (Gdm+ -NH4+) = -1.8 kJ/mol). It is noteworthy that the inverse thermodynamic origins between Gdm+ and NH4+ can merely be observed in the interfacial recognition, where the entropy-driven electrostatic and enthalpy-driven hydrogen bond interactions were amplified at the interface, respectively. Fig. 3 shows the radar plots of the thermodynamic sensing responses containing three parameters (△G°, △H° and T△S°). In bulk water, the fingerprints of NH4+ and Gdm+ are similar, which can hardly be applied for selective sensing. At self-assembled interface, the fingerprints of NH4+ and Gdm+ differ from each other definitely, mainly governed by the thermodynamic origins (△H° and T△S°). We are reasonable to envisage that the present interfacial recognition system shows potential application in discriminating peptides/proteins who is either lysine-rich or arginine-rich from the viewpoint of thermodynamics.

|
Download:
|
| Figure 3. Radar plots of the thermodynamic sensing responses (△G, △H and T△S) for discriminating NH4+ and Gdm+ by the complexation of SC4A in bulk water (a) and at selfassembled interface (b). | |
For the rest three aromatic cations, MV2+, N-mPY+ and Tpm+, the binding affinities at interface do not change too much extent in comparison with those in in aqueous phase, that is, 1.5 -4.2 times either increase or decrease. Taking the strengthened electrostatic interaction at interface into account, we inferred that the π-stacking interaction (including C -H...π, π...π and charge transfer interactions in our cases) was weakened in the interfacial recognition process. Herein we took Tpm+ as the representative example to illuminate the π-stacking interaction. Tpm+ is an electron-deficient aromatic guest with purely planer configuration. Upon lower-rim modification, SC4AB and SC4AD possess electron-rich cavity with pinched-cone shape as a result of conformational rigidification[20, 35]. During the course of complexation, strong π-stacking interaction thus occurs between calixarene and Tpm+. Comparing the thermodynamic parameters between aqueous phase and interface, we found that the entropy term at interface is less unfavorable (T△△S° = 15.6 kJ/mol) arising from the strengthened electrostatic interaction. On the contrary, the enthalpy term turns to be less favorable (△△H° = 16.1 kJ/mol).We ascribed it to the weakened π-stacking interaction at interface.The π-stacking interaction is also polarity-dependent that the strength decreases with diminishing the polarity of solvents[58].
Furthermore, we embedded sodium dodecyl benzenesulfonate (SDBS) into the HEEPCDA vesicle to declare the irreplaceable role of calixarene skeleton in the interfacial recognition. Incorporating SDBS with HEEPCDA at 1:1 molar ratio (the same ratio of benzenesulfonate units with SC4AD) generates also a vesicular aggregation with the hydrodynamic diameter of 160 nm (Fig. S2 in Supporting information). The ITC titration of Gdm+ with SDBS was performed in the same condition with SC4AD (Fig. S5 in Supporting information). Upon arranging at the interface, SDBS also shows somewhat interaction with Gdm+. We didn't obtain the binding constant between SDBS and Gdm+ because the titration curve could not be fitted well. However, it can be read from the heat effect of injection that the complexation of SDBS is much weaker than that of SC4AD, which proves the specificity of macrocyclic host. SDBS molecules are randomly dispersed in the bilayer of HEEPCDA vesicle, and thus, the unspecific ion pair interaction between SO3- and Gdm+ cannot rival the synergistic interactions offered by the pre-organization of SC4A (electrostatic, hydrogen bond, cation-π, π-stacking, etc.).
3. ConclusionIn conclusion, we have observed that the molecular recognition of SC4A is substantially different in aqueous media and at selfassembled interface, most probably due to the unique physicochemical characteristics of interfaces. The dramatic change in polarity and dielectric constant from bulk water to interface leads to strengthened electrostatic and hydrogen bond interactions but weakened π-stacking interaction. As a result, SC4A at interface exhibits pronounced enhancement of binding affinities towards NH4+, Gdm+ and DMDABCO2+ while slight alternation towards Tpm+, N-mPY+ and MV2+. Moreover, the contribution of electrostatic and hydrogen bond interactions to the binding events could be dissected in light of thermodynamics, i.e. enthalpy and entropy terms. Although in our present study SC4A has served as a specific test case for molecular recognition at interface, the conclusions are transferable to other charged, macrocyclic, acyclic, artificial, and natural receptors. We envisage that investigating molecular recognition at interface is scientifically significant to design de novo interfacial sensing systems, provide better mimic models of biological systems, and develop artificial transmembrane activators
4. Experimental 4.1. Materialsp-Sulfonatocalix[4]arene tetrabutyl ether (SC4AB)[59], p-sulfonatocalix[4]arene tetradodecyl ether (SC4AD)[40] and N-[2-(2-hydroxyethoxy)ethyl]pentacosa-10, 12-diynamide (HEEPCDA)[47] were synthesized and purified according to the respective literature procedures. Their 1H NMR spectra were supplied in Fig. S1 in Supporting information. Methyl viologen (MV2+)[60], N, N'-dimethyl-1, 4-diazabicyclo[2.2.2]octane (DMDABCO2+)[61] and N-methyl pyridinium (N-mPY+)[62] were synthesized and purified according to the respective literature procedures. Guanidinium (Gdm+), ammonium (NH4+), tropylium (Tpm+) and sodium dodecyl benzenesulfonate (SDBS) were commercially available and used without further purification.
4.2. The preparation of SC4AD/HEEPCDA vesicleSC4AD was dissolved in distilled water at a concentration of 1.0 mmol/L. A solution of 0.04 mmol HEEPCDA in chloroform was dried under vacuum. Then a certain amount of SC4AD solution and distilled water were added until got the final concentrations of 0.4 and 1.6 mmol/L for SC4AD and HEEPCDA, respectively. The samples were then sonicated at 80 ℃ for 30 min, subsequently cooled to room temperature.
4.3. Isothermal titration calorimetry (ITC) measurementsA thermostated and fully computer-operated isothermal calorimetry (VP-ITC) instrument, purchased from Microcal Inc., Northampton, MA, was used for all microcalorimetric experiments.
All titrations were performed at atmospheric pressure and 298.15 K. The titrations of Tpm+ with calixarenes were occurred in an acid aqueous solution (pH 2), while the others were in neutral aqueous solution. Each solution was degassed and thermostatted by a ThermoVac accessory before the titrations. 6 -10 μL of guest (0.5 -15.0 mmol/L) solution in a 0.2887 mL syringe was injected (25 -40 injections) into the reaction cell (1.4288 ml) charged with host solution (0.05 -0.2 mmol/L) in the same aqueous solution (Figs. S3a and S4a). The heat of dilution was measured by injecting the gust solution into a blank solution containing no host. The net heat effect was obtained by subtracting dilution heat value from the overall heat effect observed (Figs. S3b and S4b) and was analyzed by using the "one set of binding sites" model (ORIGIN software, Microcal Inc.) (Figs. S3c and S4c). In the case of interfacial recognition, the binding sites of calixarenes on the self-assembled surface were assumed as identical and independent, and therefore the isodesmic or equal-K model was implemented to analyze the data.
However, for the complexation of Gdm+ and NH4+ with SC4AB, the heat effects were too small to get accurate data by the direct titration. The measurements were performed by using the competitive method. 1.0 mmol/L MV2+ solution was sequentially injected into a 0.1 mmol/L SC4AB solution containing 8.0 mmol/L Gdm+ or 10.0 mmol/L NH4+. The data were analyzed by the "competitive binding" model (ORIGIN software, Microcal Inc.).
AcknowledgmentsThis work was supported by NSFC (Nos. 21322207 and 21672112), the Fundamental Research Funds for the Central Universities and Program of Tianjin Young Talents, which are gratefully acknowledged. We also thank Prof. Yu Liu for the convenient access to the ITC measurements.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.027.
| [1] | Q. Wang, Z. Li, D.D. Tao, Supramolecular aggregates as sensory ensembles. Chem.Commun. 52 (2016) 12929–12939. DOI:10.1039/C6CC06075G |
| [2] | K. Ariga, H. Ito, J.P. Hill, H Tsukube, Molecular recognition:from solution science to nano/materials technology. Chem.Soc.Rev. 41 (2012) 5800–5835. DOI:10.1039/c2cs35162e |
| [3] | K. Ariga, T Kunitake, Molecular recognition at air-water and related interfaces: Complementary hydrogen bonding and multisite interaction. Acc.Chem.Res. 31 (1998) 371–378. DOI:10.1021/ar970014i |
| [4] | H. Wang, D. Zhang, X. Zhao, Z Li, Supramolecular organic frameworks(SOFs): water-phase periodic porous self-assembled architectures. Acta Chim.Sinica 73 (2015) 471. DOI:10.6023/A14120880 |
| [5] | M. Onda, K. Yoshihara, H. Koyano, K. Ariga, T Kunitake, Molecular recognition of nucleotides by the guanidinium unit at the surface of aqueous micelles and bilayers.A comparison of microscopic and macroscopic interfaces. J.Am. Chem.Soc. 118 (1996) 8524–8530. DOI:10.1021/ja960991+ |
| [6] | K. Ariga, A. Kamino, X. Cha, T Kunitake, Multisite recognition of aqueous dipeptides by oligoglycine arrays mixed with guanidinium and other receptor units at the air-water interface. Langmuir 15 (1999) 3875–3885. DOI:10.1021/la981047p |
| [7] | X. Cha, K. Ariga, T Kunitake, Molecular recognition of aqueous dipeptides at multiple hydrogen-bonding sites of mixed peptide monolayers. J.Am.Chem. Soc. 118 (1996) 9545–9551. DOI:10.1021/ja961526f |
| [8] | S. Kolusheva, O. Molt, M. Herm, T. Schrader, R Jelinek, Selective detection of catecholamines by synthetic receptors embedded in chromatic polydiacetylene vesicles. J.Am.Chem.Soc. 127 (2005) 10000–10001. DOI:10.1021/ja052436q |
| [9] | O. Molt, D. Rubeling, T Schrader, A selective biomimetic tweezer for noradrenaline. J.Am.Chem.Soc. 125 (2003) 12086–12087. DOI:10.1021/ja035212l |
| [10] | R. Zadmard, M. Arendt, T Schrader, Multipoint recognition of basic proteins at a membrane model. J.Am.Chem.Soc. 126 (2004) 7752–7753. DOI:10.1021/ja049191m |
| [11] | C.C. Hayden, J.S. Hwang, E.A. Abate, M.S. Kent, D.Y Sasaki, Directed formation of lipid membrane microdomains as high affinity sites for his-tagged proteins. J.Am.Chem.Soc. 131 (2009) 8728–8729. DOI:10.1021/ja901157c |
| [12] | R. V. Vico, J. Voskuhl, B. J. Ravoo, Multivalent interaction of cyclodextrin vesicles, carbohydrate guests, and lectins: a kinetic investigation, Langmuir 27 (2011)1391-1397. |
| [13] | A. Samanta, M.C.A. Stuart, B.J Ravoo, Photoresponsive capture and release of lectins in multilamellar complexes. J.Am.Chem.Soc. 134 (2012) 19909–19914. DOI:10.1021/ja3101837 |
| [14] | J. Huskens, Multivalent interactions at interfaces, Curr. Opin. Chem. Biol. 10 (2006)537-543. |
| [15] | M.H. Mashhadizadeh, R.P Talemi, Application of diazo-thiourea and gold nano-particles in the design of a highly sensitive and selective DNA biosensor. Chin.Chem.Lett. 26 (2015) 160–166. DOI:10.1016/j.cclet.2014.09.004 |
| [16] | O. Crespo-Biel, B. J. Ravoo, J. Huskens, D. N. Reinhoudt, Writing with molecules on molecular printboards, Dalton Trans. (2006)2737-2741. |
| [17] | M.J.W. Ludden, D.N. Reinhoudt, J Huskens, Molecular printboards:versatile platforms for the creation and positioning of supramolecular assemblies and materials. Chem.Soc.Rev. 35 (2006) 1122–1134. DOI:10.1039/b600093m |
| [18] | Jong M.R.de, J. Huskens, D.N Reinhoudt, Influencing the binding selectivity of self-assembled cyclodextrin monolayers on gold through their architecture. Chem.Eur.J. 7 (2001) 4164–4170. DOI:10.1002/(ISSN)1521-3765 |
| [19] | D.S. Guo, Y Liu, Suprannolecular chemistry of p-sulfonatocalix[n] arenes and its biological applications. Acc.Chem.Res. 47 (2014) 1925–1934. DOI:10.1021/ar500009g |
| [20] | X.Y. Hu, S. Peng, D.S. Guo, F. Ding, Y Liu, Ding F., Liu Y.Molecular recognition of amphiphilicp-sulfonatocalix[4] arene with organic ammoniums. Supramol. Chem. 27 (2014) 336–345. |
| [21] | Y. Liu, C.J. Li, D.S. Guo, Z.H. Pan, Z Li, A comparative study of complexation of β-cyclodextrin, calix[4] arenesulfonate and cucurbit[7] uril with dye guests: fluorescence behavior and binding ability. Supramol.Chem. 19 (2007) 517–523. DOI:10.1080/10610270601145444 |
| [22] | Z. Xu, S. Peng, Y.Y. Wang, Broad-spectrum tunable photoluminescent nanomaterials constructed from a modular light-harvesting platform based on macrocyclic amphiphiles. Adv.Mater. 28 (2016) 7666–7671. DOI:10.1002/adma.201601719 |
| [23] | W.C. Geng, Y.C. Liu, Y.Y. Wang, A self-assembled white-light-emitting system in aqueous medium based on a macrocyclic amphiphile. Chem. Commun. 53 (2017) 392–395. DOI:10.1039/C6CC09079F |
| [24] | Y.X. Wang, D.S. Guo, Y.C. Duan, Y.J. Wang, Y. Liu, Amphiphilic p-sulfonatocalix[4] arene as drug chaperone for escorting anticancer drugs. Sci.Rep. 5 (2015) 9019. DOI:10.1038/srep09019 |
| [25] | D.S. Guo, K. Wang, Y Liu, Selective bindingbehaviors ofp-sulfonatocalixarenes in aqueous solution. J.Incl.Phenom.Macrocycl.Chem. 62 (2008) 1–21. DOI:10.1007/s10847-008-9452-2 |
| [26] | G. Arena, A. Casnati, A. Contino, Water-soluble calixarene hosts that specifically recognize the trimethylammonium group or the benzene ring of aromatic ammonium cations:a combined 1H NMR, calorimetric, and molecular mechanics investigation. Chem.Eur.J. 5 (1999) 738–744. DOI:10.1002/(ISSN)1521-3765 |
| [27] | V. Francisco, A. Pineiro, W. M. Nau, L. Garcia-Rio, The true affinities of metal cations to p-sulfonatocalix[4] arene: a thermodynamic study at neutral ph reveals a pitfall due to salt effects in microcalorimetry, Chem. Eur. J. 19(2013) 17809-17820. |
| [28] | C. Bonal, Y. Israeli, J. P. Morel, N. Morel-Desrosiers, Binding of inorganic and organic cations by p-sulfonatocalix[4] arene in water: a thermodynamic study, J. Chem. Soc. , Perkin Trans. 2(2001)1075-1078. |
| [29] | D.S. Guo, L.H. Wang, Y Liu, Highlyeffectivebindingofmethylviologendication and its radical cation by p-sulfonatocalix[4, 5] arenes. J.Org.Chem. 72 (2007) 7775–7778. DOI:10.1021/jo701304g |
| [30] | Y. Liu, D.S. Guo, H.Y. Zhang, Y.H. Ma, E.C Yang, he structure and thermodynamics of calix[n] arene complexes with dipyridines and phenanthroline in aqueous solution studied by microcalorimetry and NMR spectroscopy. J.Phys.Chem.B 110 (2006) 3428–3434. DOI:10.1021/jp0545703 |
| [31] | D.S. Guo, H.Q. Zhang, F. Ding, Y Liu, Thermodynamic origins of selective binding affinity between p-sulfonatocalix[4, 5] arenes with biguanidiniums. Org.Biomol.Chem. 10 (2012) 1527–1536. DOI:10.1039/c2ob06313a |
| [32] | K.N. Koh, K. Araki, A. Ikeda, H. Otsuka, S Shinkai, Reinvestigation of calixarene-based artificial-signaling acetylcholine receptors useful in neutral aqueous (water/methanol)solution. J.Am.Chem.Soc. 118 (1996) 755–758. DOI:10.1021/ja951488k |
| [33] | V. Francisco, N. Basilio, L. Garcia-Rio, Counterion exchange as a decisive factor in the formation of host: guest complexes by p-sulfonatocalix[4] arene, J. Phys. Chem. B 116(2012)5308-5315. |
| [34] | N. Douteau-Guével, A. W. Coleman, J. P. Morel, N. Morel-Desrosiers, Complexation of the basic amino acids lysine and arginine by three sulfonatocalix[n] arenes(n=4, 6 and 8) in water: microcalorimetric determination of the Gibbs energies, enthalpies and entropies of complexation, J. Chem. Soc. Perkin Trans. 2(1999)629-634. |
| [35] | O.I. Kalchenko, F. Perret, ${referAuthorVo.mingEn} N.Morel-Desrosiers, A.W Coleman, A comparative study of the determination of the stability constants of inclusion complexes of p-sulfonatocalix[4] arene with amino acids by RP-HPLC and 1H NMR. J.Chem. Soc.Perkin Trans. 2 (2001) 258–263. |
| [36] | J. Cui, V.D. Uzunova, D.S. Guo, Effect of lower-rim alkylation of p-sulfonatocalix[4] arene on the thermodynamics of host-guest complexation. Eur.J.Org.Chem. 2010 (2010) 1704–1710. DOI:10.1002/ejoc.v2010:9 |
| [37] | L.H. Wang, P. Du, J. Yang, D.S. Guo, Y Liu, Binding behaviour and solubilisation ofp-sulfonatocalixarenes to cinchona alkaloids. Supramol.Chem. 26 (2014) 809–816. DOI:10.1080/10610278.2014.882509 |
| [38] | F. Perret, M. Nishihara, T. Takeuchi, Anionic fullerenes calixarenes, coronenes, and pyrenes as activators of oligo/polyarginines in model membranes and live cells. J.Am.Chem.Soc. 127 (2005) 1114–1115. DOI:10.1021/ja043633c |
| [39] | T. Takeuchi, V. Bagnacani, F. Sansone, S Matile, Amphiphilic counterion activators for DNA:stimuli-responsive cation transporters and biosensors in bulk and lipid bilayer membranes. ChemBioChem 10 (2009) 2793–2799. DOI:10.1002/cbic.v10:17 |
| [40] | N. Basilio, L. Garcia-Rio, Calixarene-based surfactants:conformational-dependent solvation shells for the alkyl chains. Chem Phys Chem 13 (2012) 2368–2376. DOI:10.1002/cphc.v13.9 |
| [41] | K.P. Wang, Y. Chen, Y Liu, A polycation-induced secondary assembly of amphiphilic calixarene and its multi-stimuli responsive gelation behavior. Chem.Commun. 51 (2015) 1647–1649. DOI:10.1039/C4CC08721F |
| [42] | N. Basilio, V. Francisco, L. Garcia-Rio, Aggregation of p-sulfonatocalixarene-based amphiphiles and supra-amphiphiles, Int. J. Mol. Sci. 14(2013)3140-3157. |
| [43] | N. Basilio, L. Garci'a-Ri'o, M. Marti'n-Pastor, NMR evidence of slow monomer-micelle exchange in a calixarene-based surfactant, J. Phys. Chem. B 114(2010) 4816-4820. |
| [44] | Z.B. Qin, D.S. Guo, X.N. Gao, Y Liu, Supra-amphiphilic aggregates formed by p-sulfonatocalix[4] arenes and the antipsychotic drug chlorpromazine. Soft Matter 10 (2014) 2253–2263. |
| [45] | X.Y. Hu, Y. Chen, Y Liu, Redox-responsive supramolecular nanoparticles based on amphiphilic sulfonatocalixarene and selenocystamine dihydrochloride. Chin.Chem.Lett. 26 (2015) 862–866. DOI:10.1016/j.cclet.2015.01.003 |
| [46] | Y.X. Wang, Y Liu, Supramolecular assemblies based on p-sulfonatocalixarenes and their functions. Acta Chim.Sinica 73 (2015) 984–991. |
| [47] | S. Fernandez-Abad, M. Pessego, N. Basilio, L. Garcia-Rio, Counterion-controlled self-sorting in an amphiphilic calixarene micellar system, Chem. Eur. J. 22 (2016)6466-6470. |
| [48] | M.J. Shin, J.D Kim, Reversible chromatic response of polydiacetylene derivative vesicles in D2O solvent. Langmuir 32 (2016) 882–888. DOI:10.1021/acs.langmuir.5b03945 |
| [49] | B. Yoon, S. Lee, J. M. Kim, Recent conceptual and technological advances in polydiacetylene-based supramolecular chemosensors, Chem. Soc. Rev. 38 (2009)1958-1968. |
| [50] | D.J. Ahn, J.M Kim, Fluorogenic polydiacetylene supramolecules: immobilization, micropatterning, and application to label-free chemosensors. Acc.Chem.Res. 41 (2008) 805–816. DOI:10.1021/ar7002489 |
| [51] | T. Jin, F. Fujii, Y Ooi, Interfacial recognition of acetylcholine by an amphiphilic p-sulfonatocalix[8] arene derivative incorporated into dimyristoyl phosphatidylcholine vesicles. Sensors 8 (2008) 6777–6790. DOI:10.3390/s8106777 |
| [52] | Y.X. Wang, Y.M. Zhang, Y.L. Wang, Y Liu, Multifunctionalvehicleof amphiphilic calix[4] arene mediated by liposome. Chem.Mater. 27 (2015) 2848–2854. DOI:10.1021/cm504653k |
| [53] | J. Ma, Q. Meng, X. Hu, Synthesis of a water-soluble carboxylatobiphen[4] arene and its selective complexation toward acetylcholine. Org.Lett. 18 (2016) 5740–5743. DOI:10.1021/acs.orglett.6b03005 |
| [54] | W. Von, E. Doering, L.H Knox, Thecycloheptatrienylium(tropylium)ion. J.Am. Chem.Soc. 76 (1954) 3203–3206. DOI:10.1021/ja01641a027 |
| [55] | F. Perret, J. P. Morel, N. Morel-Desrosiers, Thermodynamicsof thecomplexation of the p-sulfonatocalix[4] arene with simple model guests in water: a microcalorimetric study, Supramol. Chem. 15(2003)199-206. |
| [56] | Y. Marcus, Ions in Water and Biophysical Implications, Springer, Netherlands, Dordrecht, 2012. |
| [57] | P.E. Mason, G.W. Neilson, C.E. Dempsey, A.C. Barnes, J.M Cruickshank, The hydration structure of guanidinium and thiocyanate ions:implications for protein stability in aqueous solution. Proc.Natl.Acad.Sci. 100 (2003) 4557–4561. DOI:10.1073/pnas.0735920100 |
| [58] | S.M. So, K. Moozeh, A.J. Lough, J Chin, Highly stereoselective recognition and deracemization of amino acids by supramolecular self-assembly. Angew. Chem.Int.Ed. 53 (2014) 829–832. DOI:10.1002/anie.201307410 |
| [59] | D.B. Smithrud, F Diederich, Strength of molecular complexation of apolar solutes in water and in organic solvents is predictable by linear free energy relationships:a general model for solvation effects on apolar binding. J.Am. Chem.Soc. 112 (1990) 339–343. DOI:10.1021/ja00157a052 |
| [60] | S. Shinkai, S. Mori, H. Koreishi, T. Tsubaki, O Manabe, Hexasulfonated calix[6] arene derivatives:a new class of catalysts, surfactants, and host molecules. J. Am.Chem.Soc. 108 (1986) 2409–2416. DOI:10.1021/ja00269a045 |
| [61] | K. Wang, D. S. Guo, H. Q. Zhang, etal. , Highlyeffective bindingof viologensbyp-sulfonatocalixarenes for the treatment of viologen poisoning, J. Med. Chem. 52 (2009)6402-6412. |
| [62] | G.M. Wang, J.H. Li, X. Zhang, Facile in situ syntheses of new templates and formations of three zinc phosphates. Inorg.Chem.Commun. 46 (2014) 295–300. DOI:10.1016/j.inoche.2014.06.025 |
| [63] | H.X. Zhao, D.S. Guo, Y Liu, Binding behaviors of p-sulfonatocalix[4] arene with gemini guests. J.Phys.Chem.B 117 (2013) 1978–1987. DOI:10.1021/jp312744d |
 2017, Vol. 28
2017, Vol. 28