b Department of Chemistry, South University of Science and Technology of China, Shenzhen 518055, China;
c Dalian Institute of Chemical Physics, Chinese Academy of Science, Dalian 116023, China;
d School of Chemical Engineering and Environment, North University of China, Shanxi 030051, China
In the past decade, low molecular weight gelators (LMWG) [1, 2] have attracted a lot of attention, due to its potential applications in organocatalysis [3, 4], stimuli-responsive materials [5–9], drug delivery systems [10], pharmaceutical crystallization [11], oil-spill recovery [12–14], and so on. The LMWG which can gelate organic liquids are organogelators. Chemical functional groups, which direct the molecules to self-assemble into one-dimensional structure, are usually needed in LMWG. The commonly used functional groups include alcohol [15, 16], amides [17, 18] and ureas [19–23]. Multiple functional groups are necessary to enhance the strength of the fibers by multivalent effect.
In urea-based organogelators, planar aromatic groups are often used in conjunction with two urea groups, and π…π stacking cooperate with hydrogen bonding to facilitate the formation of one-dimensional fibers and thus gels [24]. Two neighbouring π systems in π…π stacking are slightly off-centered rather than in perfect parallel [25]. This changes the secondary structure of the one-dimensional system and may also further affect the stability of the resulting gel. To the best of our knowledge, curved π system has not been used in conjunction with urea groups for organogelators. We wonder that whether non-planar π system can be incorporated into urea organogelator and whether this will contribute to the stability of the gels.
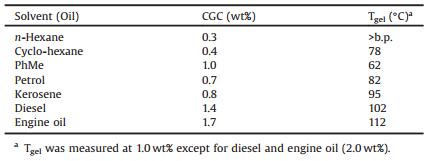
We have been interested in naphthol-based macrocyclic receptors [26]. Three classes of macrocyclic receptors have been synthesized: oxatub[n]arenes [27, 28], dynamic imine macrocycles [29, 30], and endo-functionalized molecular tubes [31–33]. The latter two are based on a curved bis-naphthalene scaffold, which can self-organize into a "supramolecular polymer" in the solid state [34]. In the "supramolecular polymer" (Scheme 1a), the neighboring two molecules are organized in a "hug from behind" fashion and are antiparallel. In this organization, C-H…π interactions and off-center π…π interactions are maximized, and the dipole moments of the neighboring molecules are also satisfied. This supramolecular polymer was not detected in solution. But we envisioned that this curved bis-naphthalene cleft may be well incorporated with two urea groups, affording a bisurea organogelator with a curved core. In the present research, we would like to report the synthesis of the first urea-based organogelator with a curved π system, the gelation properties and its application to oil-spill recovery.
|
Download:
|
| Scheme 1. (a) Chemical structure and single crystal packing of 1 and (b) Synthetic procedures of compounds 2a and 2b. Numbering on the structures is used for the assignments of NMR signals. | |
2. Results and discussion
Compounds 2a and 2b were synthesized by coupling the diamine 3 with the isocyanate 4a or 4b in the presence of Hünig's base (Scheme 1b). Both compounds 2a and 2b were obtained in good yields and carefully characterized.
Gelation abilities of 2a (1.0 wt%) in different solvents were tested by inverted-tube method. 2a is not soluble in MeOH, EtOH, n-heptanol, H2O, ethyl acetate (EtOAc), acetone, acetonitrile, and 1, 4-dioxane, but is well soluble in THF, CHCl3, CCl4, and chlorobenzene (PhCl). In contrast, petroleum ether (PE), toluene (PhMe), xylol, cyclohexane (CH), and methyl cyclohexane (MCH) can be gelated by 2a in 1.0 wt%. This gelation ability can be extended to linear alkanes, from n-hexane (n-C6H14) to n-tetradecane (n-C14H30), although n-tridecane and n-tetradecane require higher concentration of 2a (2.0 wt%) to form gels.
Linear alkanes, cyclic alkanes, and aromatic hydrocarbons are the major components in fuel oil. Logically, we wondered whether 2a can gelate fuel oil. Indeed, fuel oils, such as petrol, kerosene, diesel, and engine oil, can be gelated by 2a. The gelation parameters, including critical gelation concentration (CGC) and gelation temperature (Tgel) for these oils and n-hexane, cyclohexane, and PhMe are listed in Table 1. 2a is close to a supergelator (0.1 wt%) [35] for n-hexane and cyclohexane. For toluene and oils, higher concentration of 2a is required. The gels are all stable up to very high temperature, suggesting that the resulting gel is very robust.
|
|
Table 1 Gelation parameters of 2a in several selected solvents and oilsa. |
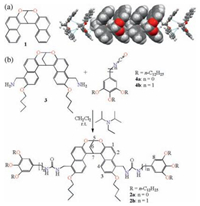
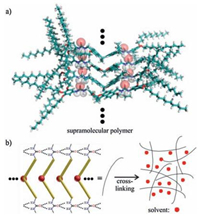
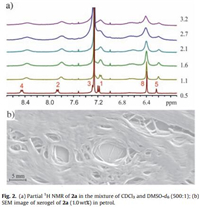
Gelator 2a contains two urea groups which are prone to selfassemble in nonpolar solvents. As shown in Fig. 1, the gelation is probably through the formation of the supramolecular polymer of 2a, which is supported by the following evidences: a) Molecular modelling indicate that the off-centered π…π stacking and C — H…π interactions of bis-naphthalene clefts can indeed cooperate with the hydrogen bonds of the urea groups (Fig. 1a). Very robust supramolecular polymer may form. In the nonpolar solvents, this effect is amplified and thus supramolecular polymers with a high degree of polymerization are formed. These polymers further entangle and thus gelate these solvents; b) 1H NMR experiments of 2a at different concentrations in the mixture of CDCl3 and DMSO-d6 (500:1) were performed (Fig. 2a). With increasing the concentration, the relative sharp signals are gradually becoming broadened, indicating the aggregation. Meantime, the aromatic protons on the bis-naphthalene clefts were shifted to upfield, suggesting that they experienced shielding effect which is in line with the above-discussed organization; c) SEM image of xerogel of 2a (1.0 wt%) in petrol shows that there are entangled networks, suggesting 2a forms one-dimensional fibers which further entangle, resulting in gelation.
|
Download:
|
| Figure 1. (a) Energy-minimized structure of the tetramer of 2a at the MMFF level of theory by using Spartan' 14 (Wavefunction Inc.), and (b) cartoon representation of the gelation process. | |
|
Download:
|
| Figure 2. (a) Partial 1H NMR of 2a in the mixture of CDCl3 and DMSO-d6 (500:1); (b) SEM image of xerogel of 2a (1.0 wt%) in petrol. | |
In great contrast, compound 2b, with one more methylene group next to each urea group than 2a, cannot gelate these solvents and oils even at much higher concentrations. This is probably due to that the additional methylene groups disturb the aggregation. This is supported by the 1H NMR spectra (Fig. S2 in Supporting information) of 2b at different concentrations in the mixture of CDCl3 and DMSO (500:1): with increasing the concentration in the same range as for 2a, only very slight peak broadening and shift were observed, indicating very weak tendency to aggregate. This suggests that the gelation result from the good balance of all these weak interactions.
One of potential applications of LMWG is to recover spilled-oil from waste water or sea water [12]. This method is considered to be effective and environmentally benign. However, large amount of carrier solvents are usually needed for the uniform distribution of gelator molecules. These solvents either diffuse into water provided they are water-miscible non-gelling solvents, or congeal along with the oil if they are water-immiscible gelling solvents. In both cases, it causes problems to the ecosystem or the application procedure of the gelator. Therefore, it would be more practical and ideal to directly apply gelators in the powder form. Very recently, two examples were reported in which gelators can be directly applied in the powder form to congeal oil in water [13, 14]. These two classes of gelators are based on sugar or aminoacid scaffolds. To the best of our knowledge, no urea gelator was reported to be able to congeal oil. Gelator 2a is promising for this purpose.
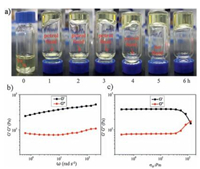
It is more important for the oil-spill recovery whether the gelator 2a can be applied in the powder form. As shown in Fig. 3a, the powder of 2a was added into petrol (95) in 1.0 wt%. The mixture was kept standing at room temperature, and the gelation was gradually formed. After ca. one hour, half of the petrol was already gelated; after five hours, the petrol was fully gelated. The similar phenomenon was observed for other solvents and oils. This indicates that gelator 2a has certain solubility in these solvents, permitting the application of 2a to the recovery of oil spill in the powder form.
|
Download:
|
| Figure 3. (a) Gelation process after adding 2a (1.0 wt%) in petrol (95); and rheological data (b, frequency sweep (ω); c, stress sweep (σ0)) of the gels made from petrol (95) with 5.0 wt% of gelator 2a. | |
The rheology of the gel (5.0 wt%) formed from 2a and petrol was also studied. In the frequency-sweep mode (Fig. 3b), the storage modulus (G') was always higher than the loss modulus (G") and both of these moduli are independent on frequency, supporting a strong gel. In the stress-sweep mode (Fig. 3c), the yield of stress (ca. 80 pa), the stress amplitude (σ0) at which a sharp decrease in moduli occurs, indicates that the gel is strong enough to hold its own weight.
Gelator 2a is not soluble in water, but can gelate petrol in the powder form. The resulting gel is quite stable and has certain mechanical property; the gelation mechanism is also understood. Therefore, the stage is set to test the real application to recover oilspill from water.
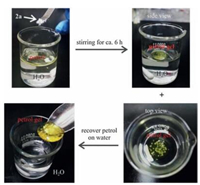
Petrol was chosen as an example and others oils worked as well. The most difficult scenario for oil spill recovery is that the spilled petrol forms a very thin layer on the water. As shown in Fig. 4, We thus prepared the mixture of 10 mL H2O and 0.5 mL petrol. Petrol as a yellow liquid floats on the surface of water. The powder of 2a (2.0 wt%) was then added on the surface. In order to accelerate the process, the water phase was stirred. After six hours, the petrol gel was formed. Surprisingly, the petrol contracts into one big piece of gel. It is very easy to scoop it from the water phase by a spatula. The recovered petrol in the gel form can be collected by distillation and the residual gelator 2a can be recycled and used again.
|
Download:
|
| Figure 4. Recovery process of spilled petrol in water by using organogelator 2a (2.0 wt%). | |
3. Conclusion
In summary, we report the first phase-selective, bis-urea organogelator with a curved bis-naphthalene core which is capable of recovering a thin layer of spilled oil in water. Its gelation property relies on the cooperative action between bis-naphthalene cleft and the urea groups. This gelator can be applied in the powder form, eliminating all the side effects of carrier solvents. In addition, it is recyclable and reusable.
4. ExperimentalAll the reagents involved in this research were commercially available and used without further purification unless otherwise noted. Solvents were either employed as purchased or dried prior to use by standard laboratory procedures. Thin-layer chromatography (TLC) was carried out on 0.25 mm Yantai silica gel plates (60F-254). Column chromatography was performed on silica gel 60 (Tsingdao 40–63 nm, 230–400 mesh). NMR spectra were recorded on Bruker Avance-400 NMR and Bruker Avance-500 NMR spectrometers. All chemical shifts are reported in ppm with residual solvents or TMS (tetramethylsilane) as the internal standards. The following abbreviations were used for signal multiplicities: s, singlet; d, doublet; dd, doublet of doublet; m, multiplet. Electrospray-ionization high-resolution mass spectrometry (ESI-HRMS) experiments were conducted on an applied Q EXACTIVE mass spectrometry system. All the computations were performed at the Semi-Empirical MMFF level of theory by using Spartan'14 (Wavefunction, Inc.).
4.1. Synthesis of compound 2aTo the solution of compound 3 (50 mg, 0.095 mmol) and N, N-diisopropyl ethylamine in dry DCM (10 mL), were added dropwise the solution of compound 4a [36] (140 mg, 0.21 mmol) in dry DCM (5 mL) during 30 min. The resulting mixture was stirred at room temperature for 5 h, and then the precipitate was filtered and washed by DCM and MeOH to get compound 2a, white solid, yield (120 mg, 68%). 1H NMR (400 MHz, CDCl3: DMSO-d6 = 99: 1, 25 ℃): δ 8.42 (d, 2H, J = 9.3 Hz), 7.80 (d, 2 H, J = 9.3 Hz), 7.23 (d, 2H, J = 8.1 Hz), 7.08 (d, 2 H, J = 9.2 Hz), 6.49 (s, 4 H), 6.16 (s, 1H), 5.24 (s, 1H), 4.70 (dd, 4H, J = 52.7, 13.4 Hz), 4.07–3.90 (m, 4H), 3.87–3.65 (m, 12H), 2.38 (s, 2 H), 1.81–1.52 (m, 16 H), 1.50–1.26 (m, 16 H), 1.16 (s, 96 H), 0.93–0.84 (m, 6 H), 0.82–0.70 (m, 18 H). 13C NMR (126 MHz, CDCl3: DMSO-d6 = 10: 1, 25 ℃): δ 155.34, 152.61, 152.46, 148.22, 135.40, 132.20, 128.67, 126.01, 123.62, 120.55, 119.03, 118.65, 96.85, 90.81, 72.86, 69.09, 68.30, 33.48, 31.39, 31.13, 29.78, 29.22, 29.20, 29.17, 29.12, 28.91, 28.85, 28.82, 25.65, 25.61, 22.21, 22.16, 18.80, 13.67, 13.47. ESI-TOF-HRMS: m/z calcd. for [M + H]+ C119H193 O12N4+, 1870.4592; found 1870.4610 (error =-1.0 ppm).
4.2. Synthesis of compound 2bCompound 4b was synthesized by following the literature procedure [37, 38]. To the solution of compound 3 (30 mg, 0.057 mmol) and N, N-diisopropyl ethylamine in dry DCM (10 mL), were added dropwise the solution of compound 4b (103 mg, 0.15 mmol) in dry DCM (2 mL) during 30 min. The resulting mixture was stirred at room temperature overnight, and then the solvent was removed in vacuum. The residue was washed by DCM for several times and subjected to column chromatography (SiO2, methanol/DCM = 1/20) to afford 2b, white solid, yield (50 mg, 46%). 1H NMR (400 MHz, CDCl3: DMSO-d6 = 99: 1, 25 ℃): δ 8.48 (d, 2 H, J = 9.5 Hz), 7.86 (d, 2 H, J = 8.8 Hz), 7.24 (d, 2 H, overlap with solvent signal), 7.20 (d, 2 H, J = 9.3 Hz), 6.36 (s, 4 H), 6.26 (s, 1 H), 5.26 (s, 1 H), 4.72 (s, 4 H), 4.20 (d, 4 H, J = 27.7 Hz), 3.99 (t, 4H, J = 6.4 Hz), 3.93–3.72 (m, 12 H), 2.42 (s, 1 H), 1.84–1.67 (m, 16 H), 1.51–1.38 (m, 16 H), 1.26 (s, 96 H), 0.95 (t, 6 H, J = 7.4 Hz), 0.88 (t, 18 H, J = 6.8 Hz). 13C NMR (126 MHz, CDCl3: DMSO-d6 = 10: 1, 25 ℃): δ 158.05, 152.52, 148.21, 136.29, 134.95, 128.75, 126.09, 123.84, 123.51, 118.90, 118.53, 114.27, 105.25, 90.82, 72.84, 69.05, 68.44, 65.31, 43.77, 33.88, 31.43, 31.14, 29.86, 29.27, 29.24, 29.22, 29.20, 29.17, 29.02, 28.95, 28.89, 28.87, 25.67, 22.29, 22.20, 18.82, 14.86, 13.71, 13.49. ESI-TOF-HRMS: m/z calcd. for [M + H]+ C121H197O12N4+, 1898.4923; found 1898.5016 (error = 4.9 ppm).
AcknowledgmentsThis research was financially supported by the National Natural Science Foundation of China (Nos. 21302090, 21572097), South University of Science and Technology of China, and the Shenzhen special funds for the development of biomedicine, internet, new energy, and new material industries (No. JCYJ20150331101823694). We thank Dr. Rui Gu for the help on SEM experiments.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.031.
| [1] | P. Terech, R.G. Weiss, Low molecular mass gelators of organic liquids and the properties of their gels. Chem.Rev. 97 (1997) 3133–3160. DOI:10.1021/cr9700282 |
| [2] | M.O.M. Piepenbrock, G.O. Lloyd, N. Clarke, J.W. Steed, Metal-and anion-binding supramolecular gels. Chem.Rev. 110 (2010) 1960–2004. DOI:10.1021/cr9003067 |
| [3] | Q.X. Jin, J. Li, X.G. Li, L. Zhang, Function and application of supramolecular gels chiral molecular recognition and asymmetric catalysis. Progress in Chemistry 30 (2014) 919–930. |
| [4] | J. Jiang, Y. Meng, L. Zhang, M.H. Liu, single-walled metal-helical nanotube(m-hn):creation of efficient supramolecular catalysts for asymmetric reaction. J.Am.Chem.Soc. 138 (2016) . DOI:10.1021/jacs.6b08808 |
| [5] | S. Dong, B. Zheng, D. Xu, A crown ether appended super gelator with multiple stimulus responsiveness. Adv.Mater. 24 (2012) 3191–3195. DOI:10.1002/adma.v24.24 |
| [6] | X. Wu, Y. Yu, L. Gao, Stimuli-responsive supramolecular gel constructed by pillar[5] arene-based pseudo[2] rotaxanes via orthogonal metal-ligand coordination and hydrogen bonding interaction. Org.Chem.Front. 3 (2016) 966–970. DOI:10.1039/C6QO00197A |
| [7] | Z.Y. Li, Y.Y. Zhang, C.W. Zhang, Cross-linked supramolecular polymer gels constructed from discrete multi-pillar[5] arene metallacycles and their multiple stimuli-responsive behavior. J.Am.Chem.Soc. 136 (2014) 8577–8589. DOI:10.1021/ja413047r |
| [8] | F. Zeng, Y. Han, Z.C. Yan, Supramolecular polymer gel with multi stimuli responsive, self-healing and erasable properties generated by host-guest interactions. Polymer 54 (2013) 6926–6953. |
| [9] | X.D. Yu, L.M. Chen, M.M. Zhang, T. Yi, Low-molecular-mass gels responding to ultrasound and mechanical stress:towards self-healing materials. Chem.Soc. Rev. 43 (2014) 5346–5371. DOI:10.1039/C4CS00066H |
| [10] | J.J. Panda, A. Mishra, A. Basu, V.S. Chauhan, Stimuli responsive self-assembled hydrogel of a low molecular weight free dipeptide with potential for tunable drug delivery. Biomacromolecules 9 (2008) 2244–2250. DOI:10.1021/bm800404z |
| [11] | D.K. Kumar, J.W. Steed, Supramolecular gel phase crystallization:orthogonal self-assembly under non-equilibrium condition. Chem.Soc.Rev. 43 (2014) 2080–2088. DOI:10.1039/C3CS60224A |
| [12] | S. Bhattacharya, Y.K. Ghosh, First report of phase selective gelation of oil from oil/water mixtures.Possible implications toward containing oil spills. Chem. Commun. (2001) 185–186. |
| [13] | A.M. Vibhute, V. Muvvala, K.M. Sureshan, A sugar-based gelator for marine oil-spill recovery. Angew.Chem.Int.Ed. 55 (2016) 7782–7785. DOI:10.1002/anie.201510308 |
| [14] | C. Ren, G.H.B. Ng, H. Wu, Instant room-temperature gelation of crude oil by chiral organogelators. Chem.Mater. 28 (2016) 4001–4008. DOI:10.1021/acs.chemmater.6b01367 |
| [15] | A. Prathap, K.M. Sureshan, A mannitol based phase selective supergelator offers a simple, viable and greener method to combat marine oil spills. Chem. Commun. 48 (2012) 5250–5252. DOI:10.1039/c2cc31631e |
| [16] | D. Wang, J. Niu, Z. Wang, J. Jin, Monoglyceride-based organogelator for broad-range oil uptake with high capacity. Langmuir 31 (2015) 1670–1674. DOI:10.1021/acs.langmuir.5b00053 |
| [17] | F. Xie, L. Qin, M.H. Liu, A dual thermal and photo-switchable shrinking-swelling supramolecular peptide dendron gel. Chem.Commun. 52 (2016) 930–933. DOI:10.1039/C5CC08076B |
| [18] | S. Mukherjee, C. Shang, X. Chen, N-Acetylglucosamine-based efficient, phase-selective organogelators for oil spill remediation. Chem.Commun. 50 (2014) 13940–13943. DOI:10.1039/C4CC06024E |
| [19] | M. George, G. Tan, V.T. John, R.G. Weiss, Urea and thiourea derivatives as low molecular-mass organogelators. Chem.Eur.J. 11 (2005) 3243–3254. DOI:10.1002/(ISSN)1521-3765 |
| [20] | C. Wang, D. Zhang, D. Zhu, A low-molecular-mass gelator with an electroactive tetrathiafulvalene group:tuning the gel formation by charge-transfer interaction and oxidation. J.Am.Chem.Soc. 127 (2005) 16372–16373. DOI:10.1021/ja055800u |
| [21] | G.O. Lloyd, M.O.M. Piepenbrock, J.A. Foster, Anion tuning of chiral bis (urea)low molecular weight gels. Soft Matter 8 (2012) 204–216. DOI:10.1039/C1SM06448G |
| [22] | C. Deng, R. Fang, Y. Guan, Sonication-induced self-assembly of flexible tris(ureidobenzyl)amine:from dimeric aggregates to supramolecular gels. Chem.Commun. 48 (2012) 7973–7975. DOI:10.1039/c2cc33408a |
| [23] | Z. Qi, P.M. de Molina, W. Jiang, Systems chemistry:logic gates based on the stimuli-responsive gel? sol transition of a crown ether-functionalized bis (urea)gelator. Chem.Sci. 3 (2012) 2073–2082. DOI:10.1039/c2sc01018f |
| [24] | M.A. Kobaisi, S.V. Bhosale, K. Latham, Functional naphthalene diimides: synthesis, properties, and applications. Chem.Rev. 116 (2016) 11685–11796. DOI:10.1021/acs.chemrev.6b00160 |
| [25] | C.R. Martinez, B.L. Iverson, Rethinking the term pi-stacking. Chem.Sci. 3 (2012) 2191–2201. DOI:10.1039/c2sc20045g |
| [26] | L.P. Yang, W.E. Liu, W. Jiang, Naphthol-based macrocyclic receptors. Tetrahedron Lett. 57 (2016) 3978–3985. DOI:10.1016/j.tetlet.2016.07.077 |
| [27] | F. Jia, Z. He, L.P. Yang, Oxatub[4] arene:a smart macrocyclic receptor with multiple interconvertible cavities. Chem.Sci. 6 (2015) 6731–6738. DOI:10.1039/C5SC03251B |
| [28] | F. Jia, H.Y. Wang, D.H. Li, Oxatub[4] arene:a molecular transformer capable of hosting a wide range of organic cations. Chem.Comm. 52 (2016) 5666–5669. DOI:10.1039/C6CC01052K |
| [29] | Z. He, G. Ye, W. Jiang, Imine macrocycle with a deep cavity:guest-selected formation of syn/anti configuration and guest-controlled reconfiguration. Chem.Eur.J. 21 (2015) 3005–3012. DOI:10.1002/chem.201405912 |
| [30] | G.B. Huang, W. Jiang, Dynamic covalent macrocycles constructed via organic templates. Prog.Chem. 27 (2015) 744–754. |
| [31] | G. Huang, Z. He, C.X. Cai, Bis-urea macrocycles with a deep cavity. Chem. Commun. 51 (2015) 15490–15493. DOI:10.1039/C5CC06768E |
| [32] | G. Huang, A. Valkonen, K. Rissanen, W. Jiang, Endo-Functionalized molecular tubes:selective encapsulation of neutral molecules in non-polar media. Chem. Commun. 52 (2016) 9078–9081. DOI:10.1039/C6CC00349D |
| [33] | G.B. Huang, S.H. Wang, H. Ke, Selective recognition of highly hydrophilic molecules in water by endo-functionalized molecular tubes. J.Am.Chem.Soc. 138 (2016) 14550–14553. DOI:10.1021/jacs.6b09472 |
| [34] | Z. He, X. Yang, W. Jiang, Synthesis, solid-state structures, and molecular recognition of chiral molecular tweezer and related structures based on a rigid bis-naphthalene cleft. Org.Lett. 17 (2015) 3880–3883. DOI:10.1021/acs.orglett.5b01871 |
| [35] | F. E. Fages, Gelation with small molecules: from formation mechanism to nanostructure architecture, in: Y. L. Xiang(Ed. ), Low molecular mass gelators: design, self-assembly, function, Springer, Berlin, 2005, pp. 1-37. |
| [36] | H. Yang, T. Yi, Z.G. Zhou, Switchable fluorescent organogels and mesomorphic superstructure based on naphthalene derivatives. Langmuir 23 (2007) 8224–8230. DOI:10.1021/la7005919 |
| [37] | D. Liu, Z. Tian, Z. Yan, Design, synthesis and evaluation of 1,2-benzisothiazol-3-one derivatives as potent caspase-3 inhibitors. Bioorg.Med. Chem. 21 (2013) 2960–2967. DOI:10.1016/j.bmc.2013.03.075 |
| [38] | V. Percec, H.J. Sun, P. Leowanawat, Transformation from kinetically into thermodynamically controlled self-organization of complex helical columns with 3D periodicity assembled from dendronized perylene bisimides. J.Am. Chem.Soc. 135 (2013) 4129–4148. DOI:10.1021/ja400639q |
 2017, Vol. 28
2017, Vol. 28