b The Co-Innovation Center of Chemistry and Chemical Engineering of Tianjin, Tianjin 300072, China;
c Renai College of Tianjin University, Tianjin 301636, China;
d Tianjin Engineering Research Center of Functional Fine Chemicals, Tianjin 300072, China
Organogel is constructed by dispersing a low molecular weight gelator (LMWG) in a liquid where it spontaneously self-assembles to create a three-dimensional network and entraps solvent molecules [1–7]. In the past few decades, LMWGs have attracted much attention owing to their potential applications in templates [8–10], drug controlled release [11], soft functional materials [12], molecular recognition [13], spilled oil recovery [14, 15], aggregation-induced emission materials [16] and hardening bitumen [17].
Most of the LMWGs have been discovered by serendipity, and a prior design of LMWGs is considered to be the "holy grail" in this field [18]. As is well known, solvents play an important role in mediating the properties of organogels. Various attempts have been made to correlate solvent parameters to gelation behaviours [19–30]. An important progress was reported by Raynal and Bouteiller [31]. They found that it was possible to define a solubility sphere and one or more gelation spheres using Hansen solubility parameters (HSPs). If the HSPs of an untested solvent fall in the solubility sphere, the LMWG is likely to be dissolved in this solvent, and the gelation sphere is in the same situation. Xu et al. [32] showed that a Teas plot derived from Hansen solubility parameters could be used to predict the gelation properties of a known gelator in untested solvents. However, gelation behaviour is normally tested in a solvent at a given concentration, and as such the results are markedly influenced by the concentration. For example, Diehn et al. [33] studied the self-assembly of 1,3: 2,4-dibenzylidene sorbitol (DBS) at three DBS concentrations: 1%, 5% and 10% (w/v) and found that samples dissolved at low concentrations became gels or precipitates at higher concentrations. The role of the concentration in gelation behaviour needs to be elucidated to better understand the gelation behaviour. Furthermore, the correlation between the gelation behaviours in single solvents and thus prepared mixed solvents is not clear and to predict the formation of gel in mixed solvents is still challenging [22, 23, 34–36]. Recently, Yan et al. [34] reported that pyrenyl-linkerglucono gelators (Pn) formed gels in tetrahydrofuran-water mixtures, and interestingly they were insoluble in each single component. We also found that a gelator that was soluble at a concentration of 2% (w/v) in one solvent (hereafter refered to as a good solvent) and insoluble in another (hereafter refered to as a poor solvent) could form a gel in the mixed solvent at a certain volume ratio [23, 36]. Therefore, it will be of interest to investigate the gelation behaviours of a given gelator in single solvents without the limitation of concentrations and how this outcome would influence its gelation behaviours in mixed solvents.
Therein, we first synthesized two structurally different compounds 1,3:2, 5:4, 6-tris(3,4-dichlorobenzylidene)-D-mannitol [37] (G1) without any hydrogen bonding site and 2,4-(3,4-dichlorobenzylidene)-N-(3-aminopropyl)-D-gluconamide (G2) with multiple hydrogen bonding sites (Scheme 1) and investigated the gelation behaviours in single solvents without limiting the concentrations of LMWG. HSPs and the Teas plot were used to correlate the gelation behaviours with the solvent parameters. Then, the mixed solvents were determined based on the Teas plot and the gelation behaviours of G1 and G2 in the mixed solvents were examined. It is hoped the results from this work would provide a method for the prediction of the gelation behaviours of a given gelator in mixed solvents, which may help to select suitable mixed solvent systems in practical industrial fields in future and reduce workloads.

|
Download:
|
| Scheme 1. Structures of gelators 1,3:2, 5:4, 6-tris(3,4-dichlorobenzylidene)-D-mannitol (G1) and 2,4-(3,4-dichlorobenzylidene)-N-(3-aminopropyl)-D-gluconamide (G2). | |
2. Results and discussion 2.1. Gelation behaviours in single solvent
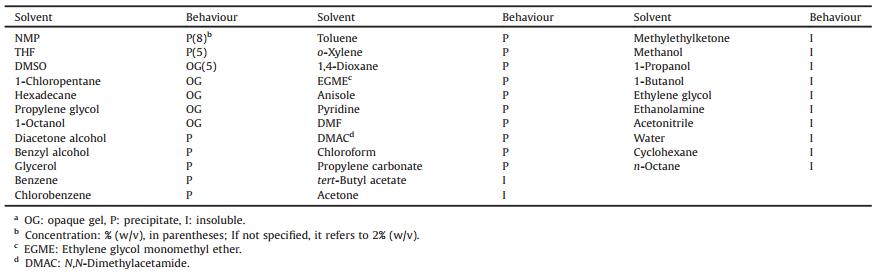
The gelation behaviours of G1 and G2 in 34 solvents which are evenly scattered in Hansen space [35] were examined. The initial concentrations of G1 and G2 were determined to be 2% (w/v). Herein, a concentration above 2% (w/v) is defined as a higher concentration. The detailed procedures of gelation experiment were described in the experiment part. The overall outcomes of G1 in single solvents are shown in Table 1. It is found that G1 is soluble in three solvents (good solvents) including NMP, THF and DMSO at 2% (w/v). A precipitate in NMP and THF was obtained at a concentration of 8% and 5% (w/v), respectively. Interestingly, an opaque gel was formed in DMSO at 5% (w/v). G1 formed precipitates at 2% (w/v) in other solvents (poor solvents) including alcohols, hydrocarbons and other polar and non-polar solvents. The gelation behaviours of G2 are shown in Table S1 in Supporting information. G2 has shown better gelation abilities. At higher concentrations, it could gel six solvents including NMP, benzyl alcohol, ethylene glycol, etc, and precipitates were formed in nine solvents such as DMSO, DMAC and EGME. It was insoluble at 2% (w/v) in solvents including alkanes and aromatic hydrocarbons. It can be seen clearly that a sample dissolved at 2% (w/v) became a gel or precipitate at higher concentrations. The rheological properties of gels which formed at higher concentrations were investigated. The results are shown in Figs. S1 and S2 in Supporting information, suggesting that the samples are true gels. Further, the SEM images of some gels and precipitates are obtained (Figs. S3 and S4 in Supporting information), showing that the gels contained dense fibres and the precipitates were lamellar or vesicular structures.
|
|
Table 1 Gelation behaviours of G1 in various solvents at room temperature.a |
2.2. Prediction of gelation behaviours in single solvent
To rationalize the solvent effects on the gelation behaviours of G1 and G2 without the limitation of concentrations, the HSPsbased framework has been adopted to connect gelation behaviours with solvent properties. The HSPs of solvents are listed in Table S2 in Supporting information and the gelation behaviours of G1 as a function of HSPs are shown in Fig. S5 in Supporting information. The solvent points gathered mostly in the precipitate domain and showed almost no correlation with the gelation behaviours. A 3-D Hansen space was then used and the result is shown in Fig. S7 in Supporting information. It can be seen that the gel and precipitate points are mixed together, suggesting that the Hansen space cannot set up a certain correlation between the solvent properties and the gelation behaviours of G1, if the concentration of the tests is not maintained constant. Next, a Teas plot was also used to correlate the gelation behaviours. The values of the solvents parameters are collected in Table S3 in Supporting information and the Teas plot is shown in Fig. S9 in Supporting information. Unfortunately, in the Teas plot still no correlation between the solvent properties and the gelation behaviours can be obtained.
G2 was found to be in the same situation, where the HSPs and Teas plot cannot correlate the solvent properties with the gelation behaviours (Figs. S6, S8 and S10 in Supporting information). The studies on how to predict gelation behaviours in single solvents without the limitation of sample concentrations are still in progress.
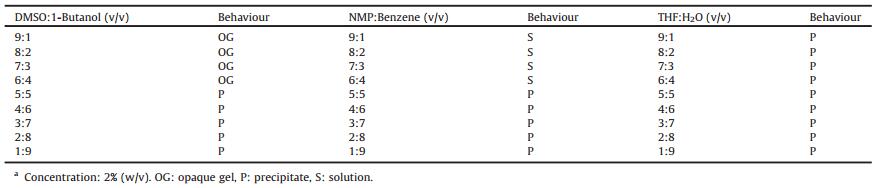
2.3. Investigation of gelation rule in solvent mixturesWhat is the relationship between the gelation behaviours in single solvents and mixed solvents? What is the gelation rule of LMWGs in mixed solvents? To answer these questions, the gelation behaviours of G1 and G2 in 23 different kinds of mixed solvents were investigated. It was mentioned above that for G1, solutions were resulted in NMP, THF and DMSO and precipitates were formed in most of the solvents at 2% (w/v). Therefore, NMP, THF and DMSO were selected as good solvents to mix with poor solvents at 2% (w/v). For the selection of poor solvents, two rules were taken into consideration. First, it must be miscible with the good solvents. Second, it should be of representative, diversity and universality. For this reason, Teas plot was used to select the poor solvents since it is more convenient to see the relative position of the solvent points in 2D plane of the Teas plot than the 3-D Hansen space. Water, 1-butanol, benzene, n-octane, DMF and chlorobenzene were selected as poor solvents, the parameters of which were widely distributed in the Teas plot and separated distantly (Fig. 1). To be specific, for DMSO solvent system (system 1) and NMP solvent system (system 2), water, 1-butanol and benzene were employed as the poor solvents. For THF solvent system (system 3), water, 1-butanol, benzene, n-octane, DMF and chlorobenzene were selected as poor solvents.

|
Download:
|
| Figure 1. Teas plot of calculated solubility parameters of the solvent mixtures versus the gelation behaviours of G1 (2% w/v). (a) filled squares: DMSO-water mixture; filled triangles: DMSO-1-butanol mixtures; filled diamonds: DMSO-benzene mixtures. (b) half-filled squares: NMP-water mixtures; half-filled triangles: NMP-1-butanol mixtures; half-filled circles: NMP-benzene mixtures. (c) open squares: THF-Water mixtures; open triangles: THF-1-butanol mixtures; open circles: THF-benzene mixtures; open diamonds: THF-n-octane mixtures; open pentagon: THF-Chlorobenzene mixtures; open hexagon: THF-DMF mixtures. Red: precipitate; green: gel; blue: solution. | |
The gelation behaviours of G1 in mixed solvents were investigated, some typical results are shown in Table 2 and more data are listed in Table S4 (Supporting information). In system 1, G1 formed gels at 2% when the compositions of DMSO are in the range of 90% to 60% in DMSO-butanol mixtures (Table 2). When the composition of poor solvent (1-butanol) increased to a certain value, a precipitate was obtained. It also formed gels in DMSO-H2O (9:1, v/v) and DMSO-benzene (9:1, v/v) as shown in Table S4 in Supporting information. For system 2, no gel was formed. Initially, a solution was formed and when the composition of the poor solvent increased, a precipitate would be observed eventually without the formation of a gel. The same results were obtained for system 3. The Teas plots of the mixed solvent systems are shown in Fig. 1 and the 3-D Hansen space are shown in Fig. S11 in Supporting information. For system 1, it can be seen that there were three solvent domains, with the blue point (DMSO solution) being the intersection of different connecting lines. As one moved far away from the intersection, some green points (gel) appeared and then a number of red points (precipitate). Similar results can be seen for system 2 and system 3, except that no gelation domain was observed. Furthermore, the gelation behaviours of the samples were also examined in the DMSO-toluene, NMP-THF and THFtoluene mixtures and the results are summarized in Table S5 in Supporting information. The gels formed in DMSO-toluene mixtures (9:1 and 8:2, v/v). A solution was obtained in NMPTHF mixtures at 2% at any volume ratio, but a precipitate appeared (1:9, v/v) when the concentration was increased to 5% and no gel formed. THF-toluene mixtures cannot form a gel either.
|
|
Table 2 Gelation behaviours of G1 in various solvent mixtures at room temperature.a |
Based on aboveresults, an interesting gelationrule can be found for mixed solvents. As for G1, the gel was formed in system 1 at certain good-poor solvent volume ratio but no gel was formed in system 2 and system 3 at any volume ratio. In the three different systems there were three common poor solvents: water, 1-butanol and benzene. It was found that when the same poor solvent was selected, for system 1, the gel appeared, but for the other two systems no gel was observed. For example, when water was selected as the poor solvent, a gel formed in DMSO-H2O mixture with a 10% of water, but no gel in NMP-H2O and THF-H2O mixture with any water composition. As mentioned above, a gel was formed in DMSO but precipitates formed in NMP and THFat higher concentrations. Thus, the combination of the self-assembly results obtained for the single solvents without the limitation of concentrations and mixed solvents suggested that the properties of good solvent are the key factor for the gel formation in the solvent mixtures. When mixing good solvents with poor solvents, whether to form gels or not in the mixed solvents depends on the gelation behaviours of the specific gelators in good solvents at higher concentrations. If the gelator can form a gel in a good solvent at higher concentrations, it may form a gel at lower concentrations when the poor solvent was added tothe system at a certain volume ratio. If the sample precipitates in a good solvent at higher concentrations, it may not form gels when the poor solvent was added at any volume ratio. These observations provide some bases for a prediction method for the gel formation in mixed solvents.
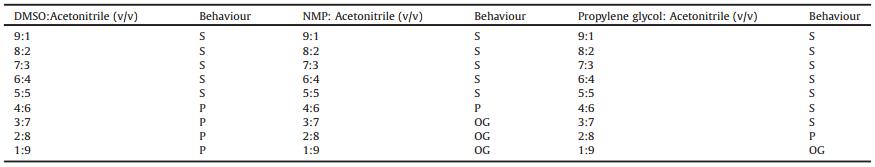
To verify the reliability of the above proposed gel formation method, the gelation behaviours of a structurally different compound (G2) were further investigated in mixed solvents. In total, 8 mixed solvent systems were selected for G2. As for the good solvents we selected, precipitates were obtained in DMSO, EGME and DMAC and gels formed in NMP, benzyl alcohol and propylene glycol at higher concentrations (Table S1 in Supporting information). The total results of gelation behaviours in the mixed solvents are shown in Table S6 in Supporting information and the teas plot and 3-D Hansen space are shown in Figs. S12 and S13 in Supporting information, respectively. Some typical results of gelation behaviours for the mixed solvents are shown in Table 3. It was found that when acetonitrile was selected as the poor solvent, no gel was formed in DMSO-acetonitrile mixtures at any volume ratio, but gels formed in NMP-acetonitrile and propylene glycol-acetonitrile mixtures at certain volume ratios. These results further demonstrate that the gelation behaviours in good solvents can be used as a guide for the gel formation in the solvent mixtures.
|
|
Table 3 Gelation behaviours of G2 in various solvent mixtures at room temperature.a |
2.4. XRD
XRD was employed to further investigate the solvent effect on the aggregate structure of the xerogels and precipitates. As shown in Fig. 2a, the DMSO xerogel of G1 displayed a series of sharp diffractions with the peaks centred at 2θ=14.34° (d=6.17nm), 2θ=15.68° (d=5.65nm), 2θ=15.98° (d=5.54nm), 2θ=22.82° (d=3.89nm), 2θ=24.94° (d=3.57nm). The DMSO-H2O (9:1, v/v) and DMSO-butanol (8:2, v/v) xerogels show similar results which indicate that the gels formed in either pure DMSO or solvent mixture possess similar ordered aggregate structures [38]. Interestingly, XRD measurements of the precipitates of G1 from NMP, NMP-H2O (7:3, v/v) mixture and NMP-Butanol (7:3, v/v) mixture all give similar diffraction patterns (Fig. 2b) with the xerogels, showing that the packing modes of G1 for the gel and precipitate obtained in pure and mixed solvents are all similar. Since no aggregation modes changed for the xerogels and precipitates in either pure or mixed solvents, the formation of gel or precipitate upon the addition of the poor solvent may be due to the reduced solubilities of the sample in the mixed solvents. However, whethertoformgel or notin themixed solvents depends on the gelation behaviours of the specific gelators in good solvents at higher concentrations.

|
Download:
|
| Figure 2. XRD spectra of the xerogels and precipitates of G1 obtained in pure and mixed solvents: (a) black line: DMSO (5% w/v); red line: DMSO-H2O mixture (9:1 v/v, 2% w/v); blue line: DMSO-butanol mixture (8:2v/v, 2% w/v). (b) black line: NMP (8% w/v); red line: NMP-H2O mixture (7:3 v/v, 2% w/v); blue line: NMP-butanol mixture (7:3 v/v, 2% w/v). | |
3. Conclusion
In summary, the gelation behaviours of G1 andG2 in34solvents were investigated without the restriction of concentrations. We found that sample dissolved at low concentrations may turn to a gel or precipitate at higher concentrations. The HSPs and Teas plots failed to provide correlation between the gelation behaviours and solvent properties, if the concentration of the tests was not maintained constant. After carefully studying the gelation behaviours of G1 in 15 different kinds of solvent mixtures, it was found that gel formation in the mixed solvents is strongly associated with the gelation behaviours in the good solvents at higher concentrations. If the sample can form a gel in a good solvent at higher concentrations, it may form a gel in mixed solvent when the poor solvent added at a certain volume ratio. If the sample precipitates in a good solvent at higher concentrations, it may not form a gel in mixed solvent. These results were further verified by examining the gelation behaviours of G2 in 8 mixed solvent systems. Therefore, in our studies, a gelation rule for prediction of gelation behaviours of a gelator in mixed solvents has been proposed, which may be useful for the determination of mixed solvent systems for practical applications involving gel formation.
4. Experimental 4.1. Materials and instrumentsD-Gluconic acid, mannitol, 3,4-dichlorobenzaldehyde, 1,3-diaminopropane and DMAP (4-dimethylaminopyridine) were purchased from Shanghai Jingchun Scientifical Co., Ltd. The chemical reagents were commercially available and directly utilized without further purification. The xerogels and precipitates were dried under ambient conditions. After coating the samples with gold, SEM images of the samples were obtained using a Hitachi S-4800 scanning electron microscope. Rheology measurements were carried out on a stress-controlled rheometer (Stress Tech) using a steel-coated parallel-plate geometry (25 mm diameter). The stress-amplitude sweep experiment was performed at a constant oscillation frequency of 1.0 Hz and the shear strain varies from 0.1% to 100%, all measurements were performed at 20 ℃. The XRD spectra of the xerogels and precipitates were collected with a Bruker D8 Focus X-ray powder diffractometer using Cu-Kα radiation. The samples were tested from 3° to 40° at a scanning rate of 0.5 s per step.
4.2. Synthesis of the gelatorsG1 was easily synthesized by reacting the D-mannitol with 3,4-dichlorobenzaldehyde using the method previously reported in the literature [37]. G2 was prepared from D-gluconic acid, 3,4-dichlorobenzaldehyde and 1,3-diaminopropane according to the following procedure. Firstly, 87.5g (0.5mol) 3,4-dichlorobenzaldehyde was dissolved in 300 mL methanol at room temperature and then 235.39 g (0.6 mol) 50wt% aqueous solution of D-gluconic acid was added to this solution followed by the addition of 200 mL hydrochloride acid (12 mol/L) under vigorously stirring for 48 h. The white solid was collected by filtration and the filter cake was washed with water for five times and hot dichloromethane for twice respectively. Secondly, to a solution of 80g (0.22mol) white solid obtained previously in 300 mL methanol was added 48.92 g (0.66 mol) 1,3-diaminopropane and 0.01 g DMAP (0.008mmol). The reaction mixture was stirred at room temperature for 36h. Subsequently, the white solid was collected by filtration. The filter cake was washed with water for twice and recrystallized with methanol to obtain gelator G2 with a yield of 76%. See Supporting information for the NMR (1H and 13C) and TOF-QⅡ high resolution mass spectrometer characterization.
4.3. Gelation experimentsThe samples were prepared by heating and then cooling to room temperature naturally. The initial concentration of sample was determined to be 2% (w/v). If the gelator formed a precipitate or gel in a certain solvent at 2% (w/v), the test would be finished. If the gelator was soluble in a certain solvent at 2% (w/v), more gelator would be added to the solution until a gel or precipitate was observed. All the samples in mixed solvents were prepared at 2% (w/v), except that it formed solutions at any volume ratio, more gelator would be added until a gel or precipitate was formed.
AcknowledgementsWe are grateful for the financial support of the National Natural Science Foundation of China (Nos. 21276188, 21476164) and Tianjin Science and Technology Innovation Platform Program (No. 14TXGCCX00017).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.024.
| [1] | Y. Lan, M.G. Corradini, R.G. Weiss, S.R. Raghavan, M.A. Rogers, To gel or not to gel:correlating molecular gelation with solvent parameters. Chem.Soc.Rev. 44 (2015) 6035–6058. DOI:10.1039/C5CS00136F |
| [2] | P. Terech, R.G. Weiss, Low molecular mass gelators of organic liquids and the properties of their gels. Chem.Rev. 97 (1997) 3133–3160. DOI:10.1021/cr9700282 |
| [3] | S.S. Babu, V.K. Praveen, A. Ajayaghosh, Functional p-gelators and their applications. Chem.Rev. 114 (2014) 1973–2129. DOI:10.1021/cr400195e |
| [4] | G.C. Yu, X.Z. Yan, C.Y. Han, F.H. Huang, Characterization of supramolecular gels. Chem.Soc.Rev. 42 (2013) 6697–6722. DOI:10.1039/c3cs60080g |
| [5] | M.O.M. Piepenbrock, G.O. Lloyd, N. Clarke, J.W. Steed, Metal-and anion-binding supramolecular gels. Chem.Rev. 110 (2010) 1960–2004. DOI:10.1021/cr9003067 |
| [6] | D.K. Smith, Lost in translation? Chirality effects in the self-assembly of nanostructured gel-phase materials. Chem.Soc.Rev. 38 (2009) 684–694. DOI:10.1039/b800409a |
| [7] | J.W. Steed, Anion-tuned supramolecular gels:a natural evolution from urea supramolecular chemistry. Chem.Soc.Rev. 39 (2010) 3686–3699. DOI:10.1039/b926219a |
| [8] | K.J.C. van Bommel, A. Friggeri, S. Shinkai, Organic templates for the generation of inorganic materials. Angew.Chem.Int.Ed. 42 (2003) 980–999. DOI:10.1002/anie.200390284 |
| [9] | Y. Liang, L.M. Tang, Y. Xia, One-pot synthesis of network supported catalyst using supramolecular gel as template. Chin.Chem.Lett. 21 (2010) 991–994. DOI:10.1016/j.cclet.2010.02.010 |
| [10] | W. Zhang, Z.G. Xie, Fabrication of palladium nanoparticles as effective catalysts by using supramolecular gels. Chin.Chem.Lett. 27 (2016) 77–80. DOI:10.1016/j.cclet.2015.09.009 |
| [11] | Y. Gao, Y. Kuang, Z.F. Guo, Enzyme-instructed molecular self-assembly confers nanofibers and a supramolecular hydrogel of taxol derivative. J.Am. Chem.Soc. 131 (2009) 13576–13577. DOI:10.1021/ja904411z |
| [12] | S. Bhattacharya, S.K. Samanta, Soft functional materials induced by fibrillar networks of small molecular photochromic gelators. Langmuir 25 (2009) 8378–8381. DOI:10.1021/la901017u |
| [13] | B. Escuder, M.L. Lusar, J.F. Miravet, Insight on the NMR study of supramolecular gels and its application to monitor molecular recognition on self-assembled fibers. J.Org.Chem. 71 (2006) 7747–7752. DOI:10.1021/jo0612731 |
| [14] | A. Prathap, K.M. Sureshan, A mannitol based phase selective supergelator offers a simple, viable and greener method to combat marine oil spills. Chem. Commun. 48 (2012) 5250–5252. DOI:10.1039/c2cc31631e |
| [15] | A.M. Vibhute, V. Muvvala, K.M. Sureshan, A sugar-based gelator for marine oil-spill recovery. Angew.Chem.Int.Ed. 55 (2016) 7782–7785. DOI:10.1002/anie.201510308 |
| [16] | M. Luo, S. Wang, M.L. Wang, Novel organogel harnessing excited-state intramolecular proton transfer process with aggregation induced emission and photochromism. Dyes Pigm. 132 (2016) 48–57. DOI:10.1016/j.dyepig.2016.04.036 |
| [17] | B. Isare, L. Petit, E. Bugnet, The weak help the strong:low-molar-mass organogelators harden bitumen. Langmuir 25 (2009) 8400–8403. DOI:10.1021/la804086h |
| [18] | R.G. Weiss, The past present, and future of molecular gels.What is the status of the field, and where is it going. J.Am.Chem.Soc. 136 (2014) 7519–7530. DOI:10.1021/ja503363v |
| [19] | M. Bielejewski, A. Łapiński, R. Luboradzki, J.Tritt-Goc, Solvent effect on 1,2-O-(1-Ethylpropylidene)-a-D-glucofuranose organogel properties. Langmuir 25 (2009) 8274–8279. DOI:10.1021/la900467d |
| [20] | A.R. Hirst, D.K. Smith, Solvent effects on supramolecular gel-phase materials: two-component dendritic gel. Langmuir 20 (2004) 10851–10857. DOI:10.1021/la048178c |
| [21] | G.Y. Zhu, J.S. Dordick, Solvent effect on organogel formation by low molecular weight molecules. Chem.Mater. 18 (2006) 5988–5995. DOI:10.1021/cm0619297 |
| [22] | K.Q. Fan, L.B. Niu, J.Q. Li, Application of solubility theory in bi-component hydrogels of melamine with di(2-ethylhexyl)phosphoric acid. Soft Matter 9 (2013) 3057–3062. DOI:10.1039/c3sm27421g |
| [23] | C.Q. Tong, K.Q. Fan, L.B. Niu, Application of solubility parameters in a D-sorbitol-based organogel in binary organic mixtures. Soft Matter 10 (2014) 767–772. DOI:10.1039/C3SM52676C |
| [24] | W. Edwards, C.A. Lagadec, D.K. Smith, Solvent-gelator interactions-using empirical solvent parameters to better understand the self-assembly of gel-phase materials. Soft Matter 7 (2011) 110–117. DOI:10.1039/C0SM00843E |
| [25] | W. Edwards, D.K. Smith, Dynamic evolving two-component supramolecular gelsâ€"hierarchical control over component selection in complex mixtures. J. Am.Chem.Soc. 135 (2013) 5911–5920. DOI:10.1021/ja4017107 |
| [26] | S. Wu, J. Gao, T.J. Emge, M.A. Rogers, Influence of solvent on the supramolecular architectures in molecular gels. Soft Matter 9 (2013) 5942–5950. DOI:10.1039/c3sm50936b |
| [27] | J. Bonnet, G. Suissa, M. Raynal, L. Bouteiller, Organogel formation rationalized by Hansen solubility parameters:dos and don'ts. Soft Matter 10 (2014) 3154–3160. DOI:10.1039/c4sm00244j |
| [28] | Y.Q. Lan, M.G. Corradini, X. Liu, Comparing and correlating solubility parameters governing the self-assembly of molecular gels using 1,3:2,4-dibenzylidene Sorbitol as the gelator. Langmuir 30 (2014) 14128–14142. DOI:10.1021/la5008389 |
| [29] | N. Yan, Z.Y. Xu, K.K. Diehn, Pyrenyl-linker-glucono gelators.Correlations of gel properties with gelator structures and characterization of solvent effects. Langmuir 29 (2013) 793–805. DOI:10.1021/la304957n |
| [30] | J. Gao, S. Wu, M.A. Rogers, Harnessing hansen solubility parameters to predict organogel formation. J.Mater.Chem. 22 (2012) 12651–12658. DOI:10.1039/c2jm32056h |
| [31] | M. Raynal, L. Bouteiller, Organogel formation rationalized by Hansen solubility parameters. Chem.Commun. 47 (2011) 8271–8273. DOI:10.1039/c1cc13244j |
| [32] | H.Q. Xu, J. Song, T. Tian, R.X. Feng, Estimation of organogel formation and influence of solvent viscosity and molecular size on gel properties and aggregate structures. Soft Matter 8 (2012) 3478–3486. DOI:10.1039/c2sm07387k |
| [33] | K.K. Diehn, H. Oh, R. Hashemipour, R.G. Weiss, S.R Raghavan, Insights into organogelation and its kinetics from Hansen solubility parameters.Toward a priori predictions of molecular gelation. Soft Matter 10 (2014) 2632–2640. DOI:10.1039/c3sm52297k |
| [34] | N. Yan, Z.Y. Xu, K.K. Diehn, How do liquid mixtures solubilize insoluble gelators? Self-assembly properties of pyrenyl-linker-glucono gelators in tetrahydrofuran-water mixtures. J.Am.Chem.Soc. 135 (2013) 8989–8999. DOI:10.1021/ja402560n |
| [35] | J. Bonnet, G. Suissa, M. Raynal, L. Bouteiller, Organogel formation rationalized by Hansen solubility parameters:influence of gelator structure. Soft Matter 11 (2015) 2308–2312. DOI:10.1039/C5SM00017C |
| [36] | H.H. Shen, L.B. Niu, K.Q. Fan, Application of solubility parameters in 1,3:2,4-bis(3,4-dimethylbenzylidene)sorbitol organogel in binary organic mixtures. Langmuir 30 (2014) 9176–9182. DOI:10.1021/la5019532 |
| [37] | X.X. Zhang, P.F. Deng, R.X. Feng, J. Song, Novel gelatinous shape-stabilized phase change materials with high heat storage density. Sol.Energy Mater.Sol.Cells 95 (2011) 1213–1218. DOI:10.1016/j.solmat.2011.01.025 |
| [38] | H. Koshima, W. Matsusaka, H. Yu, Preparation and photoreaction of organogels based on benzophenone. J.Photochem.Photobiol.A Chem. 156 (2003) 83–90. DOI:10.1016/S1010-6030(02)00431-8 |
 2017, Vol. 28
2017, Vol. 28