Rechargeable lithium-ion batteries (LIBs) have been regarded as one of the most promising power technology in the applications of energy storage stations, electric vehicles, hierarchical electric vehicles and so on especially in the circumstances of energy crisis and global environmental concerns [1, 2]. However, the present commercial lithium batteries can not meet with the requirements of increasing energy density [3, 4]. Molybdenum disulfide (MoS2), known as a typical two-dimensional (2D) transition-metal sulfide, has a structure analogous to that of graphite, in which S-Mo-S layers are held together by weak van der Waals forces, and has been considered as a promising anode material for LIBs owing to their high-lithium-storage capacity (ca. 670 mA h g-1) [5, 6]. However, the poor electronic/ionic conductivity between S-Mo-S layers limits its development for anode materials [7, 8]. Moreover, the pulverization and aggregation of MoS2 during the discharge–charge cycle can lead to fast capacity fading [9, 10].
In recent years, a variety of methods including nanostructure engineering[11, 12], construction of composite materials of MoS2 and other materials, such as carbon nanotubes, graphene, MoO2, TiO2, and so on [13–16], have been employed to address the above issues. The down-sizing strategycanpartly buffer the large volume changes of MoS2, and shorten the transport paths of electrons and Li+ ions [11]. The conductive materials such as amorphous carbon, carbon nanotubes (CNTs), graphene [13, 14], and conducting polymer [7], have been employed to construct MoS2 composite structures to effectively improve the rate capability of MoS2 obviously. For examples, Yang et al. [7] have fabricated hierarchical MoS2/ polyaniline nanowires and exhibited greatly improved Li+ storage properties. Ding et al. [13] have prepared MoS2 nanosheets on CNT backbone with assistance of glucose, which shown greatly enhanced lithium storage properties compared with the pure MoS2. Among the carbonaceous materials, nitrogen-doped carbon is of unique for improving electrochemical properties by virtue of the increased electronic conductivity and the facilitation of lithium ion transport [17, 18], to date, hierarchical architectures with porous micro/nanostructuresare of greatinterest forlithium storagedue to the dual merits of both the nanoscale primary building blocks and microscale secondary assemblies [19], which can not only improve transport of electrons and Li+ ions, but also effectively prevent the agglomeration of nanoscale building blocks [20]. Herein, we report the synthesis of MoS2 nanosheet arrays supported on hierarchical nitrogen-doped porous carbon (MoS2@C), which can protect MoS2 nanostructures from restacking during charge/discharge process. The obtained MoS2@C exhibit high specific capacity and good rate capability compared with pure MoS2 nanosheets.
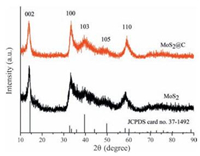
2. Results and discussionFig. 1 shows the XRD patterns of the MoS2 nanosheets and MoS2@C. All the diffraction peaks can be indexed to hexagonal MoS2 (JCPDS card No. 37-1492; space group P63/mmc; a = 3.161 Å, c = 12.299 Å). The strongest peak at 14.2° corresponds to the (0 0 2) plane, which is characteristic of the ordered stacking of 2D MoS2 layers along c-axis. Notably, the 2θ of the (0 0 2) diffraction peak was shifted to a lower-angle compared with 2H-MoS2, revealing the increased interlayer distance of the (0 0 2) plane. No other characteristic peaks from impurities are detected in the XRD pattern. According to the XRD patterns, the nitrogen-doped porous carbon is amorphous (Fig. S1 in Supporting information) and does not destroy the structure of hexagonal MoS2.
|
Download:
|
| Figure 1. XRD patterns of pure MoS2 nanosheets and hierarchical MoS2@C. | |
Fig. 2 depicts typical SEM images of MoS2@C. As shown in Fig. 2a and b, MoS2 ultrathin nanosheets can grow in situ on the surfaces of nitrogen-doped porous carbon (Fig. S2 in Supporting information) to form hierarchical MoS2@C. It is clear that the thickness of MoS2 nanosheests in MoS2@C is about 11 nm due to the strong interaction between MoS2 and nitrogen-doped carbon. Energy-dispersive X-ray spectroscopy (EDX) scanning elemental mapping of Mo, S, C, N and O elements in Fig. S3 in Supporting information also verified the formation of MoS2 nanosheets are in situ grow on the nitrogen-doped porous carbon. The hierarchical structures of MoS2@C can be further confirmed by TEM image in Fig. 2c, which shows that aligned MoS2 nanosheets are closely attached on the surfaces of the nitrogen-doped porous carbon. Typical HRTEM image of MoS2@C is represented in Fig. 2d. The observed fringes correspond to the interplanar distance of 0.69 nm, which are in good agreement with the lattice spacing of the (0 0 2) planes of pure MoS2 nanosheets as shown in Fig. 3d [21]. The amorphous nitrogen-doped porous carbon can be also found. When the nitrogen-doped porous carbon was absent in the reaction system, flower-like MoS2 nanostructures composed of nanosheets with thickness of about 20 nm are clearly observed (Fig. 3a and b), which is much larger than the thickness of MoS2 nanosheests in MoS2@C, TEM images of pure MoS2 nanosheests can also be seen in Fig. 3c, which is in agreement with the abovedescribed SEM observations. The weight content of MoS2 in MoS2@C is determined to be 84.49% through thermogravimetric (TG) analysis (Fig. S4 in Supporting information).

|
Download:
|
| Figure 2. SEM (a and b), TEM (c) and HRTEM (d) images of hierarchical MoS2@C. | |

|
Download:
|
| Figure 3. SEM (a and b), TEM (c) and HRTEM (d) images of pure MoS2 nanosheets. | |
Fig. 4a and b shows the representative CV curves of MoS2@C and pure MoS2 nanosheets electrode for the first, second, and fifth cycles, respectively. In the first cycle, the cathodic peak at about 0.8 V corresponds to the intercalation of lithium ions into the MoS2 lattice to form LixMoS2, and another peak at about 0.2 V is attributed to complete reduction of MoS2. In the following cathodic sweeps, the peaks at 0.8 V and 0.2 V disappear while two new peaks appear at about 1.7 V and 0.7 V, suggesting a multi-step lithium intercalation reaction. There is no significant change in anodic sweeps and it remains at about 2.5 V, corresponding to lithium extraction process, which is in agreement with the reports previously [22–24].

|
Download:
|
| Figure 4. Representative CV curves of pure MoS2 nanosheet electrode (a) and hierarchical MoS2@C electrode (b). | |
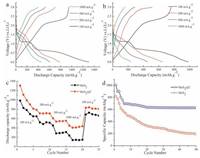
Fig. 5a and b display the galvanostatic discharge–charge profiles of MoS2@C electrode and pure MoS2 electrode, respectively. Two voltage plateaus can be observed at about 1.1 V and 0.6 V, which are attributed to the intercalation of lithium ions into the MoS2 lattice and the complete reduction of MoS2, respectively [25, 26]. The initial discharge capacity of MoS2@C electrode is 1305.5 mA h g-1 at a current density of 100 mA g-1, which is much higher than that of pure MoS2 electrode (984 mA h g-1). As the current density is increased from 200 to 500 and 1000 mA g-1, the discharge capacities of MoS2@C electrode are decreased from 713 mA h g-1 to 535 mA h g-1 and 438 mA h g-1, respectively. However, the pure MoS2 electrode delivers the discharge capacities of 521 mA h g-1 (200 mA g-1), 284 mA h g-1 (500 mA g-1), and 145 mA h g-1 (100 mA g-1). Clearly, the discharge capacity of MoS2@C electrode is much higher than that of pure MoS2 electrode, especially at high current density. Fig. 5c shows the comparison of the rate performances of MoS2 electrode and MoS2@C electrode. Both pure MoS2 and MoS2@C electrodes exhibit good cycling performances at the current density of 200–1000 mA g-1, because the restacking and condensation of MoS2 during the charge–discharge process can be avoided. It is clear that the hierarchical MoS2@C electrode exhibit good rate capability due to the introduction of nitrogen-doped carbon and hierarchical MoS2@C. When the current density reverses back to 100 mA g-1, their capacities of pure MoS2 and MoS2@C electrodes can recover to the original value immediately, indicating that the electrode materials are highly stable in spite of cycling with high rate. The cycling performance of the hierarchical MoS2@C and pure MoS2 electrode at a current density of 0.1 A g-1 is shown in Fig. 5d. After 50 cycles, the hierarchical MoS2@C can still be stable at a capacity of 628 mAh g-1, while pure MoS2 delivers fast capacity fading, which suggesting that the hierarchical porous carbon can effectively help to keep the overall structural integrity during the discharge/charge process.
|
Download:
|
| Figure 5. Galvanostatic discharge–charge profiles of hierarchical MoS2@C electrode (a) and pure MoS2 nanosheet electrode (b) at different current density; the comparison of the rate performance of pure MoS2 nanosheet electrode and hierarchical MoS2@C electrode (c); and cycling performance the pure MoS2 nanosheet and hierarchical MoS2@C electrode at 0.1 g-1 (d). | |
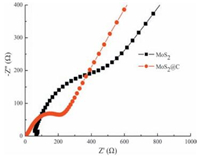
In order to understand the electrochemical reaction kinetics and interfacial behavior of the hybrid electrode, the EIS of the electrodes were further examined. Fig. 6 shows the Nyquist plots of MoS2@C electrode and the pure MoS2 electrode. Each of the curves consists of a depressed semicircle and a straight line, which are related to the charge transfer process of the electrolyte–electrode interface and the lithium ion diffusion in the bulk of the electrode, respectively. It can be seen that the charge-transfer resistance of MoS2@C electrode is much lower that of pure MoS2 electrode, indicating that the introduction of porous nitrogen-doped carbon can indeed improve the electronic conductivity of MoS2@C electrode. Furthermore, the inclined line of MoS2@C electrode is also steeper than that of pure MoS2 electrode owing to the small thickness of MoS2 nanosheets, which can facilitate Li+ ion transfer in the electrode. The entranced electrochemical performances of MoS2@C electrode can be attributed to the synergistic effects of the increased electronic conductivity and the facilitation of lithium ion transport arising from the hierarchical structures of MoS2@C.
|
Download:
|
| Figure 6. EIS spectra of the pure MoS2 nanosheet electrode and hierarchical MoS2@C electrode after test. | |
3. Conclusion
In summary, we have developed a facial hydrothermal route combined with high-temperature calcination to synthesize hierarchical MoS2@C, in which MoS2 nanosheet arrays are closely attached on the surfaces of the nitrogen-doped porous carbon. The hierarchical structures are helpful to facilitate the fast and efficient transport of electrons and Li+ ions. As expected, the MoS2@C exhibit enhanced electrochemical performances compared with pure MoS2 nanosheets. These findings based on the synergistic effect of active materials and porous carbon can be expected to open up new opportunities for designing high performance electrode materials.
4. ExperimentalThe nitrogen-doped porous carbon was prepared through a similar method using polypyrrole as the precursor [18]. In a typical synthesis for MoS2@C, nitrogen-doped porous carbon (60 mg) was dispersed into deionized water by ultrasonication for 30 min. Then ammonium molybdate ((NH4)6Mo7O24, 0.584 g) was added to the solution. After being stirred for 5 min, thiourea (1 g) was added. After being stirred for another 5 min, the solution was transferred into a Teflon-lined stainless steel autoclave (100 mL) in an electric oven at 200 ℃ for 24 h and then cooled down to room temperature naturally. The black precipitate was collected by centrifugation, washed with deionized water and ethanol several times, and finally dried overnight at 80 ℃ in an oven. The preparation of pure MoS2 was similar to that described above, but without the addition of nitrogen-doped porous carbon. The MoS2 nanosheets and MoS2@C were further calcined at 800 ℃ in an N2 atmosphere for 2 h with a heating rate of 3 ℃ min-1.
The crystalline structure of the MoS2 nanosheets and MoS2@C were characterized by X-ray powder diffraction (XRD Rigaku Dmax-γA XRD with CuKα radiation, λ = 1.54178 Å) from 10° to 90°. SEM images were measured by field-emission scanning electron microscopy (FE-SEM, JSM-6700F from JEOL) and TEM images were measured by a transmission electron microscopy (TEM, JEOL 2100F).
The electrochemical measurements were carried out using CR2032-type coin cell at room temperature. The working electrodes were fabricated by mixing the active materials, carbon black (SuperP), and poly(vinyl difluoride) (PVDF) at aweight ratio of 70:20:10 and the mixture was mixed with n-methyl pyrrolidone (NMP) to form slurry and then pasted onto pure Cu foil. The electrode area was 1.54 cm2. Pure lithium foil was used as the counter electrode and separated by a Celgard 2500 membrane separator. A solution of 1 mol L-1 LiPF6 in ethylene carbonate/dimethyl carbonate (1:1 by volume) was used as the electrolyte. The cells were assembled in a glove box filled with high purity argon gas and soaked overnight before test. The galvanostatic discharge–charge experiments were performed over a voltage range of 0.01–3.0 V (vs. Li+/Li) at different current density using a LAND CT2001A battery tester. Cyclic voltammetry (CV) curves were performed using the same workstation as EIS measurements at a scanning rate of 0.5 mV s-1. Electrochemical impedance spectroscopy (EIS) measurements were carried out on an Autolab PGSTAT302N electrochemical workstation by applying a sine wave with the amplitude of 10.0 mV over the frequency range from 100 kHz to 10 MHz.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 51272113, 51272115, 51672146), A Project of Shandong Province Higher Educational Science and Technology Program (Nos. J13LA10, J14LA15, J15LA12), Development Program in Science and Technology of Qingdao (No. 15-9-1-65-jch).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.10.007.
| [1] | J.B. Goodenough, K.S. Park, The Li-ion rechargeable battery:a perspective. J. Am.Chem.Soc. 135 (2013) 1167–1176. DOI:10.1021/ja3091438 |
| [2] | J.M. Tarascon, M. Armand, Issues and challenges facing rechargeable lithium batteries. Nature 414 (2001) 359–367. DOI:10.1038/35104644 |
| [3] | Y.X. Tang, Y.Y. Zhang, W.L. Li, B. Ma, X.D. Chen, Rational material design for ultrafast rechargeable lithium-ion batteries. Chem.Soc.Rev. 44 (2015) 5926–5940. DOI:10.1039/C4CS00442F |
| [4] | S.L. Yang, B.H. Zhou, M. Lei, Sub-100 nm hollow SnO2@C nanoparticles as anode material for lithium ion batteries and significantly enhanced cycle performances. Chin.Chem.Lett. 26 (2015) 1293–1297. DOI:10.1016/j.cclet.2015.05.051 |
| [5] | M. Chhowalla, H.S. Shin, G. Eda, The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat.Chem. 5 (2013) 263–275. DOI:10.1038/nchem.1589 |
| [6] | H.S.S. Ramakrishna Matte, A. Gomathi, A.K. Manna, MoS2 and WS2 analogues of graphene. Angew.Chem. 122 (2010) 4153–4156. DOI:10.1002/ange.201000009 |
| [7] | L.C. Yang, S.N. Wang, J.J. Mao, Hierarchical MoS2/polyaniline nanowires with excellent electrochemical performance for lithium-ion batteries. Adv. Mater. 25 (2013) 1180–1184. DOI:10.1002/adma.201203999 |
| [8] | A.B. Laursen, S. Kegnæs, S. Dahl, I. Chorkendorff, Molybdenum sulfides-efficient and viable materials for electro-and photoelectrocatalytic hydrogen evolution. Energy Environ.Sci. 5 (2012) 5577–5591. DOI:10.1039/c2ee02618j |
| [9] | L. Zhang, X.W. Lou, Hierarchical MoS2 shells supported on carbon spheres for highly reversible lithium storage. Chem.Eur.J. 20 (2014) 5219–5223. DOI:10.1002/chem.v20.18 |
| [10] | Z.Y. Guo, Y. Zhong, Z.W. Xuan, Polypyrrole-assisted synthesis of roselike MoS2/nitrogen-containing carbon/graphene hybrids and their robust lithium storage performances. RSC Adv. 5 (2015) 62624–62629. DOI:10.1039/C5RA09092J |
| [11] | C.M. Mao, Y. Zhong, H.J. Shang, Carbon encapsulated nanosheet-assembled MoS2 nanospheres with highly reversible lithium storage. Chem. Eng.J. 304 (2016) 511–517. DOI:10.1016/j.cej.2016.06.120 |
| [12] | X.X. Zuo, K. Chang, J. Zhao, Bubble-template-assisted synthesis of hollow fullerene-like MoS2 nanocages as a lithium ion battery anode material. J. Mater.Chem.A 4 (2016) 51–58. DOI:10.1039/C5TA06869J |
| [13] | S.J. Ding, J.S. Chen, X.W. Lou, Glucose-assisted growth of MoS2 nanosheets on CNT backbone for improved lithium storage properties. Chem.Eur.J. 17 (2011) 13142–13145. DOI:10.1002/chem.201102480 |
| [14] | J. Wang, J.L. Liu, D.L. Chao, Self-assembly of honeycomb-like MoS2 nanoarchitectures anchored into graphene foam for enhanced lithium-ion storage. Adv.Mater. 26 (2014) 7162–7169. DOI:10.1002/adma.v26.42 |
| [15] | Z.Q. Deng, Y.J. Hu, D.Y. Ren, Reciprocal hybridization of MoO2 nanoparticles and few-layer MoS2 for stable lithium-ion batteries. Chem. Commun. 51 (2015) 13838–13841. DOI:10.1039/C5CC05069C |
| [16] | J.Y. Liao, B. De Luna, A. Manthiram, TiO2-B nanowire arrays coated with layered MoS2 nanosheets for lithium and sodium storage. J.Mater.Chem.A 4 (2016) 801–806. DOI:10.1039/C5TA07064C |
| [17] | Z.S. Wu, W.C. Ren, L. Xu, F. Li, H.M. Cheng, Doped graphene sheets as anode materials with superhigh rate and large capacity for lithium ion batteries. ACS Nano 5 (2011) 5463–5471. DOI:10.1021/nn2006249 |
| [18] | Z.H. Zhang, Z.F. Zhou, H.R. Peng, Y. Qin, G.C. Li, Nitrogen-and oxygen-containing hierarchical porous carbon frameworks for high-performance supercapacitors. Electrochim.Acta 134 (2014) 471–477. DOI:10.1016/j.electacta.2014.04.107 |
| [19] | L. Zhang, H.B. Wu, Y. Yan, X. Wang, X.W. Lou, Hierarchical MoS2 microboxes constructed by nanosheets with enhanced electrochemical properties for lithium storage and water splitting. Energy Environ.Sci. 7 (2014) 3302–3306. DOI:10.1039/C4EE01932F |
| [20] | G.C. Li, Z.H. Zhang, H.R. Peng, K.Z. Chen, Mesoporous hydrogenated TiO2 microspheres for high rate capability lithium ion batteries. RSC Adv. 3 (2013) 11507–11510. DOI:10.1039/c3ra41858h |
| [21] | S.P. Zhang, B.V.R. Chowdari, Z.Y. Wen, J. Jin, J.H. Yang, Constructing highly oriented configuration by few-layer MoS2:toward high-performance lithium-ion batteries and hydrogen evolution reactions. ACS Nano 9 (2015) 12464–12472. DOI:10.1021/acsnano.5b05891 |
| [22] | B. Chen, E.Z. Liu, F. He, 2D sandwich-like carbon-coated ultrathin TiO2@defect-rich MoS2 hybrid nanosheets:synergistic-effect-promoted electrochemical performance for lithium ion batteries. Nano Energy 26 (2016) 541–549. DOI:10.1016/j.nanoen.2016.06.003 |
| [23] | S. Hu, W. Chen, J. Zhou, Preparation of carbon coated MoS2 flower-like nanostructure with self-assembled nanosheets as high-performance lithium-ion battery anodes. J.Mater.Chem.A 2 (2014) 7862–7872. DOI:10.1039/c4ta01247j |
| [24] | K. Chang, D.S. Geng, X.F. Li, Ultrathin MoS2/nitrogen-doped graphene nanosheets with highly reversible lithium storage. Adv.Energy Mater. 3 (2013) 839–844. DOI:10.1002/aenm.v3.7 |
| [25] | P.P. Wang, H.Y. Sun, Y.J. Ji, W.H. Li, X. Wang, Three-dimensional assembly of single-layered MoS2. Adv.Mater. 26 (2014) 964–969. DOI:10.1002/adma.v26.6 |
| [26] | N. Lingappan, N.H. Van, S. Lee, D.J Kang, Growth of three dimensional flower-like molybdenum disulfide hierarchical structures on graphene/carbon nanotube network:an advanced heterostructure for energy storage devices. J. Power Sources 280 (2015) 39–46. DOI:10.1016/j.jpowsour.2015.01.064 |
 2017, Vol. 28
2017, Vol. 28 


