b Department of Material Science and Engineering, Shanghai University, Shanghai 200444, China;
c Department of Chemical Engineering, Zaozhuang Vocational College, Zaozhuang 277800, China
Currently, lithium ion batteries have aroused an increasing attention due to their extensive applications in advanced electric vehicles and portable electronic devices. However, the current development still cannot solve the market demands of high energy density lithium-ion batteries [1, 2]. Sulfur as cathode with some advantages of abundant, inexpensive and harmlessness has a theoretical specific capacity of 1675 mAh/g, which is several times higher than that of other lithium-ion cathode materials [3–6]. Therefore, lithium–sulfur (Li–S) batteries are widely considered as very promising rechargeable lithium-ion cells, due to high energy density of 2600 Wh/kg. Nevertheless, the Li–S batteries still suffer from various challenging problems such as natural low conductivity, short cycling life and volume expansion, etc. In particular, the shuttle effect of polysulfide ions can result in a serious capacity decay and loss of active material [6], which has seriously hampered the development of Li–S batteries.
To solve the above mentioned problems, a number of approaches have been investigated carried out to optimize sulfur cathodes. Nazar et al. [7] firstly reported a mesoporous carbon for immobilizing sulfur to realize a high-sulfur content in the C/S composite. Consequently, a large number of porous carbon materials were used for sulfur loading to restrain the polysulfide species diffusion, such as meso/microporous carbon [8, 9], carbon spheres [10, 11], carbon nanotube/fiber [12, 13] and graphene [14, 15], etc. Among these designed carbon materials, microporous carbon has been successfully employed to enhance the cycling stability of batteries. Guo et al. [16] demonstrated a microporous carbon with 0.5 nm pore size for short-chain S2–4 loading, which could effectively suppress the formation of long-chain polysulfide (Li2Sx, x > 4) and exhibit a superior cycling stability. However, due to low sulfur loading and sluggish ion migration, the microporous carbon with small pore size has not been widely applied in the commercialization of lithium–sulfur batteries. In addition, researchers have also identified that nitrogen doping in porous carbon matrix for Li–S batteries can produce a higher conductivity and a stronger absorbability to the polysulfide species, which will contribute to the enhanced cycle stability of the battery [17, 18].
Considering these, it is expected that a nitrogen-doped multiporous carbon with a large pore volume and a small pore size will be designed to accelerate the actual applications of Li–S batteries. Meanwhile, it is noteworthy to explore sustainable carbon resources as electrode materials to relieve the environmental crisis and meet the increasing need of energy [19, 20]. For example, Lu et al. [20] demonstrated a 3D macro-porous carbon from cheap yeast through a template-free approach for high-performance supercapacitor. Therefore, low-cost and renewable biological resources will be a very promising sustainable carbon precursor for electrode materials.
Here, we firstly report a novel and green nitrogen-doped multiporous carbon material with renewable biological cells through a facile K2CO3 activation method. The prepared carbon material shows a large pore volume and a narrow pore size, which not only can improve S/C mass ratio, but can significantly alleviate polysulfide ion diffusion. Moreover, the natural nitrogen doping can obviously improve the electron conductivity and strongly adsorb polysulfides, which will bring about a good rate capability and remarkable cycling stability for Li–S batteries. It suggests that the C/S composite with 67 wt% sulfur as the cathode material of Li–S batteries will exhibit excellent electrochemical performance. Here, we firstly report a novel and green nitrogen-doped multiporous carbon material with renewable biological cell through facile K2CO3 activation method. The prepared carbon material shows large pore volume and narrow pore size, which not only can improve S/C mass ratio, but only can significantly alleviate the polysulfide ion diffusion. Moreover, the natural nitrogen doping can obviously improve the electron conductivity and strongly adsorb the polysulfides, which will bring about good rate capability and remarkable cycling stability for Li–S batteries. It suggests that the C/S composite with 67 wt% sulfur as cathode material of Li–S batteries will exhibit excellent electrochemical performance.
2. Experimental 2.1. Material synthesisThe yeast powder (purchased from Sunkeen Company, China) with enriched carbon and nitrogen was washed with deionized water and ethanol and dried at 50 ℃. The washed product was well-mixed with K2CO3 at a mass ratio of 1:2 and then heated in N2 atmosphere at different temperatures for 2 h. The activated carbon was washed with dilute hydrochloric acid solution and hot deionized water. Finally, the nitrogen-doped multiporous carbon from yeast powder (expressed as NYMC) was dried at 100 ℃ overnight.
Sulfur powder and NYMC sample were fully pulverized at a mass proportion of 4:1 and sealed in a Teflon autoclave with Ar protection and heated at 155 ℃ for 24 h to obtain the NYMC/S composite.
2.2. CharacterizationN2 adsorption/desorption isotherms were tested with Micrometrics ASAP 2020 + C Surface Area and Porosity Analyzer. Powder X-ray diffraction (XRD) were collected by Rigaku DLMAX-2550V diffractometer with Cu Kα radiation (λ = 1.540568 Å). The morphologies of all samples were characterized by scanning electron microscopy (SEM, S4800) and high resolution transmission electron microscope (HRTEM, JSM-2010F). Thermogravimetric analysis (TGA) was tested. Raman spectrum was characterized by a Raman spectrometer (Renishaw InVia-plus) equipped with 633 nm excitation lasers. X-ray photoelectron spectroscopy (XPS) tests were characterized.
2.3. Electrochemical measurementsThe 80 wt% NYMC/S composite, 10 wt% acetylene black and 10 wt% polyvinylidene fluoride were mixed in N-methyl pyrrolidinone. The formed slurry coating on Al foil was dried at 50 ℃ in vacuum. The coin cells (CR2016) were assembled with the NYMC/S composite as cathode and the metal lithium as counter electrode at r.t. The electrolyte was lithium bis(trifluoromethanesulfonyl)imide (1.0 mol/L) in the 1,3-dioxolane and 1,2-dimethoxyethane (1:1, v/v) with 0.1 mol/L LiNO3. All batteries were tested between 1.5 and 3 V through CT2001A battery test system at r.t. Cyclic voltammetry measurements (CV) and electrochemical impedance spectroscopy (EIS) tests were conducted on a CHI660D electrochemistry workstation. All capacities were calculated based on the mass of sulfur.
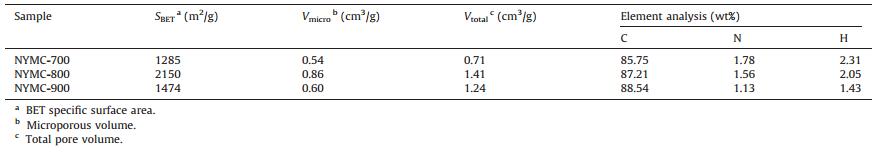
3. Results and discussionThe SEM image of the as-synthesized NYMC material is showed in Fig. 1a and obviously indicated the NYMC sample exhibits some fragmented structure from cell wall pyrolysis, as opposed to the original elliptical morphology of the cell (Inset in Fig. 1a). Additionally, Fig. 1b shows the micromorphology of the NYMC with abundant pore structure by TEM imaging, which is favorable for immobilizing sulfur and ion diffusion. Nitrogen adsorption/ desorption isotherms of the NYMC materials at 700–900 ℃ further demonstrate the existence of a porous structure (Fig. 1c). The three samples show typical Type Ⅰ adsorption/desorption isotherms, suggesting the formation of numerous rich micropores [8]. Meanwhile, the small hysteresis loop on curves means the existence of partial mesopores, which can result in a fast kinetics reaction and a high sulfur loading [21]. The pore distribution curve (Fig. 1d) calculated by the DFT method shows that the NYMC-700 and NYMC-800 samples have more micropore distribution, focusing on the 0.7 nm than NYMC-900 with wide mesopore distribution. Meanwhile, Table 1 documents the specific surface area (SBET), micropore volume (Vmicro) and total pore volume (Vtotal) of the three samples. As shown in Table 1, the NYMC-700 and NYMC-800 samples possess a higher proportion of Vmicro/Vtotal than NYMC-900. The enriched and well-defined microporous channels of NYMC materials are attributed to the K2CO3 activation at relatively low temperature, indicating that the dissolution of polysulfides will be effectively suppressed and the cycling performance will be enhanced.
|
Download:
|
| Figure 1. (a) FE-SEM image and (b) TEM image of the nitrogen-doped multiporous carbon (expressed as NYMC), the inset is the SEM image of pristine biomass material; (c) N2 adsorption/desorption isotherms and (d) pore size distribution of carbon materials. | |
|
|
Table 1 Specific surface area, pore volume and chemical composition of NYMC (700–900℃). |
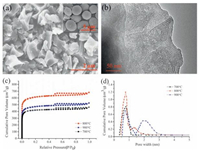
The element content of the three samples is also showed in Table 1. These samples have decreasing nitrogen content from 1.78 to 1.13 wt% with increasing temperature, resulting from the calcination of protein in cell. It is well-known that porous carbon material with high SBET, large Vtotal, narrow microporous distribution and doping nitrogen will beneficial to an improved utilization of sulfur and an excellent cycling stability of Li–S battery [22]. According to these data, the NYMC-800 material is considered to be a desired carbon network for sulfur loading in fabricating the NYMC/S composite. The actual content of sulfur in the NYMC/S composite is confirmed through TGA analysis to be 67 wt% (Fig. 2a), requiring pore volume of 0.98 cm3/g (calculated with sulfur density of 2.07 g/cm3), less than the Vtotal of NYMC-800, which will effectively accommodate volume expansion during cycling.
|
Download:
|
| Figure 2. (a)Thermogravimetric analysis oftheNYMC/S composite; (b)XRD patterns and (c)Raman spectraof NYMC, NYMC/S andsulfur; (d) TEM image, (e)X-ray photoelectron spectroscopy (XPS) spectrum and (f) N1s spectrum of the NYMC/S composite. | |
To characterize the structures of pure sulfur, NYMC and NYMC/S samples, X-ray diffraction curves were measured in Fig. 2b. The NYMC shows two typical diffraction peaks at 25° and 44°, demonstrating the formation of a graphitic carbon during the calcination. After sulfur impregnation, the characteristic peaks of S8 are not observed in the NYMC/S composite, which indicates the sulfur molecules have been highly dispersed into the pore channel of NYMC. In the Raman spectra (Fig. 2c), the D peak (1340 cm-1) and G peak (1570 cm-1), corresponding to the disordered and sp2-hybridized carbon, are observed in the NYMC material. In addition, the NYMC/S sample does not show the characteristic peaks from S8 molecule, which is attributed to the vibration of S–S bond [23]. The result suggests that S8 molecule in the pore channel does not possess the long-range ordered structure, but a highly dispersed state. The TEM (Fig. 2d) of the NYMC/S sample further reveals that the sulfur particles have been fully confined in the porous carbon without sulfur agglomeration, which will be beneficial to improve the sulfur utilization and electrochemical performance.
The surface chemical composition of NYMC/S composite was confirmed by XPS analysis. As shown in Fig. 2e, the spectrum of NYMC/S proves the existence of sulfur and nitrogen in the carbon matrix [22, 24]. The N1s spectrum of NYMC/S composite in Fig. 2f is fitted into two peaks, pyridinic nitrogen (398.6 eV) and graphitic nitrogen (401.1 eV) [24]. The doping nitrogen atoms will obviously increase the conductivity of carbon network and the adsorption to polysulfide species, which will contribute to a good cycling property and a high coulombic efficiency.
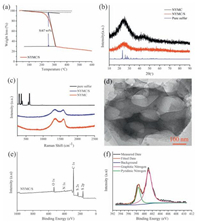
The cyclic voltammogram curve of NYMC/S cathode was recorded between 1.5 and 3 V with a constant sweep rate of 0.1 mV/s. Fig. 3a shows a typical S8 redox behavior [25]. Two reduction peaks are located at 2.34 and 2.02 V, corresponding to the reduction of S8 to long-chain polysulfide species and further to Li2S2 and Li2S. One anodic peak is observed, which is related to the oxidation process of the final discharge production to longchain polysulfide species and sulfur. Moreover, the redox peaks remain highly consistent within three cycles, meaning a relatively good electrochemical reversibility and structural stability during reaction.

|
Download:
|
| Figure 3. (a) Cycle voltammograms curves of the NYMC/S composite at a sweep rate of 0.1 mV/s between 1.5 and 3.0 V; (b) rate properties of the NYMC/S composite at different rates; (c) Electrochemical impedance spectra before and after cycle and discharge/charge voltage profiles at different rates (inset); (d) Cycling performance of the NYMC/S composite. | |
The rate capability of NYMC/S cathode is shown in Fig. 3b. During cycles, the NYMC/S cathode delivers high reversible capacities of 1422, 1091, 881, 768 and 674 mAh/g at 0.1C, 0.2C, 0.5C, 1C and 2C (1C = 1675 mA/g), confirming an excellent electron/ionic transport and enhanced reaction kinetics. The corresponding capacities were calculated based on the mass of sulfur. In particular, the capacity of the NYMC/S cathode is mostly recovered when the rate goes to 0.1C, indicating a highly reversibility rate performance of NYMC/S cathode. The fast reaction kinetics of NYMC/S cathode before and after cycles was further proved by the electrochemical impedance spectra (EIS) in Fig. 3c. After five cycles, the charge transfer resistance obviously decreases, suggesting a fast charge transfer and good electrolyte infiltration owing to the nitrogen doping with improved conduc tivity and mutliporous structure in the NYMC, respectively. In addition, we can see that the inset image of Fig. 3c depicts typical two discharge plateaus and one charge plateau at various rates, which is in accordance with CV peaks [23]. Moreover, the polarization phenomenon is not too serious at a higher rate of 2C, indicating a fast and efficient dynamics performance. The cycling performance of the NYMC/S cathode was showed at a constant current density of 0.1C (167.5 mA/g) in Fig. 3d. The initial discharge capacity of NYMC/S cathode reaches up to 1410 mAh/g, close to 84% of the theoretical capacity of 1675 mAh/g [26]. After 50 cycles, the discharge capacity obviously decreases to 1005 mAh/g, because some partial sulfur in the mesoporous carbon can diffuse into the electrolyte, resulting in a sudden capacity drop. However, in the following 150 cycles, the NYMC/S cathode still remains a high capacity of 912 mAh/g, with only 0.06% capacity loss per cycle. The outstanding cycling stability should be attributed to the mutual effect between the microporous carbon and nitrogen doping in the NYMC material. On one hand, the microporous carbon with 0.7 nm pore size from the K2CO3 activation can function as an efficient reservoir of polysulfide species and significantly relieve the shuttle phenomenon. On the other hand, the doped nitrogen from the protein pyrolysis is beneficial to immobilization of soluble polysulfide species on the surface of nitrogen atoms in the carbon network, resulting in a high utilization of sulfur.
4. ConclusionIn summary, we have developed a facile K2CO3 activation method to synthesize nitrogen-doped multiporous carbon with widespread biological cell for Li–S batteries. The microporous carbon with a highly porous structure and small pore size gives rise to a high sulfur loading and an effective suppression of polysulfides. Meanwhile, the doping nitrogen in the carbon network improved the electron conductivity and provided a strong absorption ability of polysulfides. As a result, the NYMC/S cathode exhibited an excellent electrochemical performance with high initial discharge capacity, low capacity decay rate and good rate capability. We believe that the environmentally friendly and low-cost approach for the synthesis of nitrogen-doped multi porous carbon material with renewable biological sources will be also used in large-scale production of other electrode materials.
AcknowledgmentsWe are grateful for the support from the National Natural Science Foundation of China (Nos. 61371021, 61527818), Shanghai Municipal Education Commission (Peak Discipline Construction Program) and Shanghai Education Commission Innovation Project (No. 14YZ016).
| [1] | S.L. Yang, B.H. Zhou, M. Lei, Sub-100 nm hollow SnO2@C nanoparticles as anode material for lithium ion batteries and significantly enhanced cycle perfor-mances. Chin.Chem.Lett. 26 (2015) 1293–1297. DOI:10.1016/j.cclet.2015.05.051 |
| [2] | Y.J. Li, C.X. Zhou, S. Chen, F. Wu, L. Hong, Nd3+-doped Li3V2(PO4)3 cathode material with high rate capability for Li-ion batteries. Chin.Chem.Lett. 26 (2015) 1004–1007. DOI:10.1016/j.cclet.2015.03.013 |
| [3] | X.B. Duan, Y.M. Han, L.W. Huang, Y.B. Li, Y.G. Chen, Improved rate ability of low cost sulfur cathodes by using ultrathin graphite sheets with self-wrapped func-tion as cheap conductive agent. J.Mater.Chem.A 3 (2015) 8015–8021. DOI:10.1039/C4TA06097K |
| [4] | R.J. Chen, T. Zhao, J. Lu, Graphene-based three-dimensional hierarchical sandwich-type architecture for high-performance Li/S batteries. Nano Lett. 13 (2013) 4642–4649. DOI:10.1021/nl4016683 |
| [5] | G.C. Li, G.R. Li, S.H. Ye, X.P. Gao, A polyaniline-coated sulfur/carbon composite with an enhanced high-rate capability as a cathode material for lithium/sulfur batteries. Adv.Energy Mater. 2 (2012) 1238–1245. DOI:10.1002/aenm.v2.10 |
| [6] | X. Liang, C.Y. Kwok, F. Lodi-Marzano, Tuning transition metal oxide-sulfur interactions for long life lithium sulfur batteries:the''goldilocks''principle. Adv. Energy Mater. 6 (2016) . DOI:10.1002/aenm.201501636 |
| [7] | X.L. Ji, K.T. Lee, L.F. Nazar, A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat.Mater. 8 (2009) 500–506. DOI:10.1038/nmat2460 |
| [8] | H. Ye, Y.X. Yin, S. Xin, Y.G. Guo, Tuning the porous structure of carbon hosts for loading sulfur toward long lifespan cathode materials for Li-S batteries. J.Mater. Chem.A 1 (2013) 6602–6608. DOI:10.1039/c3ta10735c |
| [9] | Z. Li, Y. Jiang, L.X. Yuan, A highly ordered meso@microporous carbon-supported sulfur@smaller sulfur core-shell structured cathode for Li-S batteries. ACS Nano 8 (2014) 9295–9303. DOI:10.1021/nn503220h |
| [10] | J. Zang, T.H. An, Y.J. Dong, Hollow-in-hollow carbon spheres with hollow foam-like cores for lithium-sulfur batteries. Nano Res. 8 (2015) 2663–2675. DOI:10.1007/s12274-015-0773-3 |
| [11] | S.K. Liu, Y.J. Li, X.B. Hong, Reduced graphene oxide-hollow carbon sphere nanostructure cathode material with ultra-high sulfur content for high perfor-mance lithium-sulfur batteries. Electrochim.Acta 188 (2016) 516–522. DOI:10.1016/j.electacta.2015.11.101 |
| [12] | W.N. Deng, A.P. Hu, X.H. Chen, Sulfur-impregnated 3D hierarchical porous nitrogen-doped aligned carbon nanotubes as high-performance cathode for lithium-sulfur batteries. J.Power Sources 322 (2016) 138–146. DOI:10.1016/j.jpowsour.2016.05.024 |
| [13] | C. Tang, Q. Zhang, M.Q. Zhao, Nitrogen-doped aligned carbon nanotube/graphene sandwiches:facile catalytic growth on bifunctional natural catalysts and their applications as scaffolds for high-rate lithium-sulfur batteries. Adv. Mater. 26 (2014) 6100–6105. DOI:10.1002/adma.201401243 |
| [14] | H.B. Zhao, Z.H. Peng, W.J. Wang, Reduced graphene oxide with ultrahigh conductivity as carbon coating layer for high performance sulfur@reduced gra-phene oxide cathode. J.Power Sources 245 (2014) 529–536. DOI:10.1016/j.jpowsour.2013.07.002 |
| [15] | M.Q. Zhao, Q. Zhang, J.Q. Huang, Unstacked double-layer templated gra-phene for high-rate lithium-sulphur batteries. Nat.Commun. 5 (2014) 3410. |
| [16] | S. Xin, L. Gu, N.H. Zhao, Smaller sulfur molecules promise better lithium-sulfur batteries. J.Am.Chem.Soc. 134 (2012) 18510–18513. DOI:10.1021/ja308170k |
| [17] | G.M. Zhou, Y.B. Zhao, A. Manthiram, Dual-confined flexible sulfur cathodes encapsulated in nitrogen-doped double-shelled hollow carbon spheres and wrapped with graphene for Li-S batteries. Adv.Energy Mater. 5 (2015) . DOI:10.1002/aenm.201402263 |
| [18] | F. Pei, T.H. An, J. Zang, From hollow carbon spheres to N-doped hollow porous carbon bowls:rational design of hollow carbon host for Li-S batteries. Adv.Energy Mater. 6 (2016) . DOI:10.1002/aenm.201502539 |
| [19] | Y. Xia, Z. Xiao, X. Dou, Green and facile fabrication of hollow porous MnO/C microspheres from microalgaes for lithium-ion batteries. ACS Nano 7 (2013) 7083–7092. DOI:10.1021/nn4023894 |
| [20] | H.M. Sun, W.H. He, C.H. Zong, L.H. Lu, Template-free synthesis of renewable macroporous carbon via yeast cells for high-performance supercapacitor elec-trode materials. Appl.Mater.Interfaces 5 (2013) 2261–2268. DOI:10.1021/am400206r |
| [21] | Y.G. Zhang, Y. Zhao, A. Konarov, Z. Li, P. Chen, Effect of mesoporous carbon microtube prepared by carbonizing the poplar catkin on sulfur cathode perfor-mance in Li/S batteries. J.Alloy Compd. 619 (2015) 298–302. DOI:10.1016/j.jallcom.2014.09.055 |
| [22] | H.C. Chen, Y.J. Wei, J.T. Wang, Controllable nitrogen doping of high-surface-area microporous carbons synthesized from an organic-inorganic sol-gel ap-proach for Li-S cathodes. Appl.Mater.Interfaces 7 (2015) 21188–21197. DOI:10.1021/acsami.5b05107 |
| [23] | X. Yang, L. Zhang, F. Zhang, Y. Huang, Y.S. Chen, Sulfur-infiltrated graphene-based layered porous carbon cathodes for high-performance lithium-sulfur batteries. ACS Nano 8 (2014) 5208–5215. DOI:10.1021/nn501284q |
| [24] | J. Yang, S.Y. Wang, Z.P. Ma, Novel nitrogen-doped hierarchically porous coralloid carbon materials as host matrixes for lithium-sulfur batteries. Electro-chim.Acta 159 (2015) 8–15. DOI:10.1016/j.electacta.2015.01.187 |
| [25] | G.M. Zhou, L. Li, C.Q. Ma, A graphene foam electrode with high sulfur loading for flexible and high energy Li-S batteries. Nano Energy 11 (2015) 356–365. DOI:10.1016/j.nanoen.2014.11.025 |
| [26] | J.L. Shi, H.J. Peng, L. Zhu, W.C. Zhu, Q. Zhang, Template growth of porous graphene microspheres on layered double oxide catalysts and their applications in lithium-sulfur batteries. Carbon 92 (2015) 96–105. DOI:10.1016/j.carbon.2015.03.031 |
 2017, Vol. 28
2017, Vol. 28