b University of Chinese Academy of Sciences, Beijing 100049, China
Photochemistry is mainly concerned with the chemical phenomena under light irradiation. By absorbing photons from light resources by compound, original bonds break and new bonds form. And then, the occurrence of chemical reaction makes molecules conformation transform from one structure to the other. Different with the photo-chemistry in solid or solution, on-surface photochemistry provides a special and simple environment to investigate the photo-chemical process [1]. As reported by previous studies, isolated species, molecular islands or steric hindrance arising from the surface atoms or the neighboring molecules were expected to affect the photo-activity of molecules, which renders surface photo-chemistry some intriguing features different from that in solid or solution [2–4].
The primary step to study photo-reactions on surface is to integrate molecules on surface. By utilizing self-assembly strategy, two-dimension (2D) self-assembled monolayer (SAM) of functional molecules can be formed through non-covalent interactions [5–12]. By introducing azobenzene, diacetylene, double bond and other photo-sensitive groups, well-formed SAMs can present various conformations when exposing to different light wavelengths. Light-regulation on well-formed SAMs is considered as an ideal external regulation strategy for in-situ chemical manipulation among numerous external stimuli [13–17]. Attractive features of these stimuli include clean reaction condition, fast response, tunable energy input and the ability to convert an optical input into a variety of useful output signals [18]. These unique advantages have led better fabrication and control on SAMs by the extensive use of light.
After gaining well-ordered photo-sensitive functional assemblies, it is necessary to have an "eye" to observe their structures as well as the photo-induced behaviors. Luckily, the invention of scanning tunneling microscope (STM) provided us handy tool to have an insight into the on-surface self-assemblies with unprecedented sub-nanometer resolution, monitor the stimuli caused structural transformation, even give stimuli to the self-assembly. By the extensive application in the field of on-surface phenomenon, STM has demonstrated its prominent advantages of high resolution at sub-molecular level and real time observation ability [19–22]. During the study of on-surface photochemistry, the combination of STM with other techniques, like UV–vis adsorption spectra or IR spectra benefited us a lot in the observation and identification of reactions.
However, in some cases, we could not observe the self-assembly of functional molecules by STM, for their interactions with the substrate or the interactions between molecules are too weak to form on-surface monolayer. To solve this problem, organic molecules which are equipped with various active groups to form 2D hydrogen-bonded porous nanostructures were introduced as molecular templates to stabilize the functional molecules [23]. The typical examples are 1, 3, 5-tris(10-carboxydecyloxy)-benzene (TCDB), NN4A, StOF-COOH and so on [24–31]. Interestingly, when molecular templates were employed into on-surface chemistry, the stabilizing effect and reaction promotion effect were both found to greatly facilitated the visualizing of photo-reaction by STM.
Herein, we focus on the STM investigation of photo-active molecules with different kinds of functional groups and their photo-induced reactions including isomerization, dimerization and polymerization on surface, thus give an overview on the onsurface photo-reactions studied in recent years. It can be predicted that the fundamental research in on-surface photo-chemistry on single molecule level will greatly benefit the future invention and fabrication of advanced on-surface devices.
2. On surface photo-reactions 2.1. Photo-reaction of azobenzene derivativesIt is well known to us that when irradiated by UV light or visible light, azobenzene derivatives can process reversible cis–trans isomerization and cause drastic changes in structures and chemical properties (Fig. 1). The two isomers can be switched with particular wavelengths of light, ultraviolet light for trans-to-cis conversion and visible light for cis-to-trans. For a variety of reasons, the cis isomer is less stable than the trans, thus, cis-azobenzene may also thermally relax back to the trans isomer without light. This isomerization property of azobenzene derivatives has been widely used in a variety of practical applications: alignment modulator in liquid crystals, nonlinear optics, molecular sensors, and photo-biological switch [32–39]. For recent years, due to the development of STM technique, more interest has been payed on its photo-reaction process on surface as the devices have to be placed on solid surface. In the study of Bazarnik et al. isomerization mechanisms of 4-anilino-40-nitroazobenzene molecules adsorbed on Si(10 0) surface were determined by a combination of STM and density functional theory (DFT) calculation [40]. Alemani et al. studied the adsorption and switching properties of 3, 30, 5, 5-tetra-tert-butylazobenzene on Au(111), Cu (111), and Au(10 0) surface respectively, discussed the substrate influence on photo-isomerization of azobenzene derivatives [41]. Mielke et al. reported a study of covalently connected multiple switching systems but failed to induce the trans–cis isomerization of azobenzene groups, electronic coupling and steric hindrance would be responsible for the suppressed switching [42]. More detailed conditions for isomerization on surface have been studied by researchers to realize better control of it.

|
Download:
|
| Figure 1. Schematic illumination of photoisomerization of azobenzene. | |
Our group also makes some helpful researches in this area. As we previously reported, 4NN-Macrocycle (Fig. 2a) is an elaborately designed macrocylic compound consisting of four azobenzene groups as the photosensitive units [43]. However, under the STM investigation, 4NN-Macrocycle cannot be observed on surface solely. To solve this problem, TCDB (Fig. 2b), which can form wellordered 2D networks on HOPG with flexible nanoscale pores through hydrogen bond was introduced as a remarkable molecular template to immobilize 4NN-Macrocycle molecules. With the TCDB/4NN-Macrocycle mixing ratio differ, mainly due to the existence of four –CH2-CH2-groups, two kinds of TCDB/4NN-Macrocycle self-assembling structures were observed at heptanoic acid/HOPG interface before light irradiation, including (t, t, t, t)1 and (t, t, t, t)2 (Fig. 3), and they were both the thermally stable trans– trans–trans–trans(t, t, t, t) state of 4NN-Macrocycle molecules. When the 4NN-Macrocycle/TCDB co-assembled network was irradiated by UV light at 366 nm wavelength, it was observed to undergo photoisomerization from trans–trans–trans–trans(t, t, t, t) to trans–trans–trans–cis(t, t, t, c) and trans–cis–trans–cis(t, c, t, c) isomers, this is related to the kinetics of azobenzene in 4NN-Macrocycle. Meanwhile, another (t, t, t, t) isomer, three (t, t, t, c) isomers and one (t, c, t, c) isomer were observed in STM images, as shown in Fig. 3 and Fig. 4, where they can be clearly distinguished by the difference in structure. It should be note that the use of flexible TCDB template greatly facilitated the molecular imaging by STM, on the other side, the structural difference of those photoisomers also dramatically affected the guest-host network structure, thus makes the TCDB template style differ.

|
Download:
|
| Figure 2. Chemical structures of (a) 4NN-Macrocycle (b) TCDB and (c) coronene. Reproduced with permission from Ref. [44]. Copyright 2011 American Chemical Society. | |

|
Download:
|
| Figure 3. Molecular models of the conformations and photoisomers in the photoisomerization process. Reproduced with permission from Ref. [43]. Copyright 2009 American Chemical Society. | |

|
Download:
|
| Figure 4. (a) High-resolution STM image (42 nm × 42 nm, I = 282 pA, V = 739 mV) of a self-assembly monolayer of irradiated TCDB/4NN-Macrocycle adlayers on HOPG surface. Domain A is the unfilled TCDB networks. Domain B is the (t, c, t, c) photoisomers in the TCDB networks. (c) An STM image (33 nm × 33 nm, I = 247 pA, V = 703 mV) of a "disordered" monolayer of irradiated TCDB/4NN-Macrocycle. The domain contains coexisting the (t, t, t, t)3 and (t, t, t, c)1 isomers. (e) An STM image (32 nm × 32 nm, I = 247 pA, V = 703 mV) of a "disordered" monolayer of irradiated TCDB/4NN-Macrocycle. The domain contains coexisting the (t, t, t, t)2 and (t, t, t, c)2 isomers. (g) An STM image (48 nm × 48 nm, I = 253 pA, V = 700 mV) of a "disordered" adlayer of (t, t, t, c)3 and (t, t, t, t)1. (b), (d), (f), (h) Molecular models for (a), (c), (e), (g), respectively. Reproduced with permission from Ref. [43]. Copyright 2009 American Chemical Society. | |
To better understand its photosensitive property, the reverse photoisomerization process was further studied [44]. As we described previously, TCDB/4NN-Macrocycle(t, c, t, c) self-assembly can be formed by UV irradiation on TCDB/4NN-Macrocycle(t, t, t, t) self-assembly, which was found to be a nanocapsule to stabilize coronene molecules (Fig. 2c). As the STM image shows (Fig. 5e), after the 4NN-Macrocycle/TCDB co-assembled structure was irradiated by UV light at 366 nm, two coronenes were entrapped in each cavity of 4NN-Macrocycle, resulting in a guest-host molecular architecture. When the three-component architecture was irradiated by subsequent visible light, due to the isomerization of azobenzene moities from cis to trans, the nanoporous complex template of TCDB/4NN-Macrocycle(t, c, t, c), which has already accommodated coronene, expelled guest molecules from the inner cavities and changed back to the original well-ordered TCDB/4NN-Macrocycle(t, t, t, t) network again (Fig. 5c), achieving the propose of selectively accommodating coronene by light-control. It can be foreseen that this switchable bistability of nanoporous network can act as a potential photoswitch to reversibly encapsulate/expel guest coronene molecules by exposing to UV–vis light in turn. Those studies on 4NN-Macrocycle provided us a facile approach to have an insight into photo-induced reversible isomerization of azobenzene derivatives and their conformational isomers, and furthermore, provided a novel strategy to design and fabricate photo-controlled functional devices at molecular scale.

|
Download:
|
| Figure 5. (a) High-resolution STM image (I = 483 pA, V = 1021 mV) of the TCDB/4NNMacrocycle(t, t, t, t) network structure. (c) STM image (I = 269 pA, V = 641 mV) of the ternary adlayer of TCDB/4NN-Macrocycle(t, t, t, t)/coronene before the UV irradiation. (e) STM image (I = 269 pA, V = 641 mV) of the TCDB/4NN-Macrocycle(t, c, t, c)/ coronene architecture after the UV irradiation. (b), (d), and (f) The molecular model for (a), (c), and (e), respectively. In all the molecular models, the red ball represents oxygen atom, the blue for carbon, and the purple for nitrogen. The hydrogen atoms are omitted for clarity. Reproduced with permission from Ref. [44]. Copyright 2011 American Chemical Society. | |
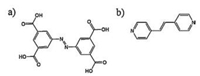
By investigating the apparent structural change of 2D selfassembly, we reported another typical study focusing on the photo-induced isomerization of co-adsorbed azobenzene derivatives. As we previously reported, NN4A (Fig. 6a) could fabricate open Kagomé networks on HOPG surface under STM investigation, as shown in Fig. 7a [28]. When mixed with DPE (Fig. 6b), the Kagomé open network of NN4A was completely replaced by wellordered rectangular network (Fig. 7b), named "boxes" [45]. This observed new structure should be attributed to the NN4A/DPE coadsorption network. Two DPE molecules and two NN4A molecules gathered together to form the basic rectangular shaped box, which arranged one by one to form the lamellae and finally resulted in a well-ordered open network.
|
Download:
|
| Figure 6. The chemical structures of (a) NN4A and (b) DPE. Reproduced with permission from Ref. [45], Copyright 2012, The Royal Society of Chemistry. | |

|
Download:
|
| Figure 7. (a) An STM image of the NN4A monolayer, Iset = 399.8 pA, Vbias = 600.0 mV. (b) A high resolution STM image of the NN4A/DPE network, Iset = 326.5 pA, Vbias = 847.4 mV. (c) A suggested molecular model of the NN4A/DPE network. (d) A high resolution STM image of NN4A/DPE assemblies after UV light irradiation for 30 min, Iset = 300.0 pA, Vbias = 599.9 mV. (e) A suggested model for the zigzag architecture. Reproduced with permission from Ref. [45], Copyright 2012, The Royal Society of Chemistry. | |
It is widely known to us and also fully verified by experiment that both azobenzene groups in NN4A and alkenyl groups in DPE could process reversible trans to cis isomerization under UV–vis light irradiation in solution. However, after exposing their 2D selfassembly on HOPG to UV light at 365 nm respectively, we could only view the assembly of isomerized NN4A by using STM, while the DPE cis-isomer could not be seen, since the assembly formed by cis-DPE molecules may be unstable on the HOPG surface. Then, NN4A/DPE co-adsorption assembly was irradiated by UV light to further study their co-assembly photo-sensitive behavior. After irradiation, the "boxes" structure changed into a new zigzag shaped structure (Fig. 7d), which is suggested to be the coadsorption of the isomerized NN4A (cis) and the non-isomerized DPE (trans) molecules. This has been demonstrated by the latter experiment of directly mixing cis-NN4A and trans-DPE on HOPG surface where the same zigzag structure could also be obtained. With subsequent irradiation at 435 nm on this zigzag shaped structure, the previous rectangle shaped "boxes" can be reconstructed, indicating that the reverse cis to trans process of NN4A has occurred on surface. This STM results suggested that not only the NN4A but also the NN4A/DPE co-adsorption can be reversibly regulated under UV/visible light irradiation, which further provided us a promising method to design highly complex and reversible supramolecular nanostructures.
Besides via the novel structural change in 2D self-assembly to confirm and study the photo-induced behavior of azobenzene compounds, Kumar et al. also demonstrated the isomerization of azobenzene derivatives by testing their protrusions height on Au {111} surface [46]. 4-[2-(4-Phenylazophenyl)-ethoxy]-butane-1-thiol (1) was designed as the azobenzene-functionalized molecule to chemisorb within decanethiolate (C10) self-assembly in ambient. The tightly self-assembled matrix of C10 isolated 1 molecules and the long tether of 1 made the azobenzene moiety protrude from the C10 matrix and stand rigidly, and both of them greatly helped to achieve the controlling over reversible photoswitching of 1. As investigated by STM, the single molecules of 1 within the C10 self-assembly were measured as 2.1±0.3 Å, which was higher than the C10 matrix, defined as the on state. When exposed to UV light at 365 nm, 1 molecules decreased their height by 1.4 Å, indicating the photo-induced switching to the off state. With the increase of UV illumination time, the number of switched molecules was increasing exponentially, as they to protrude 0.7±0.2 Å above the C10 matrix in STM images. After 160 min of UV irradiation, more than 90% of the on (trans) state molecules isomerized to off (cis) state. Then, with subsequent visible light irradiation at 450 nm for 30 min, nearly 50% switched back to the on state. Thus, it could be concluded that the azobenzenefunctionalized molecules have reversibly switched from on to off and from off to on by light-control at single-molecule level. In other words, the assembly of practical devices at nanoscale will be possible with the stability in ambient condition and more particular knowledge of photoswitches.
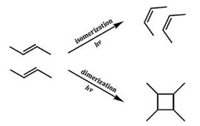
2.2. Photo-reaction of olefinsThe photoisomerization reaction (Fig. 8) of olefins is another important class of reaction that involves the internal rotation around a carbon–carbon double bond and has been heavily investigated in both liquid and gas phase. But researches on photoisomerization of olefins on solid surface are seldomly carried out by scientists in the past decades due to the lack of attention on surface phenomena. In recent years, with the fast development of modern surface analysis techniques, large progress has been achieved in this area. Tsai et al. has conducted the thorough research in the microscopic one-bond-flip mechanism of the photoisomerization reaction between trans-stilbene (TSB) and cis stilbene (CSB) on the Ag/Ge(111)-√3 surface with STM [47]. Recently, two isomers of a multifunctional π-expanded macrocyclic oligothiophene 8-mer, E, E-1 and Z, Z-1, were synthesized by Shimizu et al. [48]. With the assistance of STM technique, the reversible isomerization from E, E-1 to Z, Z-1 and Z, Z-1 to E, E-1 has been observed on liquid–solid interface due to their difference in molecular conformation. Another photochemical pathway of olefins is the [2 + 2] dimerization process (Fig. 8) which has been investigated extensively in three dimensions. According to the topochemical postulates suggested by Schmidt et al., the reactive centers must be properly placed in the crystal for the reaction to occur [49]. Except for several examples, to initiate the reaction, molecules must have a parallel structure and the distance between equivalent points of neighboring should be less than 4.2 Å. This has been also verified by the observation on surface to some degree [50–52]. Moreover, the isomerization and dimerization of olefins can be triggered sequentially by light irradiation with different irradiation time, as it is observed and verified by our group with the help of STM.
|
Download:
|
| Figure 8. Photo-induced isomerization and dimerization process of olefin. | |
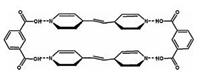
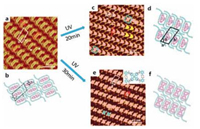
Very recently, we reported a study on both trans–cis photoisomerization and [2 + 2] photodimerization of an olefin compound trans-1, 2-bis(4-pyridyl)ethylene (4, 4'-bpe) on HOPG with the use of TCDB template [50]. 4, 4'-bpe and isophthalic (Fig. 9) with a molar ratio of 1:1 could form an olefin cocrystal in a TCDB network, resulting in a well ordered self-assembled ternary structure of 2(4, 4'-bpe)·2(iso-pa)-TCDB. Each cavity of TCDB accommodated two 4, 4'-bpe and two iso-pa molecules in a shape of polygon, as the red polygon indicated in Fig. 10a. The width between two neighboring 4, 4'-bpe molecules (L2 in both Fig. 10a and b) was measured to be 3.8–4.0 Å, which exactly met the requirement of 4.2 Å for photodimerization. After 20 min light irradiation at 365 nm, the trans–cis isomerization of 4, 4'-bpe molecules took place with the polygon changed to V-shape correspondingly, as shown in Fig. 10c. Then, with irradiation for 10 min more, [2 + 2] photodimerization took place, and most of the V-shaped structure was changed to X-shaped structure. To minimize the surface free energy on HOPG surface, two iso-pa molecules were trapped in each TCDB cavity to give a shape of X. Those photoisomerization and photodimerization processes of 2 (4, 4'-bpe)·2(iso-pa)-TCDB also have been verified in solution by using the UV–vis adsorption spectra and the IR spectra, where the absorption peaks vary with reaction time.
|
Download:
|
| Figure 9. The chemical structure of the 2(4, 4'-bpe)·2(iso-pa) cocrystal. Reproduced with permission from Ref. [50], Copyright 2014, The Royal Society of Chemistry. | |
|
Download:
|
| Figure 10. (a) A high resolution STM image of 2(4, 4'-bpe)·2(iso-pa) on the HOPG surface. Iset = 396.5 pA; Vbias = 699.8 mV. (c) An STM image of the 2(4, 4'-bpe)·2(isopa) – TCDB assembly after irradiation at 365 nm for 20 min. Iset = 341.8 pA; Vbias = 699.8 mV. (e) An STM image of 2(4, 4'-bpe)·2(iso-pa) – TCDB after irradiation for 30 min. Iset = 299.1 pA; Vbias = 699.8 mV (the inset shows the molecular model of the photodimerization of 2(4, 4'-bpe)·2(iso-pa)). (b), (d), (f) The suggested molecular models of (a), (c), (e), respectively. Reproduced with permission from Ref. [50], Copyright 2014, The Royal Society of Chemistry. | |
It is noteworthy that thanks to the flexible TCDB network, the structural change from polygon to V-shaped, then to X-shaped can easily take place. At the same time, both the shape and size of the cavities were tuned pronouncedly. This was similar to the photoisomerization of 4NN-Microcycle in TCDB network described above. By a comparative experiment of photo-irradiation on selfassembly of 4, 4'-bpe·iso-pa complex without TCDB, the template effect which greatly promoted the efficiency of the observed photo-reaction has been fully demonstrated.
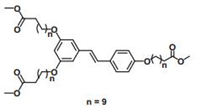
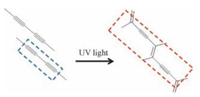
Stilbene derivatives are another kind of olefins with C=C bond locating between two phenyl groups. Recently, our group successfully investigated their photoisomerization and photodimerization (Figs. 11 and 12) on HOPG surface by using STM technique [51]. Molecule 2 (Fig. 11) was a stilbene derivative containing three long substituted alkoxy chains on its phenyl groups. Before any irradiation, 2 formed close-packed lamellae structure in dimers at 1-phenyloctane/HOPG interface (Fig. 12a). Upon irradiation by UV light at 365 nm for 5 min, the STM image showed a mixing area of unreacted 2 and its photoreaction product. Domain A in Fig. 12b stay unchanged in structure while domain B displayed a bit difference with domain A, which should be ascribed to the assembly of isomerized molecule 2 (cis-2). In the high-resolution STM image of domain B (Fig. 12c), each bright spot was composed of two cis-2 molecules, and two bright spots further form a dimer to make up each lamella. However, by careful inspection, the two bright spots in each dimer adopted a very different conformation from each other. As we artificially defined "head" as the C=C bond and "tail" as two aromatic cores of molecule 2, one spot appeared in a "head-to-head" manner (conformation 1) as shown by the white arrow in Fig. 12c while another adopted a "head-to-tail" style (conformation 2) as shown by the yellow arrow. In each row of the lamella, the bright spots adopted the same conformation. Increasing UV light irradiation time to 20 min, photo-induced [2 + 2] reaction occurred. As Fig. 13a shows, the newly formed structure was similar to that in domain B in Fig. 12b, but in each lamella, the bright spots in one row was smaller and darker than that in another. The brighter spots (red row) in Fig. 13b stay unchanged, still shown the "head-to-tail" fashion (conformation 2) while the darker one (blue row) was ascribed to the conjugation loss caused by the formation of cyclobutanes. This difference in photo-reaction activity of the two conformations could be attribute to the difference in distance between the C=C bond of two neighboring molecules. Only in conformation 1, this distance was small enough to meet the prerequisites of 4.2 Å to undergo [2 + 2] photo dimerization while conformation 2 with a distance of 6.2 Å cannot. In this research, the [2 + 2] photodimerization was "strictly" controlled by the packing of molecules, well verifying the formerly founded prerequisites for [2 + 2] photodimerization reactivities in the crystals, and thus strengthened our understanding of photodimerization of olefins.
|
Download:
|
| Figure 11. Chemical structure of molecule 2. Reproduced with permission from Ref. [51]. Copyright 2014 American Chemical Society. | |

|
Download:
|
| Figure 12. (a) An STM image of the molecule 2 monolayer obtained at a concentration below 1.7 × 10-3 mol L-1, Iset = 296 pA, Vbias = 286.4 mV, scale bar = 20 nm. (b) An STM image of the molecule 2 structure after 5 min of UV light irradiation, consisting of nonreacted area (domain A) and reacted area (domain B), Iset = 299.1 pA, Vbias = 510.9 mV, scale bar = 20 nm. (c) A high-resolution STM image of the outlined area (the black frame) in domain B of part b, Iset = 299.1 pA, Vbias = 613.4 mV, scale bar = 5 nm. (d) A suggested model corresponding to part c. Reproduced with permission from Ref. [51]. Copyright 2014 American Chemical Society. | |

|
Download:
|
| Figure 13. (a) An STM image of the molecule 2 structure after 20 min of UV light irradiation, Iset = 299.1 pA, Vbias = 535.3 mV, scale bar = 20 nm. (b) A high-resolution STM image of the outlined area (the white frame) in part a, Iset = 299.1 pA, Vbias = 535.3 mV, scale bar = 5 nm. (c) A suggested model corresponding to part b. Reproduced with permission from Ref. [51]. Copyright 2014 American Chemical Society. | |
2.3. Photo-reaction of diacetylene derivatives
Diacetylene derivatives are a kind of highly unsaturated hydrocarbon containing two triple bonds which can undergo polymerizations on surface under suitable stimulation. In the polymerization process, new covalent bond generate through the coupling between neighboring molecules, with an appearance of bright lines in the SAM under STM observation, as shown in Fig. 14. Conductive nanowires can be generated in this polymerization process, which are of great importance in the application of connecting functional organic devices [53]. There are two mainly approaches to induce the polymerization, one is by applying stimuli using an STM tip while the other is by light irradiation, and both of them are widely studied. Okawa and his co-workers successfully applied both two methods on two diacetylene compounds [15]. Through an artificial defect, they successfully initiated and terminated the polymerization at predetermined point by the probe tip of an STM to create linear polydiacetylene nanowire at designated position with designated length. In their more recent work, "chemical soldering" was realized by initiating chain polymerization with tip stimulation [54]. Ying-Hong Qiao et al. not only initiated the polymerization of a self-assembled diacetylene molecular monolayer with ultraviolet irradiation but also controlled the spacing between nanowires by the coadsorption with stearic acid on surface [55].
|
Download:
|
| Figure 14. Schematic of photo-reaction of diacetylene derivatives. | |
Recently, 10, 12-pentacosadiynoic acid was synthesised by Deshpande et al. in order to improve the performance of graphene [56]. The self-assembly and photopolymerization on surface were studied and monitored by UHV STM and AFM. After the PCDA deposited on epitaxial graphene (EG) on SiC(0 0 0 1) from the gas phase in UHV, self-assembled one-dimensional stripes were observed by STM. An "SHL-LHS" ordered monolayers was formed due to the carboxyl-terminated alkyl chain in PCDA, S for the short carboxylic-terminated alkyl chain and L for the long methylterminated alkyl chain. Then, after the self-assembly was exposed to UV light at 254 nm wavelength, one-dimensional protrusions which were higher and brighter than the unpolymerized PCDA beside appeared, due to the conjugated backbone of polymerized PCDA. The height increase after reaction was measured to be 1.15 ± 0.56 Å. What is more, this change in height was coincident with a change of (15.5 ± 5.5)° in the angle between alkyl chains and the backbone. AFM technique was employed in this study to confirm the STM result. Additionally, AFM investigation also identified the assembly and photo-reaction of PCDA at the same time, which also demonstrated the high stability and robustness of the PCDA assemblies. Moreover, semiempirical PM6 calculation result provided the theoretical support to put forward a buckled polymerization conformation as the optimal one, which agreed well with the STM image.
2.4. Photo-reaction of bifunctional compoundUp to now, most of the researches on photo-induced reactions on surface studied by STM were focused on one photo-sensitive group molecules, for their synthesis and light control are easy to realize, but multifunctional systems were seldomly achieved and discussed. To make photosensitive system more complex and gain the multi-control of it, our lab firstly combined azobenzene group with diacetylene group in one molecule to achieve photoisomerization and reversible photopolymerization process sequentially via multiple optical treatments [57]. Di-(10, 12-pentacosadiyn-1-yl) azobenzene-4, 40-dicarboxylate (Azo-DA, Fig. 15) was designed and synthesized as an azobenzene derivative containing two diactylene groups on two sides. Due to the photo-active nature of the azobenzene and diacetylene functional groups in Azo-DA, photoisomerization and photopolymerization can be triggered by light irradiation at different wavelength, thus resulting in the structural change of the self-assembly on HOPG. In order to confirm this, experiments were taken under STM investigation.

|
Download:
|
| Figure 15. Up: Chemical structure of diacetylene-substituted azobenzene derivatives (Azo-DA). Down: (a) STM image of the Azo-DA molecules (before irradiation), Iset=299pA, Vbias=700mV, scale bar=5nm. (c) STM image consisting of polymerized and unpolymerized structure of the Azo-DA molecules upon UV irradiation at 254nm. Iset=290pA, Vbias=700mV, scale bar=10nm. The polymerized diacentylene units are indicated by red arrows. (d) High resolution image of the outline area (the white frame) in (c); a schematic model and unit cell are superimposed on it. Iset=290pA, Vbias=699mV, scale bar=5nm. (f) STM image for the self-assembled structure of nonpolymerized Azo-DA and polymerized Azo-DA ((Azo-poly(DA)) upon UV irradiation at 365nm for 30min, Iset=268pA, Vbias=700mV, scale bar=10nm. (g) Submolecular resolved structures of the outlined area (the white frame) in (f), Iset=275pA, Vbias=700mV, scale bar=5nm. A yellow dashed line was drawn to separate the reacted (up) and nonreacted area (down). (b), (e), (h), (j) Molecular models for (a), (d), (g), (i), respectively. Reproduced with permission from Ref. [57]. Copyright 2012 American Chemical Society. | |
Firstly, the SAM of Azo-DA before any irradiation was observed as STM image shows a structure of well-ordered and close-packed lamellae on HOPG surface (Fig. 15a). Azobenzene of the Azo-DA presented as bright features in rows along lamella while the diacetylene units present darker on the two sides. Then, after 15min UV irradiation at 254nm, photo-polymerization occurred with the appearance of several bright lines (indicated by red arrows) (Fig. 15c), which was caused by the greater π-electron delocalization along the polymer backbone after reaction. However, some unpolymerized areas still exist, resulting in a combination of Azo-DA and Azo-poly (DA) self-assembly. Both the angle between the alkyl chains and the backbone of Azo-poly (DA) and the width of lamella were changed dramatically, while the azobenzenemoietyconformation stays unchangedwith tran-form. Thus, we can infer that Azo-DA had only underwent photopolymerization process in this case.
Then, to further realize the photoisomerization, irradiation at 365nm was carried out on both Azo-DA and Azo-poly (DA) selfassembly. In both Azo-DA and Azo-poly (DA) self-assembled area, previous two well-separated bright spots were found to be partly replaced by a bright, linear feature (Fig. 15g), as a consequence of the isomerization of azobenzene groups from tran to cis. The crosssection analysis which showed a notable difference in height between trans and cis conformation also powerfully demonstrated the structural change. Moreover, this structural transition could be further removed by subsequent visible light irradiation at 435nm for 15 min, as the conformation of azobenzene re-changed back from cis to trans (Fig. 15i). This research successfully realized the multiple and reversible remote control of organic 2D self-assembly by light illumination, so as to encourage the efforts towards constructing multifunctional optoelectronic devices at nanoscale.
3. ConclusionsTypical examples are put forward here to give an introduction on photochemistry on surface. By light irradiation, reversible photoisomerization, photodimerization and photopolymerization could take place, which resulted in structural transitions in the SAMs constructed on surfaces or interfaces by following bottom-up strategies. This transition could be directly observed or undirectly verified by means of STM technique combined with AFM, UV–vis spectra, IRspectraandothers.Meanwhile, moleculartemplateswere employed to facilitate the visualizing of target molecules and found to greatly promote the photo-reaction as well.
To successfully initiate the photo-reaction, many influence factors should be taken into account. Undoubtedly, functional groups in molecules are the key factors for the inclusion of photosensitive groups make the photo-reactions possible. Azobenzene, olefins, diacetylene are tipical and mostly studied ones that can response to light. Besides the functional groups mentioned above, photo-active behaviors of naphthacenequinone, spiropyrans, diarylethenes have been also detected by STM as reported by others [58–62]. Irradiation wavelength is another key factor for only at the correct wavelength the desired reaction can occur. This is fully utilized in the multi-control of photosensitive systems with different irradiation wavelength. Irradiation time determines both the extent of reaction and the reaction type as we can conclude from the examples of olefins that the photodimerization process requires more irradiation time than the photoisomerization process. Moreover, whether photodimerization happens or not largely depends on whether the distance between two paralleled double bond could meet the prerequisites of 4.2 Å. In summary, these studies provided us an insight into not only the mechanism of photo-chemistry but also its influence factors, and were thus of fundamental interest for photo-chemistry on surface.
Standing on the application point of view, SAMs of photosensitive molecules can be functionalized through light regulation, leading their potential application in nano-capsules, nano-swithes, nano-wires and smart surfaces and so on. For example, photopolymerization of self-assembled diacetylene derivatives can form 2D conductive nanowires which can provide inter-connections for other molecular devices to form an integrated structure. Therefore, those functional SAMs are of essential importance for constructing self-assembled nanometer function devices at molecular scale. However, current research on photo-controlled functional 2D selfassemblies is still staying in the experimental stage, therefore a lot of works are needed so as to put them into practical application. Considering the urgent demand for sub-molecular functional devices, photo-chemical reactions on surface will continue to be one of the most interesting and active fields. It can be envisioned that with improved understanding of photo-chemistry on surface and increased attempt in construction of photo-sensitive functional system at nanoscale, assembly of practical molecular devices may become possible.
AcknowledgmentThe financial support from the National Key Basic Research Program of China (Nos. 2016YFA0200700, 2013CB934203) and the National Natural Science Foundation of China (No. 21472029) are gratefully acknowledged.
| [1] | D. Wang, Q. Chen, L.J. Wan, Structural transition of molecular assembly under photo-irradiation:an STM study. Phys.Chem.Chem.Phys. 10 (2008) 6467–6478. DOI:10.1039/b810304f |
| [2] | J. Henzl, T. Bredow, Morgenstern K.Irreversible isomerization of the azobenzene derivate methyl orange on Au(111). Chem.Phys.Lett. 435 (2007) 278–282. DOI:10.1016/j.cplett.2006.12.096 |
| [3] | M. Martin, M. Lastapis, D. Riedel, Mastering the molecular dynamics of a bistable molecule by single atom manipulation. Phys.Rev.Lett. 97 (2006) 216103. DOI:10.1103/PhysRevLett.97.216103 |
| [4] | J. Henzl, M. Mehlhorn, H. Gawronski, K.H. Rieder, K. Morgenstern, Reversible cis-trans isomerization of a single azobenzene molecule. Angew.Chem.Int.Ed. 45 (2006) 603–606. DOI:10.1002/(ISSN)1521-3773 |
| [5] | H. Spillmann, A. Kiebele, M. Stöhr, A two-dimensional porphyrin-based porous network featuring communicating cavities for the templated complexation of fullerenes. Adv.Mater. 18 (2006) 275–279. DOI:10.1002/(ISSN)1521-4095 |
| [6] | S. Griessl, M. Lackinger, M. Edelwirth, M. Hietschold, W.M. Heckl, Self-assembled two-dimensional molecular host-guest architectures from trimesic acid. Single Mol. 3 (2002) 25–31. DOI:10.1002/(ISSN)1438-5171 |
| [7] | J.A. Theobald, N.S. Oxtoby, M.A. Phillips, N.R. Champness, P.H. Beton, Controlling molecular deposition and layer structure with supramolecular surface assemblies. Nature 424 (2003) 1029–1031. DOI:10.1038/nature01915 |
| [8] | S. Stepanow, N. Lin, D. Payer, Surface-assisted assembly of 2D metal-organic networks that exhibit unusual threefold coordination symmetry. Angew.Chem. 119 (2007) 724–727. DOI:10.1002/(ISSN)1521-3757 |
| [9] | M.A. Lingenfelder, H. Spillmann, A. Dmitriev, Towards surface-supported supramolecular architectures:tailored coordination assembly of 1, 4-benzenedicarboxylate and Fe on Cu(10 0). Chem.Eur.J. 10 (2004) 1913–1919. DOI:10.1002/(ISSN)1521-3765 |
| [10] | S.B. Lei, K. Tahara, X.L. Feng, Molecular clusters in two-dimensional surface-confined nanoporous molecular networks:structure, rigidity, and dynamics. J.Am.Chem.Soc. 130 (2008) 7119–7129. DOI:10.1021/ja800801e |
| [11] | X.H. Qiu, C. Wang, Q.D. Zeng, Alkane-assisted adsorption and assembly of phthalocyanines and porphyrins. J.Am.Chem.Soc. 122 (2000) 5550–5556. DOI:10.1021/ja994271p |
| [12] | A. Ulman, Formation and structure of self-assembled monolayers. Chem.Rev. 96 (1996) 1533–1554. DOI:10.1021/cr9502357 |
| [13] | Y.B. Li, C.H. Liu, Y.Z. Xie, Temperature-controlled self-assembling structure with selective guest-recognition at the liquid-solid interface. Phys. Chem.Chem.Phys. 15 (2013) 125–128. DOI:10.1039/C2CP43244G |
| [14] | F.Y. Hu, Y.N. Gong, X.M. Zhang, Temperature-induced transitions of self-assembled phthalocyanine molecular nanoarrays at the solid-liquid interface: from randomness to order. Nanoscale 6 (2014) 4243–4249. DOI:10.1039/c3nr06320h |
| [15] | Y. Okawa, M. Aono, Linear chain polymerization initiated by a scanning tunneling microscope tip at designated positions. J.Chem.Phys. 115 (2001) 2317–2322. DOI:10.1063/1.1384554 |
| [16] | Y.B. Li, J.H. Wan, K. Deng, Transformation of self-assembled structure by the addition of active reactant. J.Phys.Chem.C 115 (2011) 6540–6544. DOI:10.1021/jp1097876 |
| [17] | S.K. Mandal, Y. Okawa, T. Hasegawa, M. Aono, Rate-determining factors in the chain polymerization of molecules initiated by local single-molecule excitation. ACS Nano 5 (2011) 2779–2786. DOI:10.1021/nn103231j |
| [18] | N. Katsonis, M. Lubomska, M.M. Pollard, B.L. Feringa, P. Rudolf, Synthetic light-activated molecular switches and motors on surfaces. Prog.Surf.Sci. 82 (2007) 407–434. DOI:10.1016/j.progsurf.2007.03.011 |
| [19] | G. Binnig, H. Rohrer, C. Gerber, E. Weibel, Surface studies by scanning tunneling microscopy. Phys.Rev.Lett. 49 (1982) 57–61. DOI:10.1103/PhysRevLett.49.57 |
| [20] | S.N. Magonov, M.H. Whangbo, Interpreting STM and AFM images. Adv.Mater. 6 (1994) 355–371. DOI:10.1002/(ISSN)1521-4095 |
| [21] | S. De Feyter, A. Gesquière, M.M. Abdel-Mottaleb, Scanning tunneling microscopy:a unique tool in the study of chirality, dynamics, and reactivity in physisorbed organic monolayers. Acc.Chem.Res. 33 (2000) 520–531. DOI:10.1021/ar970040g |
| [22] | S. De Feyter, F.C. De Schryver, Self-assembly at the liquid/solid interface:STM reveals. J.Phys.Chem.B 109 (2005) 4290–4302. DOI:10.1021/jp045298k |
| [23] | X.M. Zhang, Q.D. Zeng, C. Wang, Molecular templates and nano-reactors:two-dimensional hydrogen bonded supramolecular networks on solid/liquid interfaces. RSC Adv. 3 (2013) 11351–11366. DOI:10.1039/c3ra40473k |
| [24] | J. Lu, S.B. Lei, Q.D. Zeng, Template-induced inclusion structures with copper(Ⅱ)phthalocyanine and coronene as guests in two-dimensional hydrogen-bonded host networks. J.Phys.Chem.B 108 (2004) 5161–5165. |
| [25] | Y.T. Shen, L. Guan, X.M. Zhang, Site-selective effects on guest-molecular adsorption and fabrication of four-component architecture by higher order networks. Phys.Chem.Chem.Phys. 15 (2013) 12475–12479. DOI:10.1039/c3cp50371b |
| [26] | Y.T. Shen, K. Deng, Q.D. Zeng, C. Wang, Size-selective effects on fullerene adsorption by nanoporous molecular networks. Small 6 (2010) 76–80. DOI:10.1002/smll.v6:1 |
| [27] | Y.T. Shen, K. Deng, X.M. Zhang, Selective and competitive adsorptions of guest molecules in phase-separated networks. J.Phys.Chem.C 115 (2011) 19696–19701. DOI:10.1021/jp202890y |
| [28] | M. Li, K. Deng, S.B. Lei, Site-selective fabrication of two-dimensional fullerene arrays by using a supramolecular template at the liquid-solid interface. Angew.Chem.Int.Ed. 47 (2008) 6717–6721. DOI:10.1002/anie.v47:35 |
| [29] | Y.B. Li, Z. Ma, G.C. Qi, Solvent effects on supramolecular networks formed by racemic star-shaped oligofluorene studied by scanning tunneling microscopy. J.Phys.Chem.C 112 (2008) 8649–8653. |
| [30] | Y.B. Wang, L. Niu, Y.B. Li, Single molecule studies of cyclic peptides using molecular matrix at liquid/solid interface by scanning tunneling microscopy. Langmuir 26 (2010) 16305–16311. DOI:10.1021/la101467s |
| [31] | Y.T. Shen, L.J. Zeng, D. Lei, Competitive adsorption and dynamics of guest molecules in 2D molecular sieves. J.Mater.Chem. 21 (2011) 8787–8791. DOI:10.1039/c1jm10260e |
| [32] | C.J. Barrett, J.I. Mamiya, K.G. Yager, T. Ikeda, Photo-mechanical effects in azobenzene-containing soft materials. Soft Matter 3 (2007) 1249–1261. DOI:10.1039/b705619b |
| [33] | S.K. Yesodha, C.K. Sadashiva Pillai, Tsutsumi N.Stable polymeric materials for nonlinear optics:a review based on azobenzene systems. Prog.Polym.Sci. 29 (2004) 45–74. DOI:10.1016/j.progpolymsci.2003.07.002 |
| [34] | K.G. Yager, C.J. Barrett, Novel photo-switching using azobenzene functional materials. J.Photochem.Photobiol.A 182 (2006) 250–261. DOI:10.1016/j.jphotochem.2006.04.021 |
| [35] | S. Oosaki, H. Hayasaki, Y. Sakurai, S. Yajima, K. Kimura, Photocontrol of ion-sensor performances in neutral-carrier-type ion sensors based on liquid-crystalline membranes. Chem.Commun. 522 (2005) 6–522 7. |
| [36] | P. Dietrich, F. Michalik, R. Schmidt, An anchoring strategy for photoswitchable biosensor technology:azobenzene-modified SAMs on Si (111). Appl.Phys.A 93 (2008) 285–292. DOI:10.1007/s00339-008-4828-0 |
| [37] | D. Pijper, M.G.M. Jongejan, A. Meetsma, B.L. Feringa, Light-controlled supramolecular helicity of a liquid crystalline phase using a helical polymer functionalized with a single chiroptical molecular switch. J.Am.Chem.Soc. 130 (2008) 4541–4552. DOI:10.1021/ja711283c |
| [38] | P.R. Westmark, J.P. Kelly, B.D. Smith, Photoregulation of enzyme activity. Photochromic, transition-state-analog inhibitors of cysteine and serine proteases. J.Am.Chem.Soc. 115 (1993) 3416–3419. DOI:10.1021/ja00062a003 |
| [39] | P. Gorostiza, E.Y. Isacoff, Optical switches for remote and noninvasive control of cell signaling. Science 322 (2008) 395–399. DOI:10.1126/science.1166022 |
| [40] | M. Bazarnik, L. Jurczyszyn, R. Czajka, K. Morgenstern, Mechanism of a molecular photo-switch adsorbed on Si(10 0). Phys.Chem.Chem.Phys. 17 (2015) 5366–5371. DOI:10.1039/C4CP04353G |
| [41] | M. Alemani, S. Selvanathan, F. Ample, Adsorption and switching properties of azobenzene derivatives on different noble metal surfaces:Au (111), Cu(111), and Au(1 0 0). J.Phys.Chem.C 112 (2008) 10509–10514. |
| [42] | J. Mielke, S. Selvanathan, M. Peters, Molecules with multiple switching units on a Au(111) surface:self-organization and single-molecule manipulation. J.Phys.:Condens.Matter 24 (2012) 394013. DOI:10.1088/0953-8984/24/39/394013 |
| [43] | Y.T. Shen, L. Guan, X.Y. Zhu, Q.D. Zeng, C. Wang, Submolecular observation of photosensitive macrocycles and their isomerization effects on host-guest network. J.Am.Chem.Soc. 131 (2009) 6174–6180. DOI:10.1021/ja808434n |
| [44] | Y.T. Shen, K. Deng, X.M. Zhang, Switchable ternary nanoporous supramolecular network on photo-regulation. Nano Lett. 11 (2011) 3245–3250. DOI:10.1021/nl201504x |
| [45] | X.M. Zhang, S. Wang, Y.T. Shen, Two-dimensional networks of an azobenzene derivative:bi-pyridine mediation and photo regulation. Nanoscale 4 (2012) 5039–5042. DOI:10.1039/c2nr31186k |
| [46] | A.S. Kumar, T. Ye, T. Takami, Reversible photo-switching of single azobenzene molecules in controlled nanoscale environments. Nano Lett. 8 (2008) 1644–1648. DOI:10.1021/nl080323+ |
| [47] | C.S. Tsai, J.K. Wang, R.T. Skodje, J.C. Lin, A single molecule view of bistilbene photoisomerization on a surface using scanning tunneling microscopy. J.Am. Chem.Soc. 127 (2005) 10788–10789. DOI:10.1021/ja052448b |
| [48] | H. Shimizu, J.D. Cojal González, M. Hasegawa, Synthesis, structures, and photophysical properties of '-expanded oligothiophene 8-mers and their saturn-like C60 complexes. J.Am.Chem.Soc. 137 (2015) 3877–3885. DOI:10.1021/jacs.5b00291 |
| [49] | G.M.J. Schmidt, Photodimerization in the solid state. Pure Appl.Chem. 27 (1971) 647–678. |
| [50] | J.D. Xue, J. Xu, F.Y. Hu, Highly efficient photodimerization of olefins in a nanotemplate on HOPG by scanning tunneling microscopy. Phys.Chem.Chem. Phys. 16 (2014) 25765–25769. DOI:10.1039/C4CP04154B |
| [51] | L.Y. Liao, Y.B. Li, X.M. Zhang, STM investigation of the photoisomerization and photodimerization of stilbene derivatives on HOPG surface. J.Phys.Chem. C 118 (2014) 15963–15969. DOI:10.1021/jp505511e |
| [52] | L.P. Xu, C.J. Yan, L.J. Wan, S.G. Jiang, M.H. Liu, Light-induced structural transformation in self-assembled monolayer of 4-(amyloxy)cinnamic acid investigated with scanning tunneling microscopy. J.Phys.Chem.B 109 (2005) 14773–14778. DOI:10.1021/jp052959k |
| [53] | J.M. Tour, electronics. Molecular, Synthesis and testing of components. Acc. Chem.Res. 33 (2000) 791–804. DOI:10.1021/ar0000612 |
| [54] | Y. Okawa, S.K. Mandal, C.P. Hu, Chemical wiring and soldering toward all-molecule electronic circuitry. J.Am.Chem.Soc. 133 (2011) 8227–8233. DOI:10.1021/ja111673x |
| [55] | Y.H. Qiao, Q.D. Zeng, Z.Y. Tan, Photoinduced organic nanowires from self-assembled monolayers. J.Vac.Sci.Technol.B 20 (2002) 2466–2469. DOI:10.1116/1.1526601 |
| [56] | A. Deshpande, C.H. Sham, J.M.P. Alaboson, Self-assembly and photopolymerization of sub-2 nm one-dimensional organic nanostructures on graphene. J.Am.Chem.Soc. 134 (2012) 16759–16764. DOI:10.1021/ja307061e |
| [57] | X.M. Zhang, S.D. Xu, M. Li, Photo-induced polymerization and isomerization on the surface observed by scanning tunneling microscopy. J. Phys.Chem.C 116 (2012) 8950–8955. DOI:10.1021/jp2115884 |
| [58] | W. Xu, C. Zhang, H. Gersen, A molecular conformational change induced self-assembly:from randomness to order. Chem.Commun. 49 (2013) 5207–5209. DOI:10.1039/c3cc40743h |
| [59] | N. Darwish, A.C. Aragones, T. Darwish, S. Ciampi, I. Díez-Pérez, Multi-responsive photo-and chemo-electrical single-molecule switches. Nano Lett. 14 (2014) 7064–7070. DOI:10.1021/nl5034599 |
| [60] | N. Katsonis, T. Kudernac, M. Walko, Reversible conductance switching of single diarylethenes on a gold surface. Adv.Mater. 18 (2006) 1397–1400. DOI:10.1002/(ISSN)1521-4095 |
| [61] | D. Frath, T. Sakano, Y. Imaizumi, Diarylethene self-assembled monolayers:cocrystallization and mixing-induced cooperativity highlighted by scanning tunneling microscopy at the liquid/solid interface. Chem.Eur.J. 21 (2015) 11350–11358. DOI:10.1002/chem.201500804 |
| [62] | I. Oreshkin, V.I. Panov, S.I. Vasil'ev, N.I. Koroteev, S.A. Magnitskii, Direct STM observation of electronic structure modification of naphthacenequinone molecules due to photoisomerization. J.Exp.Theor.Phys.Lett. 68 (1998) 521–526. DOI:10.1134/1.567900 |
 2017, Vol. 28
2017, Vol. 28 


