b University of Chinese Academy of Sciences, Beijing 100049, China
Antibiotics are used as life-saving drugs in the treatment of bacterial infectious diseases [1]. Different from all the other drugs, antibiotics not only affect the individual to whom they are given but also the entire community [2]. In recent years, the emergence of drug resistance or antimicrobial resistance caused by the antibiotics abuse has become a serious global potential crisis. According to the World Health Organization, "A post-antibiotic era—in which common infections and minor injuries can kill—far from being an apocalyptic fantasy, is instead a very real possibility for the 21st century" [3]. To address the challenges associated with drug resistance, great efforts have been made to explore remedial possibilities. The discovery of new antibiotics [4, 5], utilizing conjugated-polymer-based photodynamic inhibition systems [6, 7] and combining the existing antibiotics with functional materials [8, 9] are thought to be potential solutions. Antimicrobial peptides, an evolutionarily ancient weapon, represent a new era to treat resistant pathogen infections because of their complex diversity in structure. AMPs are oligopeptides with a varying number (from five to over a hundred) of amino acids, which have a broad spectrum of targeted organisms such as bacteria (Gram-positive and Gram-negative), fungi, viruses, parasites or even cancer [10]. Herein, we present a general overview about the structure and properties of AMPs. In addition, recent advances on natural, synthetic and modified AMPs are summarized as well. We further discuss the possible mechanism of antimicrobial action of AMPs. Finally, we focus on the general direction and challenges facing AMPs. With this review, we hope researchers can develop novel antimicrobial agents to fight drug resistance and other lethal diseases much more effectively.
2. Structure and properties of AMPsAMPs are produced by most living entities (microorganisms, animals and plants) to defend against invading organisms [11]. Despite the enormous diversity, most AMPs share common physiochemical properties mainly because the basic structure of them has rules to follow. AMPs are usually short, from just 5–6 amino acid residues in some synthetic peptides to approximately 50 in natural antimicrobial proteins; most of them typically consist of cationic and hydrophobic moieties because of the presence of the specific amino acid residues. In solutions, AMPs fold or arrange into a variety of amphipathic structures and conformations due to their propensity to interact with anionic lipid bilayers [12].
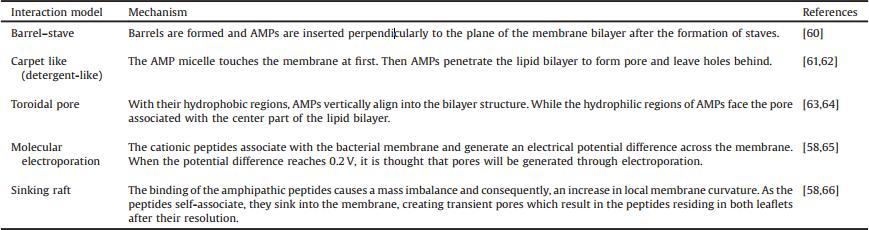
Most AMPs reported to date can be characterized as one of the following types based on their secondary structures: α-helix, β-sheet, extended and loop [13]. Among these structural groups, α-helix and β-sheet structures are more common [14]; and α-helical peptides are the most studied AMPs up to now. In α-helical structures, the angle between two adjacent amino acids with regard to the center is around 100° from the top view and the distance between them is around 0.15 nm (Fig. 1A). Magainins and cecropins are representatives of AMPs unstructured in solution that form amphipathic α-helical structures during interaction with membranes [15, 16]. β-Sheet peptides such as astachyplesins and polyphemusin Ⅱ are composed of at least two β-strands with disulfide bonds between them [17].
|
Download:
|
| Figure 1. Schematic representation of an α-helical AMP (modified from Reference [10]). This figure assumes the same α-helix propensity for all amino acids in the peptide structure. (A) Helical wheel projection of the AMP (top view). Dotted lines show two adjacent amino acids in the primary structure. (B) Side view of the α-helical AMP. The distance between two adjacent amino acids, "n" is 0.15 nm. | |
Some AMPs do not belong to any of these groups because they might contain two different structural components [18]. Only when they interacted with the membranes of target cells, these peptides could form their active structure. For instance, indolicidins show amphipathic and globular conformation in aqueous solutions while it is wedge-shaped in lipid bilayer mimicking environments. This sort of AMPs also changed its conformation during interaction with DNA evidenced with a slight shift in the wavelength of maximum emission and decreased fluorescence intensity [19]. α-Helix/β-sheet mixed structure is another important structural motif of some AMPs. Some of the well-known families (defensins) execute their antimicrobial function through this complex motif. Big defensin is an AMP composed of a highly hydrophobic N-terminal region and a cationic C-terminal region containing six cysteine residues involved in three internal disulfide bridges. The big defensin purified from horseshoe crab hemocytes is a 79 amino acid peptide with antimicrobial activities against both Gram-positive and Gram-negative bacteria and fungi [20]. Cysteine-stabilized αβ defensins are small, with a length ranging from 34–54 amino acid residues, cysteine-rich and extremely stable, normally composed of an α-helix and three β-strands stabilized by three or four disulfide bonds and commonly found in several organisms [21].
Because of the peptidic nature of AMPs, most of them are highly biocompatible and susceptible to biodegradation; in fact, increasing the stability of many short antimicrobial peptides by reducing their susceptibility to proteolysis is a major goal of AMP improvement, which would render benefits both for their practical application and their production through biotechnology [12, 22]. A very important advantage of AMPs is their rapid killing effect. Some AMPs can kill in seconds even at a low concentration after the initial contact with cell membrane [23]. Another significant benefit for the use of AMPs is their broad spectrum of antimicrobial activity not only on Gram-positive and -negative bacteria but also on fungi and virus. As explained in detail subsequently, AMPs are considered as multi-target drugs. So it is hard to develop a complete resistance against AMPs [24]. All the properties of AMPs have driven researchers to develop natural, synthetic and modified AMPs.
3. Production and modification of AMPsIn total, more than 5000 AMPs have been discovered or synthesized/biosynthesized up to date, and the number is still growing continuously [25]. The purpose of this part is not to provide a detailed survey of the extensive variety of AMPs, which has been addressed in other nice reviews [26, 27]. Only significant examples are summarized to illustrate the distinct properties of the major classes of AMPs.
3.1. Natural AMPsAMPs are widespread in nature in organisms all along prokaryotes and eukaryotes [28–30]. For example, frog skin is the source of more than 300 different AMPs [27, 28, 31]. In microorganisms, AMPs' biological significance is mainly related to competition for nutrients. In higher multicellular organisms, AMPs are considered as part of an ancestral system of defense against microbial pathogen invasion and infection. In animals, AMPs are believed to be the first line of the innate immune defense against bacteria, fungi and viruses, because they are mostly found in the tissues and organs that are exposed to airborne pathogens [26].
Bacteriocins are ribosomally synthesized peptides produced by specific bacteria, which are active against other bacterial species [32]. Producer strains have immunity mechanisms to protect themselves from peptide killing. A major group within this class is the so-called lantibiotics [33]. Nisin, one of the most studied bacteriocins, is a well-known example in this class. It is a 3.5 kDa cationic polypeptide produced by specific strains of Lactoccoccus lactis strains, which inhibits a variety of Gram-positive bacteria [34]. Non-ribosomally AMPs are a heterogeneous class also produced by microorganisms that code for specific enzymes called nonribosomal peptide synthetases (NRPS) [35]. Their structure may contain non-natural amino acids, cyclic rings, or covalently linked glycan or acyl groups [36].
Magainins and cecropins are representatives of AMPs which are well known for their antimicrobial properties; additionally, antifungal activities against human and plant pathogens were reported [15, 16]. Magainins were originally isolated from the skin of the frog, Xenopus laevis [15]. Cecropins are a family of AMPs originally isolated from the hemolymph of insects [16]. Both of them have strong nonspecific toxicity that makes their practical application difficult. Therefore, they have been extensively used in terms of structure activity studies, sequence modification, or as modules to design peptide fusions with the double objective of improving antimicrobial activity while reducing toxicity against plant or animal cells [12].
Mammalian epithelial surfaces can produce AMPs from longer inactive precursors. Thus, animals express AMPs such as defensins and thionins both constitutively and in response to microbial assault [26]. Contrary to other species, humans encode a unique member of the cathelicidin family named LL-37, which can be naturally processed into multiple peptides with distinct antimicrobial and immunomodulatory functionalities [37].
3.2. Synthetic/biosynthetic AMPsBecause of the limited natural resources of microorganisms, animals and plants origin AMPs, chemical synthesis and gene engineering have become the major means to obtain them. A continuously increasing number of synthetic peptides derived from natural AMPs has been generated and shown to have antibacterial and/or antifungal properties. Biosynthesis of AMPs by the use of bacterial and yeast producers is the trend of the future for the production.
In the last few years, the number and diversity of non-natural synthetic peptides with antimicrobial activity have increased tremendously due to the development of high-throughput peptide synthesis [25, 38]. These synthetic AMPs have been rationally designed with mimicking the structure of natural AMPs.
Although the studies above have contributed substantially to increase the number and diversity of known AMPs, synthetic libraries have limitations regarding the low production rate and high cost because of difficulty in separation and purification [10]. To overcome these limitations, a variety of expression systems, such as baculovirus (Escherichia coli), yeast, insects and plant expression systems [39-42], have been used to prepare AMPs. E. coli is the most common expression system for production of peptides. The target gene can be ligated to the plasmid and the construct is then transformed into the host E. coli cells. In this way, the corresponding protein could be changed and expressed as well. However, the hosts themselves are bacteria; expressed peptides with antimicrobial activity may kill the host once been "secreted". Thus, it is contradictory to choose prokaryotic system to express AMPs as desired manner unless fusion expression is used [43]. Recent years, researchers have tried to use yeast that is a eukaryotic system to express AMPs. For example, Albayrak et al. have constructed two different expression systems in Saccharomyces cerevisiae and Pichia pastoris to investigate the expression and secretion levels of AMPs [40].
3.3. Modification of AMPsFrom the discussion above, it is possible to make fully active peptides by chemical synthesis [44] or by using recombinant expression systems [45]. These artificial sources of AMPs are useful for designing new synthetic AMPs. Because AMPs are made with amino acids, it is relatively easy to modify the structure with phosphorylation, unnatural amino acids, methylation, amidation, glycosylation, formation of disulphide linkage, proteolytic cleavage and "graft to" or "graft from" to immobilize AMPs on surfaces [46-48]. Of course, all these approaches can be combined and applied in an iterative way to increase optimization, or they can be applied to de novo design non-natural AMP sequences [49, 50]. The goals of modified peptides are: to increase activity against microbial cells; reduce toxicity to non-target cells; increase stability against degradation [12].
Peptide analogs of natural AMP have been synthesized with deleted, extended, or substituted amino acids. Numerous examples exist of such rational designs. Synthetic analogs of cecropins, magainins, melittin, indolicidin, or lactoferricins have been produced and studied, through the modification of amino acid sequence, shortening to determine minimal antimicrobial motifs, or enlargement of peptide length, even by fusion of fragments from different peptides [12, 51].
Even though recombinant cell systems can be used to produce synthetic peptides with post-translational modifications, incorporation of unnatural amino acids may require chemical synthesis strategy. Such non-natural modifications have great influence on peptide selectivity and stability [49]. For example, incorporation of β-didehydrophenylalanine in the primary sequence of VS1 resulted in higher stability against proteases. Researchers have also been able to introduce antifungal activities to some AMPs by incorporating undecanoic acid and palmitic acid into their primary sequence [10].
The inclusion or attachment of AMPs to a variety of materials can also retain the antimicrobial properties [12]. The benefit of the incorporation of AMPs into polymers or on surfaces is that proteolytic degradation of peptides can be limited. Through the immobilization of nisin with poly (ethylene glycol) (PEG (1000)) as a linker, the antimicrobial and anti-biofilm properties of AMPs/ multi-walled-nanotubes were significantly enhanced [52]. And other reports showed that, when grafted AMPs to chitosan polymers, their antimicrobial activity was enhanced while the toxicity was reduced [53]. Similar work was carried out by preparation of AMP-grafted chitosan-based nanocapsules as an "armed" carrier of anticancer and antiepileptic drugs [54]. The successful delivery/application of AMPs can also be achieved by their inclusion into particles or liposomes that separate and protect them from the environment, enhancing their stability and efficacy as well as ensuring constant delivery [55].
Apparently, with the modification of AMPs and their mimics, the applications of these special bioactive peptides have been greatly enriched. However, a potential drawback of all these nonnatural modifications of AMPs is that they restrict the production of peptides through biotechnological approaches. Thus, the production of the antimicrobial is limited by synthetic procedures. Mechanistic characteristics of AMPs should be considered to develop their mode of applications as an antimicrobial agent.
4. Mechanism of action of AMPsIt was known that the excellent antimicrobial property of AMPs was related to their cationic capacity to interact and disrupt biological membranes. However, the concept that AMPs need to be cationic was changed later with the discovery of negatively charged AMPs in 1997 [56]. Afterwards, dermicidin [57] secreted from sweat gland tissues of human and maximin-H5 [58] from frog skin are both anionic peptides as well. Ever since, the majority of the researches have tended to divide all AMPs into two classes: membrane disruptive AMPs and non-membrane disruptive AMPs.
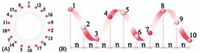
4.1. Membrane disruptive mechanismsEven if intracellular targets (non-membrane disruptive mechanism) are involved, an initial cell membrane interaction with peptides is required for the antimicrobial activities of AMPs; and this interaction determines the spectrum of target cells [10]. That is why these peptides are capable of distinguishing bacterial cells from host cells, making them desirable antimicrobials [11]. AMPs whose antimicrobial properties come from membrane disruptive mechanism are amphipathic, which means the interaction mainly includes ionic and hydrophobic interactions mostly depending on peptides' hydrophobicity and cationic state. The major types of these AMPs and the mechanisms of their actions are summarized in Fig. 2 and Table 1 [58-66].
|
Download:
|
| Figure 2. The membrane-disruptive mechanisms of action for AMPs (modified from References [10, 59]). (A)–(C) all start from the same conformation, with the peptides associating with the bacterial membrane. (A) Barrel–stave model. (B) Carpet-like model. (C) Toroidal pore model. (D) Molecular electroporation model. (E) Sinking raft model. The green color represents the hydrophobic portions of AMPs, while the red color represents the hydrophilic parts of the AMPs. | |
|
|
Table 1 The action mechanisms of disruptive mechanisms AMPs. |
4.2. Non-membrane disruptive mechanisms
As described above, in early AMPs studies, disruption and permeabilization of bacterial cell membrane were considered as the primary mechanism of killing and determinant of activity of AMPs. However, more and more evidence suggests that membrane disruption might not be the only mechanism of AMPs' antimicrobial action. Non-membrane disruptive mechanisms can be grouped into the following types: inhibiting intracellular signaling pathways; cell internalization; interacting with intracellular targets [58].
It is known that one third of the total proteins of a bacterial cell are associated with the membrane and they have many functions that are critical to the cells including active transport of nutrients, respiration, proton motive force, ATP generation, and intercellular communication [67]. The function of these proteins can be altered with AMPs' treatment even if complete cell lysis does not occur. Therefore, AMPs' rapid killing effect does not only come from membrane disruption but can also come from disturbance of these functional proteins. On the other hand, reactive oxygen species (ROS) are known as markers of cell suicide [68], and further studies broaden the number of AMP for which their effect is associated with intracellular ROS production, which also suggest an induction of intracellular signaling pathways [69, 70]. However, the role of ROS in antimicrobial action remains controversial for specific peptides such as histatin 5 [71-73].
The finding that AMPs can inhibit intracellular pathways suggests that cell internalization of AMPs might also be involved n the killing mechanisms [74]. Cell-penetrating properties are demonstrated for an increasing number of peptides such as buforin Ⅱ [75], PAF26 [76], or histatin 5 [73]. AMPs that have non membrane disruptive activity share biophysical properties with the so-called cell penetrating peptides, which questioned the differences between the two of them [38, 77]. Peptides derived from HIV-1 Tat, which were known as cell penetrating peptides nitially, have antimicrobial effects on distinct microorganisms as well. Besides, it was suggested that AMPs should be used at concentrations high enough so that they can kill microorganisms by disrupting the membrane with sufficient channels and pores [74]. While some AMPs were found to start membrane perme abilization at concentrations lower than their minimal inhibitory concentrations (MICs).
Once inside the cell of microbial, non-membrane disruptive AMPs which are also known as intracellularly active AMPs have shown to interact with multiple targets [74]. For example, some AMP such as indolicidin enters the cytoplasm and kills bacterial cells by targeting DNA synthesis without causing cells lysis directly 19]. While other AMPs such as histatin 5 [73] can also inhibit proteases of microbes. Interestingly, there are some intracellular AMPs which can only kill cells at certain growth stages, which suggests they interact with certain specific metabolic pathways during bacterial growth [74]. Seminalplasmin inhibits RNA polymerase and can stop RNA synthesis completely at concen trations lower than many other antibacterial agents [78].
AMPs kill microbial cells by disrupting membrane integrity (via interaction with negatively charged cell membrane) or by interacting with different intracellular targets such as DNA, RNA and proteins. Multiple mechanisms of action within a single molecule make the selection of microbial resistance difficult. But such a wide activity is often associated with undesirable toxicity against non-target cells/organisms including human cells, which is a negative property.
5. Trends and challengesDuring the past two decades, it has become evident that increasing drug resistance has created an urgent need for new classes of antimicrobials. At the moment, AMPs seem to represent one of the most promising future strategies for defeating this threat [79]. This statement is well represented by the fact that AMPs are subject to a continuously growing number of academic reports.
AMPs have many desirable features as a novel antibiotic class. And sometimes they can complement conventional antibiotic therapy through synergy with classical antibiotics [80]. Besides, MIC and minimal bactericidal concentration (MBC) of AMPs often coincide, indicating that fast and effective killing is generally the highly desirable bactericidal mode of action. What's more, enormous functional amino acid residuals of AMPs make these special antimicrobial polymers easy to be modified, thus these modification expanded their potential applications as antimicrobials. Last but not the least, multiple mechanisms of action within AMPs make them viewed as multi-target drugs that should not be easy to cause drug resistance [10-12].
Despite these advantageous features of AMPs, there are still some challenges hindering their applications, such as high production costs [41], potential toxicity to humans [81] and sensitivity to harsh environmental conditions (proteases and extreme pH) [10]. When AMPs are used for surface coating, the lack of selectivity against specific strains [82] and folding issues of some large AMPs may reduce their antimicrobial properties [83]. As a result, many AMPs even the natural ones (such as magainins), although active in vitro, are effective in animal models of infection only at very high doses. More than that, the bacterial resistance to some AMPs has been reported. In the future, the sources (from nature or synthesis) of AMPs and modes of actions should be investigated more deeply. New tools that can decipher the structure–function relationship should be developed to realize efficient synthesis and modification of AMPs.
6. Conclusions and prospectThe ability of bacteria to develop drug resistance rapidly, coupled with the lack of new antimicrobials, has caused an arms race between the evolution of resistance in bacteria and therapeutic development. AMPs which have direct killing or inhibitory activity against microorganisms have been extensively studied in recent years because they show great potential as novel antimicrobials. Among most AMPs reported to date, those which are based on α-helix secondary structures are the most common and studied ones. Compared with conventional antimicrobials, AMPs have similar or even better antimicrobial properties without causing complete strong drug-resistance. With rapid growth in related knowledge and lead products, more and more AMPs may enter clinical test and even our daily life in the near future. However, practical use of AMPs is still hindered by several challenges including cost-effective production, unspecific toxicity and stability around the desirable target. The modification of natural or synthetic AMPs with novel polymers or structures could represent not only a flexible use of AMPs but also part of the solution to these more general practical problems. The mechanism of antimicrobial action of AMPs has still not been fully figured out, thus research on the relationship between the structure and the action is still a major area that must be addressed for each peptide of interest. The potential for AMPs in enhancing killing efficiency and educing drug resistant infections is real, and their development should be supported by research funding, as proposed by the Chinese national action plan for combating drug resistance. It is hoped that, with this perspective, new strategies and paradigms in the application of AMPs will progressively become prosperous and lead to advances against infections caused by drug resistant strain.
AcknowledgmentsThis work was supported by National Natural Science Foundation of China (Nos. 21304103 and 21474123) and Presidential Foundation of Technical Institute of Physics and Chemistry.
| [1] | W.A. Velema, J.P.van der Berg, M.J. Hansen, Optical control of antibacterial activity. Nat.Chem. 5 (2013) 924–928. DOI:10.1038/nchem.1750 |
| [2] | M.J. Blaser, Antibiotic use and its consequences for the normal microbiome. Science 352 (2016) 544–545. DOI:10.1126/science.aad9358 |
| [3] | S.A. Beatson, M.J. Walker, Tracking antibiotic resistance. Science 345 (2014) 1454–1455. DOI:10.1126/science.1260471 |
| [4] | S.Z. Wang, S.A. Cameron, K. Clinch, New antibiotic candidates against Helicobacter pylori. J.Am.Chem.Soc. 137 (2015) 14275–14280. DOI:10.1021/jacs.5b06110 |
| [5] | L.L. Ling, T. Schneider, A.J. Peoples, A new antibiotic kills pathogens without detectable resistance. Nature 517 (2015) 455–459. DOI:10.1038/nature14098 |
| [6] | H.X. Yuan, B. Wang, F.T. Lv, L.B. Liu, S. Wang, Conjugated-polymer-based energy-transfer systems for antimicrobial and anticancer applications. Adv. Mater. 26 (2014) 6978–6982. DOI:10.1002/adma.v26.40 |
| [7] | H.T. Bai, H.X. Yuan, C.Y. Nie, A supramolecular antibiotic switch for antibacterial regulation. Angew.Chem.Int.Ed.Engl. 54 (2015) 13208–13213. DOI:10.1002/anie.201504566 |
| [8] | L. Li, H.L. Ma, G.B. Qi, Pathological-condition-driven construction of supramolecular nanoassemblies for bacterial infection detection. Adv.Mater. 28 (2016) 254–262. DOI:10.1002/adma.201503437 |
| [9] | L.L. Li, J.H. Xu, G.B. Qi, Core-shell supramolecular gelatin nanoparticles for adaptive and on-demand antibiotic delivery. ACS Nano 8 (2014) 4975–4983. DOI:10.1021/nn501040h |
| [10] | A.A. Bahar, D.C. Ren, Antimicrobial peptides. Pharmaceuticals 6 (2013) 1543–1575. DOI:10.3390/ph6121543 |
| [11] | S. Sayed, M. A. Jardine, Antimicrobial biopolymers, in: A. Tiwari, L. Uzun(Eds. ), Advanced Functional Materials, John Wiley & Sons, Inc. , Hoboken, NJ, 2015, pp. 493-533. |
| [12] | J. F. Marcos, P. Manzanares, Antimicrobial peptides, in: J. M. Lagarón, M. J. Ocio, A. López-Rubio(Eds. ), Antimicrobial Polymers, John Wiley & Sons, Inc. , Hoboken, NJ, 2011, pp. 195-225. |
| [13] | F.H. Waghu, L. Gopi, R.S. Barai, CAMP:collection of sequences and structures of antimicrobial peptides. Nucleic Acids Res. 42 (2014) D1154–D1158. DOI:10.1093/nar/gkt1157 |
| [14] | J.B. Sun, Y.Q. Xia, D. Li, Q. Du, D.H. Liang, Relationship between peptide structure and antimicrobial activity as studied by de novo designed peptides. Biochim.Biophys.Acta 1838 (2014) 2985–2993. DOI:10.1016/j.bbamem.2014.08.018 |
| [15] | M.A.S. Karal, J.M. Alam, T. Takahashi, V. Levadny, M. Yamazaki, Stretch-activated pore of the antimicrobial peptide, Magainin 2. Langmuir 31 (2015) 3391–3401. DOI:10.1021/la503318z |
| [16] | J. Juhaniewicz, L. Szyk-Warszyńska, P. Warszyński, S. Sńk, Interaction of cecropin B with zwitterionic and negatively charged lipid bilayers immobilized at gold electrode surface. Electrochim.Acta 204 (2016) 206–217. DOI:10.1016/j.electacta.2016.04.080 |
| [17] | G. Munyuki, G.E. Jackson, G.A. Venter, β-sheet structures and dimer models of the two major tyrocidines, antimicrobial peptides from Bacillus aneurinolyticus. Biochemistry 52 (2013) 7798–7806. DOI:10.1021/bi401363m |
| [18] | C.F. Maurice, H.J. Haiser, P.J. Turnbaugh, Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 152 (2013) 39–50. DOI:10.1016/j.cell.2012.10.052 |
| [19] | C. Neale, J.C.Y. Hsu, C.M. Yip, R. Pomès, Indolicidin binding induces thinning of a lipid bilayer. Biophys.J. 106 (2014) L29–L31. DOI:10.1016/j.bpj.2014.02.031 |
| [20] | R.D. Rosa, A. Santini, J. Fievet, Big defensins, a diverse family of antimicrobial peptides that follows different patterns of expression in hemocytes of the oyster Crassostrea gigas. PLoS One 6 (2011) e25594. DOI:10.1371/journal.pone.0025594 |
| [21] | R.D.O. Dias, O.L. Franco, Cysteine-stabilized αβ defensins:from a common fold to antibacterial activity. Peptides 72 (2015) 64–72. DOI:10.1016/j.peptides.2015.04.017 |
| [22] | L.J. Xia, Y.L. Wu, S. Kang, CecropinXJ, a silkworm antimicrobial peptide, induces cytoskeleton disruption in esophageal carcinoma cells. Acta Biochim. Biophys.Sin. 46 (2014) 867–876. DOI:10.1093/abbs/gmu070 |
| [23] | J.M. Loeffler, D. Nelson, V.A. Fischetti, Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294 (2001) 2170–2172. DOI:10.1126/science.1066869 |
| [24] | J.F. Marcos, M. Gandía, Antimicrobial peptides:to membranes and beyond. Expert Opin.Drug Discov. 4 (2009) 659–671. DOI:10.1517/17460440902992888 |
| [25] | G.S. Wang, X. Li, Z. Wang, APD3:the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 44 (2016) D1087–D1093. DOI:10.1093/nar/gkv1278 |
| [26] | M. Zasloff, Antimicrobial peptides of multicellular organisms. Nature 415 (2002) 389–395. DOI:10.1038/415389a |
| [27] | X.Q. Xu, R. Lai, The chemistry and biological activities of peptides from amphibian skin secretions. Chem.Rev. 115 (2015) 1760–1846. DOI:10.1021/cr4006704 |
| [28] | J. M. Conlon, A. Sonnevend, Antimicrobial peptides in frog skin secretions, in: A. Giuliani, A. C. Rinaldi(Eds. ), Antimicrobial Peptides, Humana Press, Totowa, NJ, 2010, pp. 3-14. |
| [29] | B.M. Peters, M.E. Shirtliff, M.A. Jabra-Rizk, Antimicrobial peptides:primeval molecules or future drugs. PLoS Pathog. 6 (2010) e1001067. DOI:10.1371/journal.ppat.1001067 |
| [30] | S.L. Liu, X.B. Du, J.L. Kong, H. Jiang, A novel plant defensin from Chinese mistletoe, Viscum coloratum(Kom.)Nakai. Chin.Chem.Lett. 18 (2007) 55–58. DOI:10.1016/j.cclet.2006.11.007 |
| [31] | R.L. Zhao, J.Y. Han, W.Y. Han, Molecular cloning of two novel temporins from lithobates catesbeianus and studying of their antimicrobial mechanisms. Prog.Biochem.Biophys. 36 (2009) 1064–1070. DOI:10.3724/SP.J.1206.2009.00033 |
| [32] | J.L. Anaya-López, J.E. López-Meza, A. Ochoa-Zarzosa, Bacterial resistance to cationic antimicrobial peptides. Crit.Rev.Microbiol. 39 (2013) 180–195. DOI:10.3109/1040841X.2012.699025 |
| [33] | M.A. Ortega, Y. Hao, Q. Zhang, Structure and mechanism of the tRNA-dependent lantibiotic dehydratase NisB. Nature 517 (2014) 509–512. DOI:10.1038/nature13888 |
| [34] | A.A. de Oliveira Junior, H.G. Silva de Araújo Couto, A.A.T. Barbosa, M.A.G. Carnelossi, T.R. de Moura, Stability, antimicrobial activity, and effect of nisin on the physico-chemical properties of fruit juices. Int.J.Food Microbiol. 211 (2015) 38–43. DOI:10.1016/j.ijfoodmicro.2015.06.029 |
| [35] | J. Li, P. Neubauer, Escherichia coli as a cell factory for heterologous production of nonribosomal peptides and polyketides. N.Biotechnol. 31 (2014) 579–585. DOI:10.1016/j.nbt.2014.03.006 |
| [36] | M. Strieker, A. Tanović, M.A. Marahiel, Nonribosomal peptide synthetases: structures and dynamics. Curr.Opin.Struct.Biol. 20 (2010) 234–240. DOI:10.1016/j.sbi.2010.01.009 |
| [37] | R. Lande, E. Botti, C. Jandus, The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat.Commun. 5 (2014) 5621. DOI:10.1038/ncomms6621 |
| [38] | J.F. Marcos, A. Muñoz, E. Pérez-Payá, S. Misra, B. López-García, Identification and rational design of novel antimicrobial peptides for plant protection. Annu. Rev.Phytopathol. 46 (2008) 273–301. DOI:10.1146/annurev.phyto.121307.094843 |
| [39] | P.N. Desai, N. Shrivastava, H. Padh, Production of heterologous proteins in plants:strategies for optimal expression. Biotechnol.Adv. 28 (2010) 427–435. DOI:10.1016/j.biotechadv.2010.01.005 |
| [40] | S. Albayrak, J. M. Rouillard, E. Gulari, Cloning and expression of antimicrobial peptides in yeast, Proceedings of AIChE100-2008 AIChE Annual Meeting, AIChE, Philadelphia, PA, 2008. |
| [41] | B. Bommarius, H. Jenssen, M. Elliott, Cost-effective expression and purification of antimicrobial and host defense peptides in Escherichia coli. Peptides 31 (2010) 1957–1965. DOI:10.1016/j.peptides.2010.08.008 |
| [42] | M.F. Yun, L. Hui, H.Z. Li, Expression of tandem repeat Cecropin B in Chlamydomonas reinhardtii and its antibacterial effect. Prog.Biochem. Biophys. 39 (2012) 344–351. DOI:10.3724/SP.J.1206.2010.00671 |
| [43] | H. Ishida, L.T. Nguyen, R. Gopal, T. Aizawa, H.J. Vogel, Overexpression of antimicrobial, anticancer, and transmembrane peptides in Escherichia coli through a calmodulin-peptide fusion system. J.Am.Chem.Soc. 138 (2016) 11318–11326. DOI:10.1021/jacs.6b06781 |
| [44] | J.D. Wade, F. Lin, M.A. Hossain, R.M. Dawson, Chemical synthesis and biological evaluation of an antimicrobial peptide gonococcal growth inhibitor. Amino Acids 43 (2012) 2279–2283. DOI:10.1007/s00726-012-1305-z |
| [45] | R. Ramos, S. Moreira, A. Rodrigues, M. Gama, L. Domingues, Recombinant expression and purification of the antimicrobial peptide magainin-2. Biotechnol.Prog. 29 (2013) 17–22. DOI:10.1002/btpr.1650 |
| [46] | T.J. Oman, J.M. Boettcher, H. Wang, X.N. Okalibe, W.A. van der Donk, Sublancin is not a lantibiotic but an S-linked glycopeptide. Nat.Chem.Biol. 7 (2011) 78–80. DOI:10.1038/nchembio.509 |
| [47] | A. Rifflet, S. Gavalda, N. Téné, Identification and characterization of a novel antimicrobial peptide from the venom of the ant Tetramorium bicarinatum. Peptides 38 (2012) 363–370. DOI:10.1016/j.peptides.2012.08.018 |
| [48] | F. Costa, I.F. Carvalho, R.C. Montelaro, P. Gomes, M.C.L. Martins, Covalent immobilization of antimicrobial peptides(AMPs)onto biomaterial surfaces. Acta Biomater. 7 (2011) 1431–1440. DOI:10.1016/j.actbio.2010.11.005 |
| [49] | R.W. Scott, W.F. DeGrado, G.N. Tew, De novo designed synthetic mimics of antimicrobial peptides. Curr.Opin.Biotechnol. 19 (2008) 620–627. DOI:10.1016/j.copbio.2008.10.013 |
| [50] | M. Gupta, V.S. Chauhan, De novo design of α, β-didehydrophenylalanine containing peptides:from models to applications. Biopolymers 95 (2011) 161–173. DOI:10.1002/bip.v95.3 |
| [51] | J.L. Fox, Antimicrobial peptides stage a comeback. Nat.Biotechnol. 31 (2013) 379. DOI:10.1038/nbt.2572 |
| [52] | X.B. Qi, G. Poernomo, K. Wang, Covalent immobilization of nisin on multi-walled carbon nanotubes:superior antimicrobial and anti-biofilm properties. Nanoscale 3 (2011) 1874–1880. DOI:10.1039/c1nr10024f |
| [53] | P. Sahariah, K.K. Sørensen, M.á. Hjálmarsdóttir, Antimicrobial peptide shows enhanced activity and reduced toxicity upon grafting to chitosan polymers. Chem.Commun. 51 (2015) 11611–11614. DOI:10.1039/C5CC04010H |
| [54] | C.C. Zhou, M.Z. Wang, K.D. Zou, Antibacterial polypeptide-grafted chitosan-based nanocapsules as an armed carrier of anticancer and antiepileptic drugs. ACS Macro Lett. 2 (2013) 1021–1025. DOI:10.1021/mz400480z |
| [55] | M.L. Tan, P.F.M. Choong, C.R. Dass, Recent developments in liposomes, microparticles and nanoparticles for protein and peptide drug delivery. Peptides 31 (2010) 184–193. DOI:10.1016/j.peptides.2009.10.002 |
| [56] | K.A. Brogden, M. Ackermann, K.M. Huttner, Small, anionic, and charge-neutralizing propeptide fragments of zymogens are antimicrobial. Antimicrob. Agents Chemother. 41 (1997) 1615–1617. |
| [57] | H. Steffen, S. Rieg, I. Wiedemann, Naturally processed dermcidin-derived peptides do not permeabilize bacterial membranes and kill microorganisms irrespective of their charge. Antimicrob.Agents Chemother. 50 (2006) 2608–2620. DOI:10.1128/AAC.00181-06 |
| [58] | S.R. Dennison, M. Mura, F. Harris, The role of C-terminal amidation in the membrane interactions of the anionic antimicrobial peptide, maximin H5. Biochim.Biophys.Acta 1848 (2015) 1111–1118. DOI:10.1016/j.bbamem.2015.01.014 |
| [59] | D.I. Chan, E.J. Prenner, H.J. Vogel, Tryptophan-and arginine-rich antimicrobial peptides:structures and mechanisms of action. Biochim.Biophys.Acta 1758 (2006) 1184–1202. DOI:10.1016/j.bbamem.2006.04.006 |
| [60] | P. Pieta, M. Majewska, Z.F. Su, Physicochemical studies on orientation and conformation of a new bacteriocin BacSp222 in a planar phospholipid bilayer. Langmuir 32 (2016) 5653–5662. DOI:10.1021/acs.langmuir.5b04741 |
| [61] | B. Bechinger, K. Lohner, Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim.Biophys.Acta 1758 (2006) 1529–1539. DOI:10.1016/j.bbamem.2006.07.001 |
| [62] | W.C. Wimley, Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem.Biol. 5 (2010) 905–917. DOI:10.1021/cb1001558 |
| [63] | S.C. Park, J.Y. Kim, S.O. Shin, Investigation of toroidal pore and oligomerization by melittin using transmission electron microscopy. Biochem. Biophys.Res.Commun. 343 (2006) 222–228. DOI:10.1016/j.bbrc.2006.02.090 |
| [64] | D. Sengupta, H. Leontiadou, A.E. Mark, S.J. Marrink, Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim.Biophys.Acta 1778 (2008) 2308–2317. DOI:10.1016/j.bbamem.2008.06.007 |
| [65] | M.O. Lee, H.J. Jang, J.Y. Han, J.E. Womack, Chicken NK-lysin is an alpha-helical cationic peptide that exerts its antibacterial activity through damage of bacterial cell membranes. Poult.Sci. 93 (2014) 864–870. DOI:10.3382/ps.2013-03670 |
| [66] | A. Pokorny, P.F.F. Almeida, Permeabilization of raft-containing lipid vesicles by δ-lysin:a mechanism for cell sensitivity to cytotoxic peptides. Biochemistry 44 (2005) 9538–9544. DOI:10.1021/bi0506371 |
| [67] | Y.M. Zhang, C.O. Rock, Transcriptional regulation in bacterial membrane lipid synthesis. J.Lipid Res. 50 (2009) S115–S119. |
| [68] | F. Madeo, E. Herker, S. Wissing, Apoptosis in yeast. Curr.Opin.Microbiol. 7 (2004) 655–660. DOI:10.1016/j.mib.2004.10.012 |
| [69] | K. Vriens, T.L. Cools, P.J. Harvey, The radish defensins RsAFP1 and RsAFP2 act synergistically with caspofungin against Candida albicans biofilms. Peptides 75 (2016) 71–79. DOI:10.1016/j.peptides.2015.11.001 |
| [70] | M.L. Narasimhan, B. Damsz, M.A. Coca, A plant defense response effector induces microbial apoptosis. Mol.Cell 8 (2001) 921–930. DOI:10.1016/S1097-2765(01)00365-3 |
| [71] | S. Puri, M. Edgerton, How does it kill? Understanding the candidacidal mechanism of salivary histatin 5. Eukaryot.Cell 13 (2014) 958–964. DOI:10.1128/EC.00095-14 |
| [72] | D.S. Borgwardt, A.D. Martin, J.R. Van Hemert, Histatin 5 binds to Porphyromonas gingivalis hemagglutinin B(HagB)and alters HagB-induced chemokine responses. Sci.Rep. 4 (2014) 3904. |
| [73] | S. Orrapin, S. Intorasoot, Recombinant expression of novel protegrin-1 dimer and LL-37-linker-histatin-5 hybrid peptide mediated biotin carboxyl carrier protein fusion partner. Protein Expr.Purif. 93 (2014) 46–53. DOI:10.1016/j.pep.2013.10.010 |
| [74] | P. Shah, F. Shih-Hsiao, Y.H. Ho, C.S. Chen, The proteome targets of intracellular targeting antimicrobial peptides. Proteomics 16 (2016) 1225–1237. DOI:10.1002/pmic.v16.8 |
| [75] | J.H. Jang, Y.J. Kim, H. Kim, S.C. Kim, J.H. Cho, Buforin Ⅱb induces endoplasmic reticulum stress-mediated apoptosis in HeLa cells. Peptides 69 (2015) 144–149. DOI:10.1016/j.peptides.2015.04.024 |
| [76] | A. Muñoz, J.F. Marcos, N.D. Read, Concentration-dependent mechanisms of cell penetration and killing by the de novo designed antifungal hexapeptide PAF26. Mol.Microbiol. 85 (2012) 89–106. DOI:10.1111/j.1365-2958.2012.08091.x |
| [77] | D.M. Copolovici, K. Langel, E. Eriste, Ü. Langel, Cell-penetrating peptides: design, synthesis, and applications. ACS Nano 8 (2014) 1972–1994. DOI:10.1021/nn4057269 |
| [78] | N. Sitaram, Structure-function Correlations in Antibacterial and Cytolytic Peptides: Studies on Seminalplasmin: Seminalplasmin-derived and Related Synthetic Peptides, Jawaharlal Nehru University, Delhi, 2014. |
| [79] | A. Giuliani, G. Pirri, S. Nicoletto, Antimicrobial peptides:an overview of a promising class of therapeutics. Open Life Sci. 2 (2007) 1–33. DOI:10.2478/s11535-007-0010-5 |
| [80] | S.J. Kang, S.J. Park, T. Mishig-Ochir, B.J. Lee, Antimicrobial peptides:therapeutic potentials. Expert Rev.Anti Infect.Ther. 12 (2014) 1477–1486. DOI:10.1586/14787210.2014.976613 |
| [81] | X. Zhu, N. Dong, Z.Y. Wang, Design of imperfectly amphipathic α-helical antimicrobial peptides with enhanced cell selectivity. Acta Biomater. 10 (2014) 244–257. DOI:10.1016/j.actbio.2013.08.043 |
| [82] | Y.L. Ding, W. Wang, M. Fan, Antimicrobial and anti-biofilm effect of Bac8c on major bacteria associated with dental caries and Streptococcus mutans biofilms. Peptides 52 (2014) 61–67. DOI:10.1016/j.peptides.2013.11.020 |
| [83] | M. Bagheri, M. Beyermann, M. Dathe, Immobilization reduces the activity of surface-bound cationic antimicrobial peptides with no influence upon the activity spectrum. Antimicrob.Agents Chemother. 53 (2009) 1132–1141. DOI:10.1128/AAC.01254-08 |
 2017, Vol. 28
2017, Vol. 28