b College of Environmental & Energy Engineering, Beijing University of Technology, Beijing 100124, China
Microencapsulation is a technology by which a solid or liquid is enclosed with some other materials such as the polymer. The preparation of thermally expandable microspheres is an example of microencapsulation [1, 2]. The thermally expandable particles were originally invented by Dow Chemical Co., [3], and had been further developed by others for several decades [4-8]. Thermally expandable microspheres are polymeric core/shell particles, in which the hydrocarbon is encapsulated by thermoplastic polymer shells. The particles expand when heated above the glasstransition temperature of the polymer shell, thereby reducing the density from approximately 1000 kg m-3 to 30 kg m-3 [9, 10]. If the thermoplasticity of polymers and the vaporization pressure of blowing agents fit properly, a good expansion performance of the microspheres will appear [11, 12]. Generally speaking, the diameter of microsphere will increase to several times and the volume nearly 100 times after expansion. Meanwhile, the expanded microcapsules have relative morphological stability and the particle volumes are retained upon cooling [13-15]. At present, thermally expandable microspheres are widely used in light industry as textile printing foam and braille printing because of their unique expansion properties. Furtherly, the preparation of foaming ink and the application of printing technology will be widely developed to obtain a series of novel products with large area, low cost and three-dimensional foaming effects [16]. Recently, TEMs have been utilized for controlled handing of nano-liter volumes in microfluidic-type applications [17]. TEM particles have also been evaluated for the production of reversible adhesive joints, since the in situ volume expansion can induce rapid and effective delamination [18, 19]. Moreover, TEMs can also be used in some composite materials [20, 21].
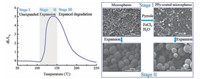
Being used in such a wide variety of application, tailoring the surface properties is interesting in order to improve the microsphere/matrix interactions as these are crucial for the final product properties [22, 23]. In this study, a novel functional component for TEMs is introduced. The conducting polymers are of excellent optical (light absorbing and emitting) and electronic (charge transport) properties. Therefore, the conducting polymers have been useful as the active component in various microelectronics [24-26], microfluidic and strong heavy-metal ion adsorption (Pb (Ⅱ) ion, silver (Ⅰ) ion, mercury (Ⅱ), Fe (Ⅲ) ion, etc.) applications [27-32] and also could be an additional tool in TEMs applications [33-35]. Thereinto, polypyrrole is a relatively air-stable organic conducting polymer that can be readily deposited onto various substrates by chemical oxidative polymerization [36-39]. This combination of polypyrrole and TEMs could be very useful in printed electronics and antistatic materials fields. Meanwhile, the dominating manufacturing technique of TEMs is based on suspension polymerization [40-42], which combined the surface modification and the use of functional conducting polymers. In this paper, the thermally expandable microspheres and polypyrrolecoated TEMs with core/shell structure were successfully prepared by in situ deposition from solution and the structure and property were characterized. The schematic representations of expansion of the TEMs and PPy-coated TEMs were shown in Fig. 1.
|
Download:
|
| Figure 1. Schematic representation of the expansion of the TEMs and PPy-coated TEMs. | |
2. Results and discussion 2.1. Particle size distribution
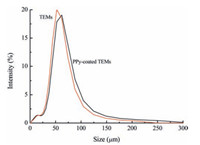
Fig. 2 and Table 1 show the particle size distributions of TEMs and PPy-coated TEMs. The volume average particle diameter (MV) of the TEMs is 50.96 μm. Moreover, the volume average particle diameter (MV) of the PPy-coated TEMs is 55.84 μm. Particle size distribution curves differed very little between the PPy-coated TEMs and TEMs. This suggests that the ultrathin conducting polymer overlayer has a negligible effect on the degree of dispersion of the TEM particles in dilute aqueous solution.
|
Download:
|
| Figure 2. Particle size distribution of TEMs and PPy-coated TEMs. | |
|
|
Table 1 Particle size characteristics parameters of TEMs and PPy-coated TEMs. |
2.2. Particle morphology
Fig. 3 shows the particle size distribution of TEMs before (Fig. 4a) and after expansion (Fig. 4b) with the image software analysis and calculation in the SEM photos. It was easy to see that the distribution zone of particle sizes was very wide before expansion. The maximum particle size distribution region of unexpanded microspheres was located in 30-35μm and the average diameter was 28.25 μm. After expansion, the particle size distribution became more relatively concentrated and most TEMs articles located in 150-200 μm with the average diameter of 184.75 μm. Therefore, the TEMs had a relatively good performance of expansion under optimum conditions and specific temperature, and that the maximum expansion ratio in diameter was obtained, i.e. e=(D2 -D1)/D1=5.53.

|
Download:
|
| Figure 3. SEM images of TEMs. (a) unexpanded (b) expanded (c) expanded section. | |

|
Download:
|
| Figure 4. Particle size distribution of TEMs. | |
When TEMs were coated by polypyrrole, the surface of the microcapsule exhibited the characteristic black color of PPy, thus indicating the presence of PPy as a coating. As the particles expanded, the black color became less intense. Scanning electron micrographs of PPy-coated TEMs before and after expansion are shown in Fig. 5. The unexpanded image indicated the relatively polydisperse nature of the products. None of the conducting PPycoated TEMs exhibited significant morphological differences compared to the uncoated TEMs.

|
Download:
|
| Figure 5. SEM images of PPy-coated TEMs. (a) Unexpanded (b) expanded. | |
2.3. Dilatometer (DIL)
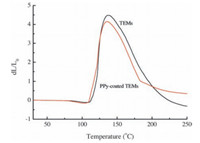
In the investigation of the expansion properties between the TEMs and PPy-coated TEMs, the results showed that the expansion properties were not constrained by the conducting polymer overlayer and the expansion properties were almost exactly alike contrast to the uncoated TEMs. An example of that can be seen in Fig. 6, in which the expansion of the microspheres before and after immobilization is shown by the dilatometer (DIL). There are several important temperature spots obtained, including Tstart of TEMs (108.7 ℃), Tstart of PPy-coated TEMs (105.2 ℃), Tmax of TEMs (137.0 ℃) and Tmax of PPy-coated TEMs (136.1 ℃). The expansion temperature of PPy-coated TEMs is slightly higher than that of TEMs, indicating that the conducting polymer is sensitive to temperature. Moreover, the maximum expansion ratio dL/L0 of TEMs is 4.5, while the maximum expansion ratio dL/L0 of PPycoated TEMs is 4.15. The expansion ratio of TEMs is a little higher than PPy-coated TEMs which is because the volume fraction of microcapsules in PPy-coated TEMs is decreased compared with TEMs in the same volume.
|
Download:
|
| Figure 6. Dilatometer (DIL) curves of TEMs and PPy-coated TEMs. | |
3. Conclusion
In this study, the conductive thermal expandable microspheres were prepared via suspension polymerization. The particle size distribution was analyzed and compared by the laser particle size analyzer and scanning electron microscope. The expansion property of PPy-coated TEMs was almost exactly alike contrast to the uncoated TEMs. Meanwhile, the results showed that the unexpanded TEMs were about 30 μm in diameter and the maximum expansion ratio was nearly 125 times of original volume. The particle morphology of unexpanded PPy-coated TEMs are shriveled spherical and gradually become spherical as the temperature increases. Through the thermal analysis investigation, the thermal expansion ratio of PPy-coated TEMs is 4.15. Therefore, the combination of polypyrrole and TEMs could have a potential use in printed electronics and antistatic materials fields.
4. Experimental 4.1. Materials and instrumentsUnless otherwise noted, the reagents were obtained from commercial suppliers and were used without further purification. Acrylonitrile (AN), AR; methyl methacrylate (MAN), AR; methyl acrylate (MA), CP; 2, 2-azobisisobutyronitrile (AIBN), AR; dimethyl 1, 4-butanediol acrylate (BDDMA), AR; n-hexane, AR; isooctane, AR; n-octane, AR; 2-methyl butane, AR; cyclohexane, AR; sodium hydroxide, AR; MgCI2·6H2O, AR; lauryl sodium sulfate, CP; sodium chloride, AR; HCl, AR; ferric chloride hexahydrate; pyrrole, AR.
Dilatometer (DIL), NETZSCH DIL 402 PC, Germany; scanning electron microscope (SEM), the HITACHI SU8020, Japan; particle size analysis, MICROTRAC S3500, the United States.
4.2. Preparation of TEMsThe preparation method of thermally expandable microspheres is based on suspension polymerization, and the mechanism is insitu polymerization. The general procedure for preparing thermally expandable microspheres was described as follows:
4.2.1. Oil phaseBDDMA (0.043 g), AIBN (0.43 g) and n-hexane (7 g) were added into the monomers with AN-MMA-MA in the proportion of 70%/ 20%/10% (m/m/m) and stirred to dissolve.
4.2.2. Water phaseThe dispersion of Mg (OH)2 was prepared by mixing sodium hydroxide solution (2.5 g in 30 g of deionized water) with MgCl2·6H2O solution (6 g MgCI2·6H2O in 30 g of deionized water) followed by vigorous stirring for at least 0.5 h. After that, this dispersion including lauryl sodium sulfate (0.01 g) and sodium chloride (1.1 g) were fully mixed for a period of time and set aside until ready to serve.
The oil phaseand water phase were mixed and emulsified using a homogenizer for 3 min at 8000rpm. Polymerization was transferred under agitation into a 250 mL boiling three-necked flask with a water bath at 65 ℃ and reacted for 15 h-20 h. Agglomerates and larger particles were removed from the dispersions using the sieve of 106 mm after polymerization. Besides, the suspending agent was removed from the particles by acidifying pH to 3-4 with diluted hydrochloric acid under stirring. The residual products were washed repeatedly with water and filtered, and then the particles were finally dried for 24 h at 50 ℃. The pictures of TEMS before and after expansion were shown in Fig. 7a.

|
Download:
|
| Figure 7. (a), (b) for TEMs before and after expansion; (c), (d) for PPy-coated TEMs before and after expansion. | |
4.3. Preparation of conducting polymer-coated TEMs
TEMs were coated by polypyrrole (PPy) and the preparation of PPy-coated particles was showed as follows. Firstly, TEMs (5 g) suspended in deionized water (25 mL) of a sample bottle by magnetic stirring. And then, ferric chloride hexahydrate (0.17 g) dissolved in 6.0 mL water was added into the aqueous TEM suspensions and mixed with mechanical stirring. After that, pyrrole monomer (0.075 g) was added into the reaction system and continued to stir for 22-24 h at room temperature. Moreover, the amounts of oxidant and monomer were controlled in 1.5%. The resulting suspensions were vacuum-filtered and the PPy-coated TEMs were washed several times with deionized water. Finally, the black dry free-flowing powders (Fig. 7c) were obtained through vacuum drying overnight.
4.4. Characterization 4.4.1. Particle size analysisThe microcapsules were dissolved in deionized water with ultrasonic dispersion for 5 min. The average size and size distribution of microspheres were measured by the MICROTRAC S3500 laser particle size analyzer. MV (μm) is a volume average particle diameter, D50 represents the number of particles with size larger than this value accounted for 50% of the total. The dispersion of particle size distribution was expressed by the span, span=(D90 -D10)/D50. The smaller the span, the narrower the particle size distribution it is. However, the microspheres stuck together after the expansion, so that these microspheres were difficult to disperse in water. Meanwhile, the density of microspheres was far less than that of water and floated on the surface of water.
4.4.2. Scanning electron microscope (SEM)The particle size and morphology of both the uncoated TEM particles and the PPy-coated TEMs were examined by scanning electron microscopy using the HITACHI SU8020 instrument. Each sample was sputter-coated with a thin layer of gold to prevent the sample-charging problems. The scanning electron microscope method was used to measure and count the average particle sizes of about 300 microspheres before and after expansion. The particle diameters of the TEMs were respectively showed as D1 (before expansion) and D2 (after expansion), and the microcapsules expansion ratio was obtained as follows, e=(D2 -D1)/D1.
4.4.3. Dilatometer (DIL)The thermal expansion ratio was measured by the NETZCSH DIL 420 PC. The samples were heated from 30 ℃ to 300 ℃ at 5 ℃/min with 25 cN load. Several determined expansion parameters are tested as follows, Tstart (the onset temperature of expansion), Tmax (the temperature of maximum of expansion) and the maximum expansion ratio dL/L0.
AcknowledgementsThe authors are thankful to the National Natural Science Foundation of China (Nos. 21206171, 21376010), the Project of Natural Science Foundation of Beijing (No. 2152012), the Young Elite Teacher Project (No. 27170115004/027), the Project of 2011 Collaborative Innovation for Green Printing and Publishing Technology and the Project of Beijing Municipal Commission of Education (No. km201410005007) for the financial supports.
| [1] | S. Gouin, Microencapsulation:industrial appraisal of existing technologies and trends. Trends Food Sci. Technol. 15 (2004) 330–347. DOI:10.1016/j.tifs.2003.10.005 |
| [2] | S. Benita, Microencapsulation:Methods and Industrial Applications, 2nd ed., CRC Press, Boca Raton, 2005. |
| [3] | S.D. Morehouse Jr., Expansible thermoplastic polymer particles containing volatile fluid foaming agent and method of foaming the same, U.S. Patent 3615972. |
| [4] | J.L. Garner, P.A. Tiffany, Method for expanding microspheres and expandable composition, U.S. Patent 4179546. |
| [5] | G.E. Melber, W.A. Oswald, L.E. Wolinski, Composition and process for drying and expanding microspheres, U.S. Patent 4722943. |
| [6] | H.S. Wu, F.M. Sun, V.L. Dimonie, A. Klein, Expandable hollow particles, U.S. Patent 583526. |
| [7] | L.O. Svedberg, G. Hovland, T. Holmlund, Method and expansion device for preparing expanded thermoplastic microspheres, U.S. Patent 7192989. |
| [8] | L.O. Svedberg, P. Ajdén, Method and a device for preparation of expanded microspheres, WIPO Patent Application WO/2014/198532. |
| [9] | S.Y. Chen, Z.C. Sun, L.H. Li, Preparation and characterization of thermally expandable microspheres. Mater. Sci. Forum 852 (2016) 596–600. DOI:10.4028/www.scientific.net/MSF.852 |
| [10] | Z.S. Hou, C.Y. Kan, Preparation and properties of thermoexpandable polymeric microspheres. Chin. Chem. Lett. 25 (2014) 1279–1281. DOI:10.1016/j.cclet.2014.04.011 |
| [11] | M. Jonsson, O. Nordin, A.L. Kron, E. Malmström, Thermally expandable microspheres with excellent expansion characteristics at high temperature. J. Appl. Polym. Sci. 117 (2010) 384–392. |
| [12] | M. Fujino, T. Taniguchi, Y. Kawaguchi, M. Ohshima, Mathematical models and numerical simulations of a thermally expandable microballoon for plastic foaming. Chem. Eng. Sci. 104 (2013) 220–227. DOI:10.1016/j.ces.2013.09.010 |
| [13] | M. Safajou-Jahankhanemlou, F. Abbasi, M. Salami-Kalajahi, Synthesis and characterization of thermally expandable PMMA-based microcapsules with different cross-linking density. Colloid Polym. Sci. 294 (2016) 1055–1064. DOI:10.1007/s00396-016-3862-2 |
| [14] | M. Jonsson, O. Nordin, A.L. Kron, E. Malmström, Influence of crosslinking on the characteristics of thermally expandable microspheres expanding at high temperature. J. Appl. Polym. Sci. 118 (2010) 1219–1229. |
| [15] | M. Jonsson, O. Nordin, E. Malmström, Increased onset temperature ofexpansion in thermally expandable microspheres through combination of crosslinking agents. J. Appl. Polym. Sci. 121 (2011) 369–375. DOI:10.1002/app.v121.1 |
| [16] | R. Urbas, U.S. Elesini, Color differences and perceptive properties of prints made with microcapsules. J. Graph. Eng. Des. 6 (2015) 15. |
| [17] | J.W. Jeong, J.G. Mccall, G. Shin, Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162 (2015) 662–674. DOI:10.1016/j.cell.2015.06.058 |
| [18] | M.D. Banea, L.F.M. da Silva, R.J.C. Carbas, R.D.S.G. Campilho, Mechanical and thermal characterization of a structural polyurethane adhesive modified with thermally expandable particles. Int. J. Adhes. Adhes. 54 (2014) 191–199. DOI:10.1016/j.ijadhadh.2014.06.008 |
| [19] | M.D. Banea, L.F.M. da Silva, R.J.C. Carbas, Debonding on command of adhesive joints for the automotive industry. Int. J. Adhes. Adhes. 59 (2015) 14–20. DOI:10.1016/j.ijadhadh.2015.01.014 |
| [20] | J. Loomis, P. Xu, B. Panchapakesan, Stimuli-responsive transformation in carbon nanotube/expanding microsphere-polymer composites. Nanotechnology 24 (2013) 185703. DOI:10.1088/0957-4484/24/18/185703 |
| [21] | L.J. Wang, X. Yang, J. Zhang, C. Zhang, L. He, The compressive properties of expandable microspheres/epoxy foams. Compos.Part B-Eng. 56 (2014) 724–732. DOI:10.1016/j.compositesb.2013.09.030 |
| [22] | M. Jonsson, D. Nyström, O. Nordin, E. Malmström, Surface modification of thermally expandable microspheres by grafting poly (glycidyl methacrylate) using ARGET ATRP. Eur. Polym. J. 45 (2009) 2374–2382. DOI:10.1016/j.eurpolymj.2009.05.002 |
| [23] | Y.C. Lu, J. Broughton, P. Winfield, Surface modification of thermally expandable microspheres for enhanced performance of disbondable adhesive. Int. J. Adhes. Adhes. 66 (2016) 33–40. DOI:10.1016/j.ijadhadh.2015.12.007 |
| [24] | S.Y. Yu, Y.C. Li, T. Xiong, A ladder conjugated polymer transducer for solidcontact Cu2+-selective electrodes. Chin. Chem. Lett. 25 (2014) 364–366. DOI:10.1016/j.cclet.2013.11.015 |
| [25] | X.Y. Hu, Q.X. Liu, D. Ma, One-step synthesis of MnO2 doped poly (anilineco-o-aminophenol) and the capacitive behaviors of the conducting copolymer. Chin. Chem. Lett. 26 (2015) 1367–1370. DOI:10.1016/j.cclet.2015.06.003 |
| [26] | Y.Y. Yao, L. Zhang, Z.F. Wang, J.K. Xu, Y.P. Wen, Electrochemical determination of quercetin by self-assembled platinum nanoparticles/poly (hydroxymethylated-3, 4-ethylenedioxylthiophene) nanocomposite modified glassy carbon electrode. Chin. Chem. Lett. 25 (2014) 505–510. DOI:10.1016/j.cclet.2014.01.028 |
| [27] | M.R. Huang, Y.B. Ding, X.G. Li, Synthesis of semiconducting polymer microparticles as solid ionophore with abundant complexing sites for long-life Pb (Ⅱ) sensors. ACS Appl. Mater. Interfaces 6 (2014) 22096–22107. DOI:10.1021/am505463f |
| [28] | M.R. Huang, H.J. Lu, X.G. Li, Synthesis and strong heavy-metal ion sorption of copolymer microparticles from phenylenediamine and its sulfonate. J. Mater. Chem. 22 (2012) 17685–17699. DOI:10.1039/c2jm32361c |
| [29] | X.G. Li, H. Feng, M.R. Huang, G.L. Gu, M.G. Moloney, Ultrasensitive Pb (Ⅱ) potentiometric sensor based on copolyaniline nanoparticles in a plasticizerfree membrane with a long lifetime. Anal. Chem. 84 (2011) 134–140. |
| [30] | X.G. Li, R. Liu, M.R. Huang, Facile synthesis and highly reactive silver ion adsorption of novel microparticles of sulfodiphenylamine and diaminonaphthalene copolymers. Chem. Mater. 17 (2005) 5411–5419. DOI:10.1021/cm050813s |
| [31] | X.G. Li, X.L. Ma, J. Sun, M.R. Huang, Powerful reactive sorption of silver (Ⅰ) and mercury (Ⅱ) onto poly (o-phenylenediamine) microparticles. Langmuir 25 (2009) 1675–1684. DOI:10.1021/la802410p |
| [32] | X.G. Li, Y.Z. Liao, M.R. Huang, V. Strong, R.B. Kaner, Ultra-sensitive chemosensors for Fe (Ⅲ) and explosives based on highly fluorescent oligofluoranthene. Chem. Sci. 4 (2013) 1970–1978. DOI:10.1039/c3sc22107e |
| [33] | A. Schmid, L.R. Sutton, S.P. Armes, P.S. Bain, G. Manfrè, Synthesis and evaluation of polypyrrole-coated thermally-expandable microspheres:an improved approach to reversible adhesion. Soft Matter 5 (2009) 407–412. DOI:10.1039/B811246K |
| [34] | H.E. Cingil, J.A. Balmer, S.P. Armes, P.S. Bain, Conducting polymer-coated thermally expandable microspheres. Polym. Chem. 1 (2010) 1323–1331. DOI:10.1039/c0py00108b |
| [35] | G. Vamvounis, M. Jonsson, E. Malmström, A. Hult, Synthesis and properties of poly (3-n-dodecylthiophene) modified thermally expandable microspheres. Eur. Polym. J. 49 (2013) 1503–1509. DOI:10.1016/j.eurpolymj.2013.01.010 |
| [36] | X.G. Li, Q.F. Lü, M.R. Huang, Self-stabilized nanoparticles of intrinsically conducting copolymers from 5-sulfonic-2-anisidine. Small 4 (2008) 1201–1209. DOI:10.1002/smll.v4:8 |
| [37] | X.G. Li, F. Wei, M.R. Huang, Y.B. Xie, Facile synthesis and intrinsic conductivity of novel pyrrole copolymer nanoparticles with inherent self-stability. J. Phys. Chem. B 111 (2007) 5829–5836. DOI:10.1021/jp0710180 |
| [38] | X.G. Li, Z.Z. Hou, M.R. Huang, M.G. Moloney, Efficient synthesis of intrinsically conducting polypyrrole nanoparticles containing hydroxy sulfoaniline as key self-stabilized units. J. Phys. Chem. C 113 (2009) 21586–21595. DOI:10.1021/jp9081504 |
| [39] | X.G. Li, A. Li, M.R. Huang, Efficient and scalable synthesis of pure polypyrrole nanoparticles applicable for advanced nanocomposites and carbon nanoparticles. J. Phys. Chem. C 114 (2010) 19244–19255. DOI:10.1021/jp107435b |
| [40] | M. Jonsson, O. Nordin, E. Malmström, C. Hammer, Suspension polymerization of thermally expandable core/shell particles. Polymer 47 (2006) 3315–3324. DOI:10.1016/j.polymer.2006.03.013 |
| [41] | Z.S. Hou, Y.R. Xia, W.Q. Qu, C.Y. Kan, Preparation and properties of thermoplastic expandable microspheres with P (VDC-AN-MMA) shell by suspension polymerization. Int. J. Polym. Mater. Polym. Biomater. 64 (2015) 427–431. DOI:10.1080/00914037.2014.958831 |
| [42] | J.G. Kim, J.U. Ha, S.K. Jeoung, Halloysite nanotubes as a stabilizer:fabrication of thermally expandable microcapsules via Pickering suspension polymerization. Colloid Polym. Sci. 293 (2015) 3595–3602. DOI:10.1007/s00396-015-3731-4 |
 2017, Vol. 28
2017, Vol. 28 



