Presently, the fabrication or designing the luminescent nanoparticles (NPs) is an emerging field because of their potential applications in applied material sciences as well as in biomedical sciences [1-6]. The representative luminescent nanoparticle probes include semiconductor NPs (quantum dots) [1, 3, 4], plasmon-resonant NPs, gold NPs and dye-doped silica NPs [2-8]. Compared with conventional organic dyes, the luminescent NPs have higher photo-stability and stronger luminescence, which allows them favorably to be used as luminescence probes for bioassays. The main problem using the nanoparticle luminescence probes in bioassays is that the luminescence measurement is easily affected by the strong nonspecific scattering lights, such as Tyndall, Rayleigh, and Raman scatterings. This problem has limited their effective application for quantitative bioassays. In fact, the uses of the nanoparticle luminescence probes in bioassays are mainly qualitative or semi-quantitative simultaneously.
To resolve these problems, the development of an alternative biomaterial applications through the use of lanthanide doped NPs is gaining popularity due to their distinctive luminescence properties, such as sharp absorption and emission lines, high quantum yields, large stokes shifts (≥150 nm) long lifetimes, superior photo-stability, biocompatibility and low toxicity, which make them particularly advantageous for use as bio-lables/optical bio-probe [5, 6, 9]. Many of these lanthanide ion-doped NPs consist of a crystalline host doped with a small amount of luminescent rare-earth cations. Among the possible host matrices, rare-earth fluorides are preferred because they have lower vibrational energies than oxides and consequently, the quenching of the exited state of the Ln3+ cations is minimized, resulting in a higher quantum efficiency of the luminescence [10, 11]. Due to the high ionicity and coordination numbers, which could lead to wide band gap and low vibrational energies, the rare-earth fluorides are considered as good candidates for host materials. YF3 is one of the most important rare-earth fluorides and has been actively studied; especially it is an important host crystal for lanthanide doped phosphors. YF3 provides a wide band gap (>10 eV) and suitable Y3+ sites at which trivalent rare-earth elements can be easily substituted without additional charge compensation [10-13]. Based on these virtues, it has potential applications in the fields of new laser materials, up/down-conversion biolabels, and efficient UV phosphors in the near-UV region [10-15]. Due to their variety of applications in applied material sciences and biomedical sciences it is urgent need to improve luminescence efficiency of nanomaterials, which may be affected by two fundamental factors: crystallinity and surface effect. Insufficient crystallinity means unordered structure and more defects which act as quenching centers consuming excited photoelectrons without radiation. And the large specific surface area of nanomaterials, unsaturated bonds on the surface and high surface energy will result in surface quenching and greatly decrease the lifetime and luminescent efficiency of nanomaterials. In recent years, core/shell structure is utilized to weaken the surface effect, reduce surface quenching so as to enhance efficiency of light-emitting materials [16-22]. The shell of similar lattice constant provides a chemically-designed protection and prevents quenching by external environment. Up to now, several core/shell nanostructure such as LaPO4:Ln@LaPO4, LnF3:Eu@LnF3, NaLnF4:Ln/Ln@NaLnF4, CaMoO4:Ln@CaMoO4 (Ln=La, Gd, Y, Eu, Tb, Yb, Er, Tm, Ho, ) etc. have been reported in the literature [16-25]. Additionally, due to hydrophobic nature of these core/shell-NPs in aqueous solvent, their application in biomedical sciences is limited. To overcome this problem, it is better to develop hydrophilic nature functional groups on the surface of luminescent core/shell-NPs. Currently silica has been used as a surface coating materials, since silica layer is easily grown on the surface of luminescent-NPs, which is biocompatible, nontoxic and easily soluble in aqueous and non-aqueous solvents to make colloidal solution [16, 22, 26, 27]. Besides that, silica has high chemical/thermal stability, optical transparency, easily controllable shell thickness, low cytotoxicity, biocompatibility and inexpensiveness.
In this study, we presented simple and effective strategy to enhance the luminescence efficiency along with solubility and colloidal stability in aqueous and non-aqueous solvent. In this report, we presented successfully synthesized luminescent coreNPs and their after surface coating of inert LaF3 and silica layers surrounding the luminescent core-NPs via polyol and sol-gel Stober chemical routs, respectively. The developed inert crystalline LaF3 shell surrounding the luminescent core-NPs enhanced the luminescent intensity, whereas, amorphous silica layer improves the solubility and colloidal character in aqueous and non-aqueous solvent. We discussed comparative spectral analysis such as X-ray diffraction pattern (XRD), transmission electron microscopy (TEM), UV/vis absorption (UV/vis), Fourier transform infrared (FTIR), photoluminescence (PL) spectra to investigate the effect of surface coating on their crystal structure, crystalinity, morphology, surface properties and optical properties of the as-prepared core/shell/ SiO2-NPs. Their high colloidal stability in aqueous medium with enhanced luminescent intensity, large specific surface area, and low toxicity make them extremely attractive for application in bio-labeling/optical bio-probe and optical bio-sensing.
2. Results and discussion 2.1. Crystallographic and morphological structureX-ray diffraction pattern was performed to gain the crystal structure, phase purity and crystalinity of the prepared samples. Fig. 1 displays the powder XRD pattern of the terbium doped YF3: Tb (core), YF3Tb@LaF3 (core/shell) and YF3:Tb@LaF3@SiO2 core/ shell/SiO2 NPs. XRD shows all reflection planes of pure cubic phase YF3 microcrystals, which are closely matched with the literature reports and standard data (JCPDS Card No. 072-0579) [10, 11, 28, 29]. Note that there is a small residual amount of orthorhombic phase also present in the sample (Fig. 1a). None of the samples was found to show any extra peak corresponding to terbium oxide and salt and thus ruled out the formation of secondary phase. The high purity of all samples demonstrates the formation of homogeneous Y-F-Tb solid solution. In addition, a relatively diminish diffraction peak intensity is observed for the peaks of the silica surface modified core/shell/SiO2 NPs as compared to those of the core/shell NPs, owing to amorphous silica surface has been grafted effectively around the core/shell NPs. It is worth noticing, that the XRD pattern of the core/shell NPs is more similar to those of the core-NPs, it could be due to the similar cubic phase of LaF3 shell. However, increase peak intensity could have resulted from the increase of the grain size and similar crystal structure between YF3 and LaF3 (JCPDS No. 072-0579) [28, 29]. Furthermore, we observed that after growing a LaF3 shell, the XRD peaks at 2θ=44.27° and 2θ=52.36° corresponding to (221) and (022) diffraction planes, increase more than other peaks, suggesting that the LaF3 layer might prefer to grow along the (111) direction of YF3:Tb nanocrystals. On the other hand the reflection peaks are notably broadened, possibly because the structure of core/shell NPs brings partial disorder to the ordered mesoporous framework of the amorphous silica shell. The grain size of all samples is calculated from X-ray line broadening from the most strong diffraction peak according to the Scherrer formula and they are 16, 23 and 44 nm for core, core/shell and core/shell/SiO2 NPs, respectively [11, 12, 28-30].

|
Download:
|
| Figure 1. X-ray diffraction pattern of core, core/shell and core/shell/SiO2 nanoparticles. | |
Transmission electron microscopy (TEM) and selected area electron diffraction (SAED) pattern were performed to measure the size, morphology, phase purity and crystalline nature of the prepared samples. It can be seen in Fig. 2a, the core-NPs are small grain size irregular shaped, narrow size distributed, which are highly aggregate. The aggregations of the particles are mainly attributed to the presence of little amount ethylene glycol on the surface of nanoparticle. On the other hand, these NPs are prepared in aqueous environment and their surface may be covered with large amount of hydroxyl groups, which are either chemically bonded or physically adsorbed on the surface. Owing to the presence of surface hydroxyl groups, these particles connected to each other through hydrogen bonding. This hydrogen bonding is responsible to aggregation as well as high dispersibility of the NPs in aqueous solvents. The average grain size of the core-NPs is 15-25 nm, which is in good agreement with the values calculated from the XRD pattern. Furthermore, there are no obvious discrepancies in grain size and morphology, which means chelating or doping components do not effect on the morphological features. Their cubic crystal structure was further confirmed by selected area electron diffraction (SAED) pattern as shown in Fig. 2b. The well resolved rings in SAED are assigned to the (020), (111) and (210) planes, which indicate the well crystalline nature of the cubic phase YF3, in accordance with the XRD results [11, 12, 28-30]. The high magnified FETEM image in Fig. 2e displays well resolved lattice fringes which indicates the excellent crystallization of core/shell/SiO2 crystalline grain. The observed distances between the adjacent lattice fringes correspond to the inter-planer spacing of YF3 (111) planes agreeing well with the d (111) spacing of the literature value (JCPDS No. 072-0579) [28, 29]. Owing to the presence of hydroxyl groups on the surface of as-prepared core/shell NPs, TEOS can easily react with the surface of core/shell NPs and formed homogeneous smooth amorphous silica layer surrounding the core/shell-NPs. The core/shell/SiO2 nanostructured of the YF3:Tb@LaF3 @SiO2-NPs can be clearly seen in Fig. 2c-e due to the different electron penetrabilities of the cores and shells. The particle size measurement is performed on randomly selected particles on the TEM image, suggesting that the dark cores have an average size of~21 nm for irregular spherical and 5-8 nm thickness of silica shell. EDX analysis confirmed the chemical composition and doped element into the lattice site of core NPs. The confirmation and identification of the core and shell is important for fundamental understanding of NPs based on structure and composition. The incorporation of Tb3+ cation in the YF3 matrix is corroborated by EDX analyses since the spectra obtained for several single particles are closely similar. Fig. 2f clearly shows all assigned peaks of corresponding elements such as Y, La, F, Tb, O and Si for the designed core/shell/SiO2-nanostructured. The observed strong peaks of C and Cu belong to the carbon coated copper TEM grid. These results indicate the phase purity and successful silica surface coating on the surface of core/shell/SiO2-NPs, supporting the XRD and FE-TEM results.

|
Download:
|
| Figure 2. FETEM images of (a–e) core/shell/SiO2 NPs (b) SAED of the YF3:Tb@LaF3@SiO2 core/shell/SiO2 NPs and (f) energy dispersive X-ray analysis of core/shell/SiO2 NPs. | |
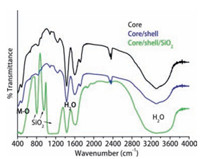
FTIR spectroscopy provides the surface chemistry of the asprepared samples before and after silica surface modification. Fig. 3 shows the broadened transmission band between 3000-3600 cm-1 attributed to symmetrical stretching vibration of O-H groups. Another two weaken intensity bending and wagging vibration modes of surface anchored hydroxyl groups are also observed at around 1622 and 1415 cm-1 [16, 21, 22]. This measurement confirmed that surface of the samples may be covered by a large amount of hydroxyl groups either by chemically surface bound or physically adsorbed residual water molecules. These surface anchored hydroxyl groups are responsible for their solubility and colloidal stability in aqueous media. To improve hydrophilicity of the core/shell NPs modified them with amorphous silica through sol-gel chemical rout. Silica surface not only improve their hydrophilic nature but also improve their colloidal stability as well as non-toxic nature of the NPs. The silica surface modified NPs spectrum shows characteristic infrared bands such as a strong doublet band located at 1180 cm-1 and another two weaken intensity peaks at around 946 and 804 cm-1, which originate from symmetrical stretching and bending vibrational modes of (Si-O-Si), (Si-OH) and (Si-O), it indicates that amorphous silica has been effectively grafted around the core/ shell-NPs [16, 21, 22]. These observations are consistent with the TEM and EDX analysis and good agreement with published literature reports [21, 22, 27]. Another weak intensity band located at 475 cm-1 attribute to the M-O stretching vibration [21, 22, 27].
|
Download:
|
| Figure 3. FTIR spectra of core, core/shell and core/shell/SiO2 NPs. | |
In parallel to this, we measured UV-vis spectra of all samples in dist. water over the range from 200 to 600 nm to confirm their water dispersibility and colloidal stability at room temperature. It is seen in Fig. 4A core/shell/SiO2 NPs, a significant enhancement is observed in the absorption spectrum of amorphous silica surface modified core/shell/SiO2 NPs than other two spectrums, suggesting that the optically active amorphous silica layer has been successfully encapsulated on the surface of core/shell-NPs. Furthermore, we observed similar trend in the absorption spectra of these samples in absolute ethanol (Fig. 4B). Furthermore, we utilized optical absorption spectra to investigate the correlation between energy band gap and gain size of the nanomaterials. According to the Tauc and Menth, the experimentally estimated band gap energies for core, core/shell and core/shell/SiO2-NPs are 2.11, 1.99 and 1.97 in H2O and 1.78, 1.63 and 1.61 in absolute ethanol, respectively [31]. The experimentally estimated band gap energies decrease gradually when shell growth surrounding the core-NPs increases the grain size of the nanomaterials, indicate the existence of quantum confinement effect.

|
Download:
|
| Figure 4. (a) UV–vis absorption spectra of core, core/shell and core/shell/SiO2 NPs in de-ionized water and in inset shows the plot of (αhν)2 vs. photon energy (hν) of the core, core/shell and core/shell/SiO2 NPs. (b) UV–vis absorption spectra of core, core/shell and core/shell/SiO2 NPs in absolute ethanol and in inset shows the plot of (αhν)2 vs. photon energy (hν) of the core, core/shell and core/shell/SiO2 NPs. | |

|
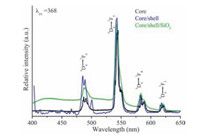
Photoluminescence properties were determined to examine the impact of surface coating on luminescent core-NPs. Fig. 5 illustrated the excitation spectrum of core, core/shell and core/ shell/SiO2-NPs with a monitoring emission 542 nm. As seen in Fig. 5, the spectrum consists of many sharp 4f-4f emission transitions of Tb3+ ion located at 315, 329, 339, 350, 368, 375 and 485, which correspond to the transitions 7F6→5D0, 7F6→5D1, 7F6→5L8, 7F6→5L10 & 5G5, 7F6→5G6, 7F6→5D3 and 5D4→7F5, respectively [16, 17, 32-34]. All three excitation spectra revealed similar trend and all excitation transitions are identical to the excitation of Tb3+ ion in powder samples [25]. As observed in Fig. 5, the peak intensities of 4f-4f transitions of Tb3+ ions are increase, it may be related to surface growth of undoped LaF3 layer around the core-nanoparticles. Whereas, the relatively peak intensity decrease after silica surface modification, due to the influence of amorphous silica. Fig. 6 illustrates the emission spectra of core, core/shell and core/shell/SiO2-NPs observed at room temperature with in the 400-650 nm UV/vis spectral range under excitation from 368 nm. All spectrums show the well-known 4f-4f emission transitions of trivalent Tb3+ ion, it is an evidence that terbium ions have been successfully doped inside the YF3 crystal lattice. The PL spectra of core-NPs revealed four well-known 4f-4f emission transitions in the visible region observed at 485, 543, 582 and 617 nm, which can be attribute to the transitions 5D4→7F6, 5D4→7F5, 5D4→7F4, and 5D4→7F3, respectively [5, 9, 35, 36]. The green emission transition (5D4→7F5) located at 543 nm as the most prominent as the other transitions because it is magnetically dipole transition with △J=1 [5, 9, 35]. It is called hypersensitive transition, and can provide more information about the chemical environment surrounding the Tb3+ ion. This hypersensitive emission transition is the true fingerprint of the characteristic emission lines corresponding to the 4fn-4fn transition of the Tb3+ ion, which is induced by the change in chemical environment of Tb3+ ions during the formation of a new chemical bond between host and the Tb3+ metal ion [5, 35, 36]. As seen in Fig. 6 this transition has very peculiar features in respect to other emission transitions, indicating that the spectrum is dominated by the hypersensitive transition from 5D4 to the 7F5 manifold, whereas comparatively other emission peaks are weak. Due to the shielding effect of 4f-electrons by 5s25p6 electrons in outer shells in the Tb3+ ion, narrow emission peaks are observed, consistent with the sharp peak in Fig. 6 [5, 35, 36]. The comparative emission spectra show that the peak positions of Tb3+ ions are not affected, the structure and luminescence properties of YF3:Tb3+ phosphor are not destroyed after coating of the inert crystalline LaF3 and amorphous silica layers surrounding the luminescent YF3:Tb3+ seed core-NPs, this could be due to no alteration of symmetry of the core crystal lattice. As shown in Fig. 6, a remarkable luminescent intensity is observed in the case of core/shell-NPs in respect to core-NPs, it could be due to the coating of an inert crystalline LaF3 shell in the seed core-NPs. The inert crystalline shell protects the luminescent core-NPsfromincident and emission light scattering. Furthermore, after surface coating a significant amount of non-radiative centers existing on the surface of seed core-NPs are eliminated by the shielding effect of the inert crystalline layer. In this core/shell structure, the distance between the luminescent Tb3+ ions and the surface quenchers is increased, thus reducing the non-radiative pathways and suppressing the energy quenching in energy transfer processes. Although, in the case of core/shell/SiO2-NPs, the luminescent intensity is reduced to some extent than the respective core/shell-NPs. The reduction in luminescent intensity could be due to light scattering effect on both emission and incident light by silica shell. It indicated that surface of core/shell/ SiO2-NPs are covered with large number of high energy hydroxyl groups, as confirmed by FTIR. These high energy hydroxyl groups scattered the incident and emission light or enhanced nonradiative transitions, resulting in reduction of the emission intensity of luminescent lanthanide ions. Notably, core/shell-NPs are weakly soluble in aqueous solvents due to core-NPs were capped with a layer of hydrophobic inert crystalline LaF3 shell. Therefore, their surface modification is necessary before their use in biological sciences. So that, these core/shell-NPs were coated with amorphous silica shell via sol-gel process. The resultant silica surface functionalized core/shell/SiO2-NPs could be easily dispersed in aqueous solvents to form colloidal solution. It is worth noting that silica surface modification can provide high solubility and good biocompatibility, which can be employed in bioapplications without any adverse toxic effects. The emission efficiency is an important factor; however, the biocompatibility and toxicity of the luminescent nano-materials are much more critical for their applications in bio-medical sciences and should be taken as the priority in the design of nano-labels.
|
Download:
|
| Figure 6. Emission spectra of the core, core/shell and core/shell/SiO2 NPs. | |
3. Conclusions
A novel aqueous soluble with enhanced luminescent intensity silica surface modified core/shell/SiO2-NPs was fabricated with simple sol-gel chemical rout. The designed core/shell/SiO2-nanostructure was examined with XRD, FETEM, FTIR, UV/vis, energy band gap and photoluminescence spectroscopy. Comparative spectral results indicate that the surface coating affect the crystalinity, morphology, optical and emission properties of the seedcore-NPs.These core/shell/SiO2-NPs rangingfrom35to40nm are well crystalline, irregular spherical shaped with smooth silica surface coated and could be well dispersed in aqueous and nonaqueous solvents to form colloidal solutions. The reduction in experimentally estimated optical energy band-gap from core to core/shell/SiO2-NPs was attributed to the quantum-size effect due to the growth of an inert crystalline LaF3 and amorphous silica layers increase the grain size of the luminescent core-NPs. The silica surface modified NPs showed lower luminescent intensity in respect to non-silica modified seed NPs, causing the absorption of UV exciting radiation by amorphous silica shell and its quenching properties. We expected that this method can be applied to design other core/shell-nanostructured materials for their potential applications in optical bio-probe/bio-imaging and luminescent bio-detection etc.
4. Experimental 4.1. MaterialsYttrium oxide (99%, BDH Chemicals Ltd., England), lanthanum oxide (99%, BDH Chemicals Ltd., England), terbium oxide (99.99%, Alfa Aesar, Germany), ethanol (E-Merck, Germany), Tetraethyl orthosilicate (TEOS, 99wt% analytical reagent), NH4F, ethylene glycol (EG), HNO3 and NH4OH were used as the starting materials without any further purification. Y (NO3)36H2O, La (NO3)37H2O and Tb (NO3)36H2O were prepared by dissolving the corresponding oxides in diluted nitric acid. The double distilled H2O was prepared using a Milli-Q system (Millipore, Bedford, MA, USA). All other chemicals used were of reagent grade.
4.2. Preparation of YF3:Tb3+ NPsFor preparation of YF3:Tb3+ NPs (Y0.95Tb0.05F3), 0.2 mol/L stock solutions of Y (NO3)36H2O and Tb (NO3)36H2O in de-ionized water were prepared. In brief, 9.50 mL of Y (NO3)36H2O and 0.50 mL of Tb (NO3)36H2O were dissolved in 50 mL of ethylene glycol. Then an equiv. molar aqueous solution of NH4F was added drop wise under magnetically stirred forgoing reaction, and kept whole solution with magnetic stirring on hotplate at 80 ℃ to obtain homogenously mixing. Later on, this homogenously mixed solution was transferred in a round bottle 250 mL flask fitted with reflux condenser for 4 h until complete precipitation. On cooling to room temperature, the white precipitates then segregated to the bottom. The product was collected by centrifugation and washed with distilled water and absolute ethanol several times, and dried in oven at 60 ℃ for 6 h for further characterization. The obtained solid product can be re-dispersed in de-ionized water to form a waterdispersible solution.
4.3. Preparation of YF3:Tb3+@LaF3 core/shell NPsFor the preparation of YF3:Tb3+@LaF3 core/shell NPs, similar polyol process was used as discussed above. The as-prepared 0.500 g YF3:Tb3+ was dispersed with the help of ultra-sonication in 10 mL of distilled water. These dispersed NPs solution mixed into magnetically stirred hot EG dissolved Y (NO3)36H2O (0.500 g) solution. After thirty minutes an equiv. molar aqueous solution of NH4F was injected into the foregoing mixed system under magnetically stirred at 80 ℃. Afterward this suspension was refluxed at 80 ℃ for 3 h until the complete precipitation is occurred. This white precipitate was centrifuged and washed many times with ethanol and dist. water to remove excess un-reacted reactants. The core/shell NPs were collected after centrifugation and allowed to dry in ambient temperature for further characterization.
4.4. Preparation of silica coated YF3:Tb3+@LaF3@SiO2 core/shell NPsthe yf3:tb3+@laf3@sio2 core/shell-nps were prepared through a versatile solution sol-gel method as follows [16, 22, 26, 27]. the synthesized yf3:tb3+@laf3 nps (50 mg) were well dispersed in a mixed solution of deionized water (50 ml), ethanol (70 ml) and concentrated aqueous ammonium hydroxide (1.0 ml) in a three-neck round-bottom flask. afterward, 1.0 ml of teos was added drop-wise in 2 min, and the reaction was allowed to proceed for 5-6 h under continuous mechanical stirring. after continuous stirring at room temperature, the silica-coated yf3: tb3+@laf3 core/shell nps were separated by centrifugation, washed several times with ethanol and dried at room temperature for further analysis.
4.5. CharacterizationThe crystalinity of the powder samples was examined by X-ray diffraction (XRD) at room temperature with the use of Rigaku X-ray diffractometer equipped with a Ni filter using Cu Kα (λ=1.54056 Å) radiations as X-ray source. The size and morphology of the samples were inspected using a field emission transmission electron microscope (FE-TEM) equipped with the EDX (FETEM, JEM-2100F, JEOL, Japan) operating at an accelerating voltage of 200 kV. EDX analysis was used to confirm the presence of the elements. The samples for TEM were prepared by depositing a drop of a colloidal ethanol solution of the powder sample onto a carbon-coated copper grid. The FTIR spectra were recorded on a Perkin-Elmer 580B IR spectrometer using KBr pellet technique in the range 4000-400 cm-1. The UV/vis absorption spectra were measured in the Perkin-Elmer Lambda-40 spectrophotometer in the range 190-600 nm, with the sample contained in 1 cm3 stoppered quartz cell of 1 cm path length. Photoluminescence spectra were recorded on Fluorolog 3 spectrometer (model: FL3-11, Horiba Jobin Yvon, Edison, NJ, USA). All measurements were performed at room temperature.
AcknowledgmentThis work was supported through the project funded by National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, award number (No. 13-Bio1246-02).
| [1] | H. Yang, S. Santra, G.A. Walter, P.H. Holloway, GdⅢ-functionalized fluorescent quantum dots as multimodal imaging probes. Adv. Mater. 18 (2006) 2890–2894. DOI:10.1002/(ISSN)1521-4095 |
| [2] | Z.Q. Ye, M.Q. Tan, G.L. Wang, J.Q. Yuan, Novel fluorescent europium chelatedoped silica nanoparticles:preparation, characterization and time-resolved fluorometric application. J. Mater. Chem. 14 (2004) 851–856. DOI:10.1039/b311905j |
| [3] | C.L. Zhang, X.H. Ji, Y. Zhang, One-pot synthesized aptamer-functionalized CdTe:Zn2+ quantum dots for tumor-targeted fluorescence imaging in vitro and in vivo. Anal. Chem. 85 (2013) 5843–5849. DOI:10.1021/ac400606e |
| [4] | P. Reiss, M. Protière, L. Li, Core/shell semiconductor nanocrystals. Small 5 (2009) 154–168. DOI:10.1002/smll.200800841 |
| [5] | P. Huhtinen, M. Kivelä, O. Kuronen, Synthesis, characterization, and application of Eu (Ⅲ), Tb (Ⅲ), Sm (Ⅲ), and Dy (Ⅲ) lanthanide chelate nanoparticle labels. Anal. Chem. 77 (2005) 2643–2648. DOI:10.1021/ac048360i |
| [6] | Z. Chen, W. Zheng, P. Huang, Lanthanide-doped luminescent nanobioprobes for the detection of tumor markers. Nanoscale 7 (2015) 4274–4290. DOI:10.1039/C4NR05697C |
| [7] | S. Schultz, D.R. Smith, J.J. Mock, D.A. Schultz, Single-target molecule detection with nonbleaching multicolor optical immunolabels. Proc. Natl. Acad. Sci. U. S. A. 97 (2000) 996–1001. DOI:10.1073/pnas.97.3.996 |
| [8] | M.A. Hayat, Colloidal Gold:Principles, Methods and Applications, Academic Press, New York, 1989. |
| [9] | I. Hemmilä, V. Laitala, Progress in lanthanides as luminescent probes. J. Fluoresc. 15 (2005) 529–542. DOI:10.1007/s10895-005-2826-6 |
| [10] | J.W. Stouwdam, F.C.J.M. van Veggel, Near-infrared emission of redispersible Er3+, Nd3+, and Ho3+ doped LaF3 nanoparticles. Nano Lett. 2 (2002) 733–737. DOI:10.1021/nl025562q |
| [11] | R.X. Yan, Y.D. Li, Down/up conversion in Ln3+ doped YF3 nanocrystals. Adv. Funct. Mater. 15 (2005) 763–770. DOI:10.1002/(ISSN)1616-3028 |
| [12] | S.L. Zhong, Y. Lu, M.R. Gao, Monodisperse mesocrystals of YF3 and Ce3+/Ln3+(Ln=Tb, Eu) co-activated YF3:shape control synthesis, luminescent properties, and biocompatibility. Chem. Eur. J. 18 (2012) 5222–5231. DOI:10.1002/chem.v18.17 |
| [13] | C. Peng, C.X. Li, G.G. Li, S.W. Li, J. Lin, YF3:Ln3+(Ln=Ce, Tb, Pr) submicrospindles:hydrothermal synthesis and luminescence properties. Dalton Trans. 41 (2012) 8660–8668. DOI:10.1039/c2dt30325f |
| [14] | G.X. Liu, X. Li, X.T. Dong, J.X. Wang, Architectures of YF3:Eu3+ solid and hollow sub-microspheres:a facile arginine-assisted hydrothermal synthesis and luminescence properties. J. Nanopart. Res. 13 (2011) 4025–4034. DOI:10.1007/s11051-011-0332-0 |
| [15] | N.O. Nuñez, M. Ocaña, An ionic liquid based synthesis method for uniform luminescent lanthanide fluoride nanoparticles. Nanotechnology 18 (2007) 455606. DOI:10.1088/0957-4484/18/45/455606 |
| [16] | A.A. Ansari, R. Yadav, S.B. Rai, Enhanced luminescence efficiency of aqueous dispersible porous NaYF4:Yb/Er nanoparticles and the effect of surface coating. RSC Adv. 6 (2016) 22074–22082. DOI:10.1039/C6RA00265J |
| [17] | A.K. Parchur, A.I. Prasad, A.A. Ansari, S.B. Rai, R.S. Ningthoujam, Luminescence properties of Tb3+-doped CaMoO4 nanoparticles:annealing effect, polar medium dispersible, polymer film and core-shell formation. Dalton Trans. 41 (2012) 11032–11045. DOI:10.1039/c2dt31257c |
| [18] | F. Vetrone, R. Naccache, V. Mahalingam, C.G. Morgan, J.A. Capobianco, The active-core/active-shell approach:a strategy to enhance the upconversion luminescence in lanthanide-doped nanoparticles. Adv. Funct. Mater. 19 (2009) 2924–2929. DOI:10.1002/adfm.v19:18 |
| [19] | F. Wang, R.R. Deng, J. Wang, Tuning upconversion through energy migration in core-shell nanoparticles. Nat. Mater. 10 (2011) 968–973. DOI:10.1038/nmat3149 |
| [20] | J.C. Boyer, J. Gagnon, L.A. Cuccia, J.A. Capobianco, Synthesis, characterization, and spectroscopy of NaGdF4:Ce3+, Tb3+/NaYF4 core/shell nanoparticles. Chem. Mater. 19 (2007) 3358–3360. DOI:10.1021/cm070865c |
| [21] | A.A. Ansari, A.K. Parchur, M. Alam, J. Labis, A. Azzeer, Influence of surface coating on structural and photoluminescent properties of CaMoO4:Pr nanoparticles. J. Fluoresc. 24 (2014) 1253–1262. DOI:10.1007/s10895-014-1409-9 |
| [22] | A.A. Ansari, M. Alam, J.P. Labis, Luminescent mesoporous LaVO4:Eu3+ core-shell nanoparticles:synthesis, characterization, biocompatibility and their cytotoxicity. J. Mater. Chem. 21 (2011) 19310–19316. DOI:10.1039/c1jm12871j |
| [23] | K. Kömpe, H. Borchert, J. Storz, Green-emitting CePO4:Tb/LaPO4 coreshell nanoparticles with 70% photoluminescence quantum yield. Angew. Chem. Int. Ed. 42 (2003) 5513–5516. DOI:10.1002/(ISSN)1521-3773 |
| [24] | G.S. Yi, G.M. Chow, Water-soluble NaYF4:Yb, Er (Tm)/NaYF4/polymer core/shell/shell nanoparticles with significant enhancement of upconversion fluorescence. Chem. Mater. 19 (2007) 341–343. DOI:10.1021/cm062447y |
| [25] | K.A. Abel, J.C. Boyer, F.C.J.M. van Veggel, Hard proof of the NaYF4/NaGdF4 nanocrystal core/shell structure. J. Am. Chem. Soc. 131 (2009) 14644–14645. DOI:10.1021/ja906971y |
| [26] | A.A. Ansari, S.P. Singh, N. Singh, B.D. Malhotra, Synthesis of optically active silica-coated NdF3 core-shell nanoparticles. Spectrochim. Acta A 86 (2012) 432–436. DOI:10.1016/j.saa.2011.10.063 |
| [27] | A.A. Ansari, M. Alam, Optical and structural studies of CaMoO4:Sm, CaMoO4:Sm@CaMoO4 and CaMoO4:Sm@CaMoO4@SiO2 core-shell nanoparticles. J. Luminesc. 157 (2015) 257–263. DOI:10.1016/j.jlumin.2014.09.001 |
| [28] | S. Sarkar, V. Mahalingam, Tuning the crystalline phase and morphology of the YF3:Eu3+ microcrystals through fluoride source. CrystEngComm 15 (2013) 5750–5755. DOI:10.1039/c3ce40554k |
| [29] | L.Y. Wang, Y. Zhang, Y.Y. Zhu, One-pot synthesis and strong near-infrared upconversion luminescence of poly (acrylic acid)-functionalized YF3:Yb3+/Er3+ nanocrystals. Nano Res. 3 (2010) 317–325. DOI:10.1007/s12274-010-1035-z |
| [30] | M. Darbandi, T. Nann, One-potsynthesis of YF3@silica core/shell nanoparticles. Chem. Commun. (2006) 776–778. |
| [31] | J. Tauc, A. Menth, States in the gap. J. Non-Cryst. Solids 8-10 (1972) 569–585. DOI:10.1016/0022-3093(72)90194-9 |
| [32] | Y.C. Li, Y.H. Chang, Y.S. Chang, Y.J. Lin, C.H. Laing, Luminescence and energy transfer properties of Gd3+ and Tb3+ in LaAlGe2O7. J. Phys. Chem. C 111 (2007) 10682–10688. |
| [33] | D.M. Krol, R.P. van Stapele, J.H. Haanstra, Luminescence and absorptionof Tb3+ in mo A12O3·B2O3·Tb2O3 glasses. J. Luminesc. 37 (1987) 293–302. DOI:10.1016/0022-2313(87)90011-1 |
| [34] | E. Cavalli, P. Boutinaud, R. Mahiou, M. Bettinelli, P. Dorenbos, Luminescence dynamics in Tb3+-doped CaWO4 and CaMoO4 crystals. Inorg. Chem. 49 (2010) 4916–4921. DOI:10.1021/ic902445c |
| [35] | A.A. Ansari, J.P. Labis, One-pot synthesis and photoluminescence properties of luminescent functionalized mesoporous SiO2@Tb (OH)3 core-shell nanospheres. J. Mater. Chem. 22 (2012) 16649–16656. DOI:10.1039/c2jm33583b |
| [36] | F.S. Richardson, Terbium (Ⅲ) and europium (Ⅲ) ions as luminescent probes and stains for biomolecular systems. Chem. Rev. 82 (1982) 541–552. DOI:10.1021/cr00051a004 |
 2017, Vol. 28
2017, Vol. 28 

