b Organic Research Laboratory, Department of Bioresources and Food Science, College of Life and Environmental Sciences, Konkuk University, Seoul 143 701, Republic of Korea;
c College of Life Science and Biotechnology, Department of Biological and Environmental Science, Dongguk University, 32, Ilsandong-gu, Goyang-si, Gyeonggi-do 410-820, Republic of Korea;
d Department of Food Science and Biotechnology, Dongguk University-Seoul, Ilsandong-gu, Goyang-si, Gyeonggi-do 410-820, Republic of Korea
Schiff bases, named after Hugo Schiff and their very first formation was accomplished in the nineteenth millennium [1]. They are known as azomethine or imine as nitrogen analogue an aldehyde or ketone structurally, where imine or azomethine group found to replace the carbonyl group [2]. Schiff base is very widely used and the most appreciated organic building blocks to have a diverse range of pharmacological importance as well as a versatile tool to explore in many other fields as biological, inorganic and analytical chemistry. They hold a spectrum of biological importance as antioxidant, anthelmintic, antitubercular, antiinflammatory, anticancer, antimicrobial, anticonvulsant and so forth [3]. The active centers of cell constituents are supposed to get interacted with azomethine's nitrogen atom via forming a hydrogen bond which interferes with normal cell processes [4] and results in the destruction of enzymatic activity of cancerous cells, thereby presents Schiff base as a potential target to discover anticancer chemotherapeutics. Hence, in the current research, we were directed to construct Schiff base derivatives involving the presence of three different types of heterocycles as imidazole, thiazole, and other heteroaromatics. Imidazole ring, highly polar heterocycle, is an important five-membered aromatic heterocycle widely present in natural products and synthetic molecules. It readily binds with different targeted enzymes in a biological system via ion-dipole, coordination, van der Waals forces, π-π stacking, hydrophobic effects, cation-π, and so on, because of its unique electronrich characteristic which establishes diverse weak interactions, thereby exhibiting broad bioactivities [5]. Hence, we have selected imidazole as a starting heterocyclic core to build the desire Schiff bases with intermediate thiazole entity which represents a versatile tool to generate anticancer chemotherapeutics [6]. Finally, different N, S and O-based heterocycles were attached to the Schiff base function because, such heterocyclic compounds have a considerable active role as antibacterial [7, 8], anti-viral [9], anti-fungal [10], anti-inflammatory [11], and antitumor drugs [12-14].
Heterocycles represent a common architectural device of most promoted drugs and hence, we effort here to produce multiheterocyclic systems to be examined for their in vitro anticancer results, because cancer provides being a major health issue in developed along with developing countries. With >100 types of cancer exist, 8.2 million people die each year from cancer, an estimated 13% of all deaths worldwide and 70% the increase in new cases of cancer expected over the next 2 decades [15]. In the attempts to distinguish a variety of chemical substances that may provide as leads for creating novel anticancer agents, nitrogen-and sulfur-that contains heterocycles are of specific attention [16-18] and the fact prompted us to generate anticancer multi-heteroaromatic systems as imidazolylphenylheterocyclic-2-ylmethylenethiazole-2-amines. Recently, we have observed many structures those represent similar features as ours with Schiff bases function linked to 2-aminothiazole nucleus presenting interesting pharmacological effects [19-22]. Hence, we attempt here to generate Schiff base derivatives holding all three other building block as diverse heterocycles which anticipated delivering significant anticancer effects.
2. Results and discussion 2.1. ChemistryScheme 1 represents the chemical steps followed to obtain desired Schiff base 4a-4i. 4'-(Imidazol-1-yl) acetophenone was selected as a starting material to be treated with aqueous HBr with glacial acetic acid as well as bromine to give the first intermediate derivative 1-(4-(1H-imidazol-1-yl) phenyl)-2-bromoethanone (2). Intermediate 2 was subsequently reacted with thiourea in the presence base to generate final thiazole-2-amine intermediate 3. FT-IR spectra of intermediate 2 revealed aromatic stretching at 2976 cm-1, carbonyl group characteristic peak at 1697 cm-1 as well as C-Br peak at 691 cm-1 which ensure its correct formation. In addition, FT-TR solid state spectrum obtained for intermediate 3 revealed NH2 characteristic peak at 3438 cm-1 and thiazole ring peaks were observed as 1523 for C=N as well as 691 as C-S-C linkage. 1H NMR spectra for intermediate 2 revealed peak for COCH2Br linkage as a singlet 4.80 ppm, whereas, a peak at 7.71 ppm was assigned to thiazole-H and such data confirmed the correct formation of both intermediates. In further steps, intermediate 3 was reacted with various desired heterocyclic aldehyde entities (a-i) in the presence of organocatalyst piperidine with molecular sieve (4A) to furnish final thiazolyl Schiff bases 4a-4i [24]. All FT-IR and 1H NMR data were in accurate accordance with the proposed structural features of the final compounds and 1H NMR for compound 4a as a representative analogue; N=CH peak was observed at 8.91 ppm which apparently confirms the accurate synthesis. Mass spectral data were presented as M+1 molecular ion peaks and which were also in accordance with the molecular weights for the 4a-4i and elemental analysis data were in a considerable range acceptable to confirm the correct formation of anticipated structures.

|
Download:
|
| Scheme 1. Schematic presentation for the synthesis of imidazolylphenyl-heterocyclic-2-ylmethylene-thiazole-2-amines (4a–4i). | |
2.2. Pharmacology
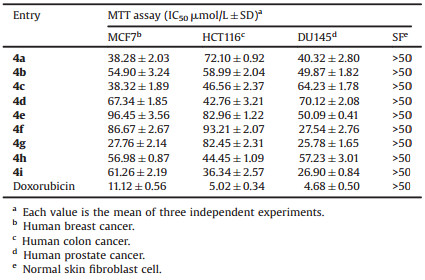
The newly yielded multi-heterocomponent Schiff base 4a-4i were subjected to the screening of their in vitro anticancer potencies against a panel of three cancerous cell lines as MCF7 (human breast cancer), HCT116 (human colon cancer), and DU145 (human prostate cancer) as well as one non-cancer normal skin fibroblast (SF) to inspect the cytotoxic character of the said molecules toward the healthy cell line. The results of anticancer screening are presented in Table 1 in terms of IC50s in μmol/L, and the bioassay data suggested that these new Schiff base displayed a variable degree of anticancer activity with a broad scope of SAR studies leading to a tool which can facilitate further rationale in drug designing. It was noticed that primary imidazole and intermediate thiazole rings were having an essential role to provide promising cancerous cell inhibitory effects, whereas, varying the type of coupled heterocyclic entity to the Schiff base function has a key role in determining the final anticancer potency of resultant molecule.
|
|
Table 1 Results of anticancer activity investigation for final Schiff base 4a–4i |
A molecule with a benzothiazole entity (4g) connected to the azomethine function showed the highest anticancer activity against MCF-7 cell line with IC50 of 27.76 × 2.14 μmol/L which can be comparable to that of control drug doxorubicin with 11.12 × 0.56 μmol/L of IC50. Moreover, the presence of two heteroatoms as S and N was found beneficial to gain activity against MCF-7 as compounds 4a with thiazole ring and 4c with imidazole moiety presented 38.28 × 2.03 μmol/L and 38.32 × 1.89 μmol/L of IC50s, respectively. All these three derivatives indicated low cytotoxic character toward normal skin fibroblast cell with >50 μmol/L of IC50. It will be suffice to mention here that S heteroatom has a good role to play for the anticancer potency against MCF-7 because a compound with S-heterocyclic entity in terms of thiophene (4b) demonstrated good anticancer action with 54.90 × 3.24 μmol/L of IC50, whereas, compounds (4e and 4f) bearing oxygenated heterocycles showed poor anticancer potencies with IC50s >85 μmol/L. Finally, molecules holding single N atom as 4d, 4h and 4i appeared to have moderate anticancer effects with IC50s, 67.34 × 1.85 μmol/L, 56.98 × 0.87 μmol/L and 61.26 × 2.19 μmol/L, respectively. Furthermore, quinolone ring (4i) had the most influence on HCT116 cell line with 36.34 × 2.57 μmol/L of the IC50 level and >50 μmol/L of IC50 against non-cancer skin fibroblast cell being the most active analogue of the series against the mentioned cancerous cell line. Similar to the activity results observed against MCF-7, nitrogen-based heterocycles were found remarkably active against HCT116 cell line as compounds 4c, 4d, and 4h with imidazole, pyrrole and pyridine rings demonstrated 46.56 × 2.37 μmol/L, 42.76 × 3.21 μmol/L and 44.45 -1.09 μmol/L, respectively, however their cytotoxicity towards SF was observed beyond 50 μmol/L, thus suggesting these N-heterocyclic candidates as potentially active anticancer agent against human colon cancer. The above data suggested that heterocycle with single N atom were active against human colon cancer than those carrying two N atoms. In fact, the presence of an additional heteroatom in the form of S lead to the reduced activity of the molecule as 4g and 4a showed >70 μmol/L of IC50s against HCT116 cell line, respectively. However, molecules bearing only a single sulfur atom as compound 4b observed to have moderate anticancer activity against HCT116 cell line with IC50 of 58.99 × 2.04 μmol/L. Lastly, with O-based heterocycles, similar to activity recorded against MCF-7, compounds 4e and 4f were found least sensitive against HCT116 cell line with >80 μmol/L of IC50s. Presence of an additional aromatic ring along with a heterocyclic core in terms of benzofuran (4f), benzothiazole (4g) and quinolone (4i) was a key to have successive anticancer action against DU-145 prostate cancer cell line with IC50s, 27.54 × 2.76 μmol/L, 25.78 × 1.65 μmol/L and 26.90 × 0.84 μmol/L, respectively and it was found that heterocyclic with two different heteroatoms (4g) was most active. Similarly, further data recorded against DU-145 cell line proved that S has an excellent role to play as molecules with thiazole (4a) and thiophene (4b) entities showed 40.32 × 2.80 μmol/L and 49.87 × 1.82 μmol/L of IC50s, respectively; and >50 of IC50s toward SF cells presenting themselves as lead anticancer candidates against prostate cancer. However, with an exception to the activity data for MCF-7 and HCT116, a compound with O heteroatom in the form of furan (4e) was as active as that with thiophene ring towards DU-145 cell line with 50.09 × 0.41 μmol/L of IC50. Finally, a derivative with a pyridine (4h) ring has good action than imidazole (4c: IC50, 64.23 -1.78 μmol/L) and pyrrole (4d: 70.12 × 2.08 μmol/L) with 57.23 × 3.01 of IC50 against DU-145 cell line. Overall, many of the final Schiff base derivatives showed < 50 μmol/L of IC50s against all three cancerous cell lines and >50 μmol/L of IC50s against non-cancer cells which present them as leading candidates for anticancer drug discovery.
3. ConclusionTo conclude, a successful attempt has been made to furnish a new series holding multi-hetero component resides with tremendous anticancer action against three different cancerous cell lines. Imidazole and thiazole core were kept constant in all final molecules to get a regular anticancer data with a variable degree of efficacy observed while varying heterocyclic moieties connected to the Schiff base link. The correct formation of the desired molecules was ensured utilizing different analytical techniques followed by the inspection of their in vitro anticancer effects, in which, all compounds presented < 100 μmol/L of IC50s. Core imidazole liked to the thiazole ring was very beneficial, as final results also displayed anticipated potencies with thiazole core lined to the Schiff base function. The presence of particular heteroatom, as well as presence of an additional benzene ring, had a significant effect against MCF-7 and DU-145, and heterocycles with S atoms were more active, whereas, against HCT116, Nheterocycles played a major role. Overall, 4a, 4g, and 4i showed tremendous activity against all cancerous cell lines. In addition, all compounds presented >50 μmol/L of IC50s against non-cancer normal skin fibroblast (SF) cells which indicated tolerable cytotoxic nature of these Schiff bases toward healthy cells. As some substituent were found inactive, for example, those with O heteroatom, more studies are obviously warranted to replace basic core from imidazole and thiazole to other heteroatom-containing rings to examine their overall influence on anticancer effects.
4. ExperimentalReichert Thermover instrument was utilized to record melting points of the final compounds which were uncorrected. FT-IR spectra were recorded using KBr on Perkin Elmer RXI spectrometer. Proton and carbon NMR data were recorded on Bruker AVANCE Ⅲ 400 instrument spectrometer (1H NMR, 400 MHz; 13C NMR, 100 MHz) in the presence of TMS internal standard and with DMSO. Mass spectra have been registered on JEOL-Accu TOF JMST100LC DART-MS spectrometer. Microanalytical data were collected using Carlo Erba analyzer model 1108. TLC plates were used to monitor the reaction as well as to identify the purity of the prepared products.
4.1. Synthetic procedure for 1-(4-(1H-imidazol-1-yl) phenyl)-2-bromoethanone (2)In a flask charged with 4'-(imidazol-1-yl) acetophenone (0.01 mol) with glacial acetic acid (20 mL) and aqueous hydrobromic acid (HBr) [48% (w/w)]. The resulting mixture was immersed in an ice-salt mixture (0-5 ℃), and bromine (0.01 mol) was added, and temperature of the reaction mixture does not exceed 5 ℃. After completion of addition, it was stirred at room temperature for 2-3 h. The progress of the reaction was monitored by using TLC. After the completion of the reaction, the suspension was poured onto crushed ice. The colorless precipitate was filtered, repeatedly washed with water and dried at room temperature. Yield: 55%-57%, m.p: 147-149 ℃; FT-IR (KBr) cm-1: 2976 (Ar-stretching), 1697 (C=O), 691 (C-Br); 1H NMR (400 MHz, DMSO-d6): δ 8.38 (s, 1H), 8.13 (d, 1H, J=7.6 Hz, ), 8.02 × 7.96 (m, 2H), 7.94 × 7.87 (m, 2H), 7.84 (d, 1H, J=7.5 Hz, ), 4.80 (s, 2H). 13C NMR (100MHz, DMSO-d6): δ 181.34, 150.12, 148.34, 137.6, 134.2, 132.7, 129.9, 125.4, 123.7, 122.5, 34.23.
4.2. Synthetic procedure for 4-(4-(1H-imidazol-1-yl) phenyl) thiazol-2-amine (3)In a flask charged with compound 2 (0.01 mol), thiourea (0.01 mol) and triethylamine (0.01 mol) and the reaction mixture was refluxed for 10-12h in 30 mL acetonitrile until the complete consumption of starting material as detected by TLC. The reaction mixture was then cooled, poured onto ice. The precipitate was filtered, dried and recrystallised from ethanol. Yield: 45%-53%, m. p: 160-163 ℃; FT-IR (KBr) cm-1: 3438 (NH2), 1577 (aromatic C=C stretch), 1523 (C=N) thiazole, 1154 (C-N), 691 (C-S-C) thiazole; 1H NMR (400MHz, DMSO-d6): δ 5.08 (s, 1H, NH2), 8.13 (d, 1H, J=7.5Hz, ), 7.98-7.91 (m, 2H), 7.84 (d, 1H, J=7.5Hz, ), 7.71 (s, 1H), 7.61 (s, 2H), 7.48-7.42 (m, 2H). 13C NMR (100MHz, DMSO-d6): d 167.52, 153.71, 147.89, 146.21, 138.7, 134.6, 131.8, 128.7, 125.6, 124.6, 122.8, 121.9. Anal Calcd. for C22H22N2O3: C, 72.91; H, 6.12; N, 7.73; Found: C, 72.76; H, 6.29; N, 7.62.
4.3. General method for the preparation of imidazolylphenylheterocyclic-2-ylmethylene-thiazole-2-amines (4a-4i)A mixture of substituted compound 3 (0.01 mol), substituted heterocyclic aldehyde (0.01 mol) and piperidine (0.01 mol) was refluxed in 30 mL ethanol for 6-8h. The reaction mixture was poured into an ice-water mixture to get a product, which was filtered, dried, and recrystallized using ethanol to give the final compounds 4a-4i, for example, 4-(4-(1H-imidazol-1-yl) phenyl)-N-(thiazol-2-ylmethylene) thiazol-2-amine (4a): Yield 65%. m.p. 213-214 ℃; FT-IR (KBr) cm-1: 3067 (aromatic C-H stretch), 1608 (N=CH stretch, azomethine), 1572 (aromatic C=C stretch), 1509 (C=N) thiazole, 1147 (C-N), 685 (C-S-C) thiazole; 1H NMR (400MHz, DMSO-d6): δ 8.91 (s, 1H, N=CH); 8.38 (s, 1H), 8.13 (d, 1H, J=7.5Hz), 8.05 (d, 1H, J=7.5Hz), 7.99-7.92 (m, 2H), 7.84 (d, 1H, J=7.5Hz), 7.75 (s, 1H), 7.55=(d, 1H, J=7.5Hz), 7.49-7.43 (m, 2H). 13C NMR (100MHz, DMSO-d6): δ 164.71, 164.03, 152.22, 147.72, 146.81, 144.11, 135.26, 131.25, 130.19, 125.67, 123.88, 122.45, 121.37, 118.30, 113.17, 112.61. EI-MS m/z (M+): 338.74; Anal Calcd. for C16H11N5S2: C, 56.95; H, 3.29; N, 20.76; Found: C, 56.88; H, 3.35; N, 20.64.
4.4. In vitro evaluation of anticancer activityHuman cancer cell lines namely, MCF7 (human breast cancer), HCT116 (human colon cancer), and DU145 (human prostate cancer) and one normal skin fibroblast (SF), MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide], DMEM, trypsin-EDTA, RPMI and 96-well flat bottom tissue culture plates were obtained from the commercial suppliers. Cancerous were grown in DMEM medium, and SF cells were grown in RPMI medium supplemented with 10% fetal bovine serum, 200lg/mL streptomycin, 100 lg/mL penicillin, 2 μmol/L L-glutamine, and culture was maintained with 5% CO2. A stock solution of for compounds to be tested was prepared in DMSO with sterile PBS to get required concentration. A standard MTTcolorimetric assay was performed for investigating the anticancer potential of 4a-4i because MTT is a photosensitive compound and is consumed by living organisms and a mitochondrial dehydrogenase enzyme performs its reduction to a purple formazan product that is impermeable to the cell membrane. DMSO solubilization of test compounds yields liberation and resulting formazan product with purple color is directly related to the cell viability. Cells placed in 96-well plates were incubated with test compounds and a controlled drug (doxorubicin) with variable dilutions at 37 ℃ for 24h in DMEM medium carrying 10% FBS medium. After that, 90 μL of fresh serum free media and 10 μL of MTT reagent (5 mg/mL) was placed in the place of old media followed by the incubation at 37 ℃ for 4h and lastly, this media was changed to 200 μL of DMSO followed by last incubation at 37 ℃ for 10 min. The absorbance at 570nm was calculated on a spectrophotometer (Spectra Max, Molecular devices) IC50 principles were identified from plot: % cell viability (from control) versus concentration [23].
Conflicts of interestThe authors report no conflicts of interest.
AcknowledgmentsThis work is supported by Dongguk University-Seoul, Republic of Korea, research funds 2016-2017. This article was supported by the KU Research Professor Program of Konkuk University, Seoul, Republic of Korea.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.10.021.
| [1] |
(a) H. Schiff, Mittheilungen aus dem universitätslaboratorium in Pisa:eine neue reihe organischer Basen, Eur. J. Org. Chem. 131(1864) 118-119; (b) C.M. Da Silva, D.L. Da Silva, L.V. Modolo, et al., Schiff bases:a short review of their antimicrobial activities, J. Adv. Res. 2(2011) 1-8. |
| [2] | I.A. Mohammed, E.V.S. Subrahmanyam, Synthesis, characterization and antimicrobial activity of some substituted N'-arylidene-2-(quinolin-8-yloxy) aceto hydrazides. Acta Pharm. Sci. 51 (2009) 163–168. |
| [3] | A. Kajal, S. Bala, S. Kamboj, N. Sharma, V. Saini, Schiff bases:a versatile pharmacophore, J. Catal. 2013(2013) Article ID 893512. |
| [4] | K. Vashi, H.B. Naik, Synthesis of novel Schiff base and azetidinone derivatives and their antibacterial activity. Eur. J. Chem. 1 (2004) 272–276. |
| [5] | L. Zhang, X.M. Peng, G.L. Damu, R.X. Geng, C.H. Zhou, Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 34 (2014) 340–437. DOI:10.1002/med.2014.34.issue-2 |
| [6] | V. Gupta, V. Kant, A review on biological activity of imidazole and thiazole moieties and their derivatives. Sci. Int. 1 (2013) 253–260. DOI:10.17311/sciintl.2013.253.260 |
| [7] | N.M.A. El-Salam, M.S. Mostafa, G.A. Ahmed, O.Y. Alothman, Synthesis and antimicrobial activities of some new heterocyclic compounds based on 6-chloropyridazine-3(2H)-thione. J. Chem. 2013 (2013) 890617. |
| [8] | M.E. Azab, M.M. Youssef, E.A. El-Bordany, Synthesis and antibacterial evaluation of novel heterocyclic compounds containing a sulfonamido moiety. Molecules 18 (2013) 832–844. DOI:10.3390/molecules18010832 |
| [9] | M.S. Salem, S.I. Sakr, W.M. El-Senousy, H.M.F. Madkour, Synthesis, antibacterial, and antiviral evaluation of new heterocycles containing the pyridine moiety. Arch. Pharm. 346 (2013) 766–773. DOI:10.1002/ardp.v346.10 |
| [10] | X.F. Cao, Z.S. Sun, Y.B. Cao, Design, synthesis, and structure-activity relationship studies of novel fused heterocycles-linked triazoles with good activity and water solubility. J. Med. Chem. 57 (2014) 3687–3706. DOI:10.1021/jm4016284 |
| [11] | E.R. El-Sawy, M.S. Ebaid, H.M. Abo-Salem, A.G. Al-Sehemi, A.H. Mandour, Synthesis, anti-inflammatory, analgesic and anticonvulsant activities of some new 4, 6-dimethoxy-5-(heterocycles) benzofuran starting from naturally occurring visnagin. Arab. J. Chem. 7 (2014) 914–923. DOI:10.1016/j.arabjc.2012.12.041 |
| [12] | Y. Chen, K. Yu, N.Y. Tan, Synthesis, characterization and anti-proliferative activity of heterocyclic hypervalent organoantimony compounds. Eur. J. Med. Chem. 79 (2014) 391–398. DOI:10.1016/j.ejmech.2014.04.026 |
| [13] | E.R. El-Sawy, A.H. Mandour, S.M. El-Hallouty, K.H. Shaker, H.M. Abo-Salem, Synthesis, antimicrobial and anticancer activities of some new Nmethylsulphonyl and N-benzenesulphonyl-3-indolyl heterocycles:1st Cancer Update. Arab. J. Chem. 6 (2013) 67–78. DOI:10.1016/j.arabjc.2012.04.003 |
| [14] | Y.N. Mabkhot, A. Barakat, A.M. Al-Majid, Synthesis, reactions and biological activity of some new bis-heterocyclic ring compounds containing sulphur atom. Chem. Cent. J. (2013) 112. |
| [15] | WHO, World Health Organization, Cancer Accessed from, http://www.who.int/cancer/en/. |
| [16] | M. Kidwai, R. Venkataramanan, R. Mohan, P. Sapra, Cancer chemotherapy and heterocyclic compounds. Curr. Med. Chem. 9 (2002) 1209–1228. DOI:10.2174/0929867023370059 |
| [17] | J. Salimon, N. Salih, E. Yousif, A. Hameed, H. Ibraheem, Synthesis and antibacterial activity of some new 1, 3, 4-oxadiazole and 1, 3, 4-thiadiazole derivatives. Aust. J. Basic Appl. Sci. 4 (2010) 2016–2021. |
| [18] | H.A. Mohamed, B.R. Lake, T. Laing, R.M. Phillips, C.E. Willans, Synthesis and anticancer activity of silver (Ⅰ)-N-heterocyclic carbene complexes derived from the natural xanthine products caffeine, theophylline and theobromine. Dalton Trans. 44 (2015) 7563–7569. DOI:10.1039/C4DT03679D |
| [19] | S. Rajasekaran, G.K. Rao, P.N.S. Pai, A. Ranjan, Design, synthesis, antibacterial and in vitro antioxidant activity of substituted 2H-benzopyran-2-one derivatives. Int. J. ChemTech Res. 3 (2011) 555–559. |
| [20] | X. Zhou, L. Shao, Z. Jin, Synthesis and antitumor activity evaluation of some schiff bases derived from 2-aminothiazole derivatives. Heteroat. Chem. 18 (2007) 55–59. DOI:10.1002/(ISSN)1098-1071 |
| [21] | P.M.G. Swamy, B.R. Sri, D. Giles, Synthesis, anticancer, and molecular docking studies of pyranone derivatives. Med. Chem. Res. 22 (2013) 4909–4919. DOI:10.1007/s00044-013-0478-7 |
| [22] | V.S. Shruthy, Y. Shakkeela, In silico design, docking, synthesis and evaluation of thiazole schiff bases. Int. J. Pharm. Pharm. Sci. 6 (2014) 271–275. |
| [23] | J. McCauley, A. Zivanovic, D. Skropeta, Bioassays for anticancer activities, in:U. Roessner, D.A. Dias (Eds.), Methods in Molecular Biology, Metabolomics Tools for Natural Product Discovery, Humana Press, 2013, pp. 191-205. |
| [24] | P.P. Bhosale, R.S. Chavan, A.V. Bhosale, Design, synthesis, biological evaluation of thiazolyl Schiff base derivatives as novel anti-inflammatory agents. Ind. J. Chem. 51B (2012) 1649–1654. |
 2017, Vol. 28
2017, Vol. 28