b Key Laboratory of Systems Bioengineering (Ministry of Education), Tianjin University, Tianjin 300350, China
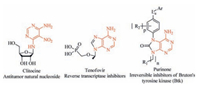
Growing efforts in the past decades were paid to develop methods toward the functionalization of pyrimidine derivatives [1]. These six-membered heterocycles are an important class of nitrogen-containing heterocyclic compounds, which either display a wide variety of biological activities by themselves [2] or can be used as key intermediates of medicinally relevant molecules [3]. A particularly interesting subset of these molecules is chlorosubstituted 5-nitropyrimidines, which are intermediates of several bioactive nucleoside analogs. For example, clitocine, an antitumor nucleoside isolated from the mushroom Clitocybe inversa, is featured with a 5-nitropyrimidine moiety [4]. Reverse transcriptase inhibitors tenofovir [5] and kinase inhibitor purinone derivatives [6] can also be synthesized from chloro-substituted 5-nitropyrimidines, which demonstrates their unique roles as milestones for substituted purine syntheses (Fig. 1) [7].
|
Download:
|
| Figure 1. Biologically active compounds synthesized from chloro-substituted 5-nitropyrimidines. | |
In the course of our ongoing syntheses of clitocine analogs, a series of modified bases are needed for studies on structureactivity relationships. The present synthetic method under aromatic nucleophilic substitution reaction (SNAr) of 4, 6-dichloro-5-nitropyrimidine and nucleophiles suffers from low selectivity, long reaction time and troublesome operation procedure [8]. On the other hand, palladium-catalyzed coupling processes have become an indispensable tool for the synthesis of bioactive substance and organic building blocks, and C-N coupling reactions of aryl halides with amines catalyzed by palladium have become an important tool for aromatic amines [9]. But to our surprise, there have been scarce investigations on Pd-catalyzed coupling of pyrimidine halides and amines [10], presumably due to their ability to inhibit and/or deactivate the palladium catalyst [11]. In this contribution, we report the scope of Pd-catalyzed amination for a number of chloro-substituted 5-nitropyrimidines with different amines.
2. Results and discussionWe initiated our studies by screening conditions for the coupling of 4, 6-dichloro-5-nitropyrimidine 1 and 4-anisidine 2 (Table 1) [12]. To improve the yield of the reaction, various temperatures, molar ratios, solvents, bases and ligands were examined. For this Pd-catalyzed coupling reaction system, the best yield is 10%-20% higher than that under original nucleophilic reaction conditions [8a]. The experimental results also prove that palladium and ligand are beneficial for this reaction (Table 1, entries 1-3). The Pd-catalyzed aryl amination reactions most likely proceed by the oxidative addition of chloro-substituted 5-nitropyrimidines to a Pd0 species, followed by the formation of an intermediate "(BINAP) Pd (Ar)(Cl)(NR1R2)" which BINAP may switch to a monodentate binding mode. The chlorine is then dissociated from the intermediate with the help of base, to yield neutral complex "(BINAP) Pd (Ar)(NR1R2)" which BINAP may switch to a bis-ligated mode. Finally the neutral complex undergoes reductive elimination to afford the desired arylamine and regenerates the Pd0 catalyst [13].
|
|
Table 1 Pd-catalyzed cross-coupling of 1 with 2 under various conditions.a |
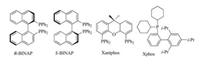
As depicted in Table 1, using Pd2(dba)3 as the precatalyst, five phosphine ligands (R-BINAP, S-BINAP, Rac-BINAP, Xantphos and Xphos, Fig. 2) [14] were screened for this reaction (entries 3-7). On the choice of ligands, we screened the most general ones for the cross-coupling reaction without regard to chirality. All other ligands examined here afforded yields inferior to BINAP for this reaction, and unsurprisingly all R-BINAP, S-BINAP and Rac-BINAP give similar results. So we chose R-BINAP as the ligand because of the highest yield. Although bases played a very important role in the Buchwald-Hartwig coupling reaction, which had different results in different solvents [13a], replacement of cerium carbonate with potassium carbonate gave similar results (yield 52.4% and 53.0%; Table 1, entries8 and 3).Sopotassium carbonatewaschosen as base for the rest of experiments due to cost. Three different temperatures are screened, and results showed that low temperature was preferable for the synthesis of the title product 3 (Table 1, entries 9-11). It could be explained by strong nucleophilicity of 4-anisidine at higher temperature and selectivity issue due to existence of two labile chlorine groups. A high molar ratio between 4, 6-dichloro-5-nitropyrimidine 1 and 4-anisidine 2 was needed for a better yield. Substantially low yields were found when lower molar ratios were used (Table 1, entries 8, 11, 12). Another commonly used solvent THF was also tried in this reaction, but it gave lower yield (Table 1, entry 13). Four experiments were carried under different strong bases and high polarity solvents to compare with the result obtained through Pd-catalyzed cross-coupling reaction. As shown inTable 1, the Pd-catalyzed reaction system has a higher yield (entries 8, 14-17).
|
Download:
|
| Figure 2. Structures of the five phosphine ligands. | |
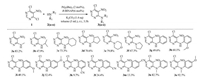
Using this optimized protocol, with Pd2(dba)3 (2mol%), R-BINAP (6mol%), and K2CO3 (1.4 equiv) in toluene, we assessed the scope of the amine coupling partners for the amination of 4, 6-dichloro-5-nitropyrimidine (Scheme 1). The palladium-catalyzed coupling reaction was proved to be efficient for a number of amines, but the yields varied greatly among different amines. As shown in Scheme 1, this system was found to be effective for electron-rich aliphatic primary and secondary amines (3a-3f). Medium yields (3g-3j) are usually afforded when primary arylamines with electron-rich substituents were used as substrates, while arylamines with electron-deficient groups such as cyano suffered from substantial drop in yields (3k-3m).
|
Download:
|
| Scheme 1. Scope of Pd-catalyzed aminations of 1 with different alkylamines and arylamines. Reaction conditions: 1 (1.5mmol), amine 2 (0.5mmol), base (0.7mmol), 5mL toluene as solvent, 25 ℃, 3.5h. All yields are isolated yield. | |
We also observed that ortho substituent exerts more influence on arylamine coupling reaction than para substituent, and meta substituent has smallest influence on reactant's activity. For example, a methoxy in ortho, meta and para position of aniline (2h-2j) gave yields as 60.1%, 49.1% and 52.4% respectively, compared with 49.6% of aniline. Another case was the ortho, meta and para-substituted cyano analogs showed the similar results to give yields as 9.7%, 24.4% and 13.3% respectively. The reason of these three low yields (3k-3m) were the substrates did not react completely. Arylamines appear to be more sensitive to the electronic effect than steric crowding and typically arylamines with electron-deficient substituents would require more reaction time or higher temperature and afford products in diminished yields. Secondary arylamines gave higher yields than corresponding primary ones (3g vs. 3n, 3j vs. 3o), which is probably due to the difficulty of further coupling of secondary amines and monosubstituted products under current reaction conditions. The results also illustrate unambiguously again that electron-rich group in secondary arylamines (methoxy, 2o) is favorable to the coupling reaction.
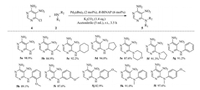
To further demonstrate the accessibility of this method to C-N coupling reaction of mono-substituted pyrimidines, we examined the reaction of 4-amino-6-chloro-5-nitropyrimidine with multiple aliphatic, heterocyclic and aromatic amines under the condition as shown in Scheme 2. Acetonitrile was selected as solvent because of products' high solubility. We can see that this reaction system was effective for synthesizing amino-substituted pyrimidines. These compounds could be used as intermediates of 6-substituted purine derivatives [15].
|
Download:
|
| Scheme 2. Scope of 4-amino-6-chloro-5-nitropyrimidine coupling with aliphatic, heterocyclic and aromatic amines. Reaction conditions: 4 (0.5 mmol), amine 2 (1.0 mmol), base (0.7 mmol), 5 mL acetonitrile as solvent, 25 ℃, 3.5 h. All yields are isolated yield. | |
Finally, we turned our attention to amination reactions using 6-chloro-5-nitropyrimidines as electrophiles to synthesize disubstituted 5-nitropyrimidines. Under the slightly modified reaction conditions, various aliphatic, heterocyclic and aromatic amines of different electronic and steric properties represent useful coupling partners for the transformation (Scheme 3). This reaction system provide a complementary tool for the synthesis of 4-((5-nitropyrimidine-4-yl) amino) benzimidamide derivatives, a potent PRMT1 inhibitor [16], and 2, 6, 9-trisubstituted purine derivatives which act as antitumor agents [17]. This synthetic methodology can be very important to exploit practical value in medicine and pharmacology.
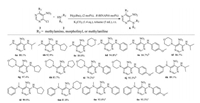
|
Download:
|
| Scheme 3. Scope of 4-amino-6-chloro-5-nitropyrimidine coupling with aliphatic, heterocyclic and aromatic amines. Reaction conditions: 4 (0.5 mmol), amine 2 (1.0 mmol), base (0.7 mmol), 5 mL acetonitrile as solvent, 25 ℃, 3.5 h. All yields are isolated yield. | |
3. Conclusion
In summary, we have developed a palladium-catalyzed C-N coupling reaction of chloro-substituted 5-nitropyrimidines and amines with high yields. Yields depended greatly on amines' electronic effect and steric crowding when synthesizing monosubstituted products. High yields are afforded when monosubstituted 5-nitropyrimidines worked as aryl halides. And the scope of this reaction system is much broad. We believe that this methodology would provide an effective and practical tool for the synthesis of pyrimidine derivatives and correlative purine compounds.
4. ExperimentalAll reactions were performed under argon atmosphere. Toluene, tetrahydrofuran and acetonitrile were dried over Å molecular sieve, fractionally distilled under reduced pressure and stored under argon atmosphere. Pd2(dba)3 was purchased from Creasyn, R-BINAP, S-BINAP, Rac-BINAP, Xantphos and Xphos were purchased from Hwrk Chem. Co., Ltd. and used as received. 5-Nitropyrimidines were purchased from commercial vendors and used as received. Amines were purchased from TCI Co., Ltd., Aladdin Co., Ltd. or J & K Co., Ltd. CDCl3 and DMSO were purchased from Henwos Co., Ltd. and used as received. 6-Chloro-N-methyl-5-nitropyrimidin-4-amine, 4-(6-chloro-5-nitropyrimidin-4-yl) morpholine and 6-chloro-N-methyl-5-nitro-N-phenylpyrimidin-4-amine were prepared according to the procedures reported in this paper. All other chemicals were obtained from commercial vendors and used as received. Reaction courses and product mixture were routinely monitored by TLC on silica gel purchased from Merck Co., Ltd. and visualized with UV and aqueous KMnO4. Melting points were determined on a Gallenkamp apparatus. NMR spectra were recorded on a Bruker Avance Ⅲ 600 spectrometer at ambient temperature. High-resolution mass spectra were obtained on a miorOTOF-QⅡ.
4.1. General procedure for reaction of amines with 4, 6-dichloro-5-nitropyrimidine (3a-3o)To a stirred solution of 4, 6-dichloro-5-nitropyrimidine (1.5 mmol), amine (0.5 mmol), Pd2(dba)3 (0.01 mmol), R-BINAP (0.03 mmol) and potassium carbonate (0.7 mmol) in toluene (5 mL) at room temperature and the mixture was under an argon atmosphere for 3.5 h. The resulting reaction mixture was filtered and evaporated. The residue was purified by column chromatography on silica gel.
4.2. General procedure for reaction of amines with 6-chloro-5-nitropyrimidine-4-amine (5a-5l)6-Chloro-5-nitropyrimidine-4-amine (0.5 mmol), amine (1.5 mmol), Pd2(dba)3 (0.01 mmol), R-BINAP (0.03 mmol) and potassium carbonate (0.7 mmol) were dissolved in acetonitrile (5 mL). The solution was stirred at room temperature for 3.5 h under an argon atmosphere. The resulting reaction mixture was treated with saturated brines (50 mL) and extracted with acetonitrile (3 × 25 mL), and dried with anhydrous Na2SO4. The anhydrous Na2SO4 was removed by filtration and the filtrate was concentrated. The residue was washed with ethyl acetate (3 × 2 mL) and diethyl ether (3 × 2 mL), filtered and dried under vacuum.
4.3. General procedure for synthesis of di-substituent pyrimidines (6a-6o)Amine (1.5 mmol), 3b (0.5 mmol), Pd2(dba)3 (0.01 mmol), R-BINAP (0.03 mmol), potassium carbonate (0.7 mmol) and toluene (5 mL) were successively added into a reactor. After stirring at room temperature for 3.5 h under an argon atmosphere, water was added. The resulting reaction mixture was extracted with EA (3 × 20 mL). The combined organic phases were dried over anhydrous Na2SO4, concentrated by rotary evaporation and purified by chromatography on silica gel.
The detailed spectral data and NMR spectra of synthesized compounds are attached as electronic Supporting information.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.11.019.
AcknowledgmentsThis research is financially supported by the National Natural Science Foundation of China (No. 21202116), Independent Innovation Foundation of Tianjin University of China (No. 2016XZC-0071), and Natural Science Foundation of Tianjin of China (No. 16JCYBJC20300).
| [1] | K. Ohta, E. Kawachi, N. Inoue, Retinoidal pyrimidinecarboxylic acids. Unexpected diaza-substituent effects in retinobenzoic acids. Chem. Pharm. Bull 48 (2000) 1504–1513. DOI:10.1248/cpb.48.1504 |
| [2] |
(a) L.M. De Coen, T.S. Heugebaert, D. Garcia, et al., Synthetic entries to and biological activity of pyrrolopyrimidines, Chem. Rev. 116(2016) 80-139; (b) A. Palasz, D. Ciez, In search of uracil derivatives as bioactive agents. Uracils and fused uracils:synthesis, biological activity and applications, Eur. J. Med. Chem. 97(2015) 582-611; (c) M. Chauhan, R. Kumar, A comprehensive review on bioactive fused heterocycles as purine-utilizing enzymes inhibitors, Med. Chem. Res. 24(2014) 2259-2282. |
| [3] |
(a) K. Toti, M. Renders, E. Groaz, et al., Nucleosides with transposed base or 4'-hydroxymethyl moieties and their corresponding oligonucleotides, Chem. Rev. 115(2015) 13484-13525; (b) Z. Jahnz-Wechmann, G. Framski, P. Januszczyk, J. Boryski, Bioactive fused heterocycles:nucleoside analogs with an additional ring, Eur. J. Med. Chem. 97(2015) 388-396. |
| [4] |
(a) T. Kamikawa, S. Fujie, Y. Yamagiwa, M. Kim, H. Kawaguchi, Synthesis of clitocine, a new insecticidal nucleoside from the mushroom Clitocybe inversa, J. Chem. Soc. Chem. Commun. (1988) 195-196; (b) K. Eger, E.M. Klunder, M. Schmidt, Synthesis of new acyclic pyrimidine nucleoside analogs as potential antiviral drugs, J. Med. Chem. 37(1994) 3057-3061; (c) H. Fortin, S. Tomasi, J.G. Delcros, et al., In vivo antitumor activity of clitocine, an exocvclic amino nucleoside isolated from Lepista inversa, ChemMedChem 1(2006) 189-196; (d) X.H. Guo, C. Liu, L.X. Zheng, et al., Novel exocyclic nucleoside related to clitocine:a convergent synthesis of 3'-azido-2'3'-dideoxy clitocine, Synlett (2010) 1959-1962; (e) X.H. Guo, H. Kang, L.X. Zheng, S.D. Jiang, Synthetic methods and biological activities of clitocine and its analogues, Chin. J. Org. Chem. 31(2011) 176-186; (f) J.G. Sun, H. Li, X. Li, et al., Clitocine targets Mcl-1 to induce drug-resistant human cancer cell apoptosis in vitro and tumor growth inhibition in vivo, Apoptosis 19(2014) 871-882. |
| [5] |
(a) M.M. Baum, I. Butkyavichene, S.A. Churchman, et al., An intravaginal ring for the sustained delivery of tenofovir disoproxil fumarate, Int. J. Pharm. 495(2015) 579-587; (b) C. Smit, J. Arends, L. Peters, et al., Effect of abacavir on sustained virologic response to HCV treatment in HIV/HCV co-infected patients, Cohere in Eurocoord, BMC Infect. Dis. 15(2015). |
| [6] |
(a) T.D. Owens, Preparation of purinone derivatives as tyrosine kinase inhibitors, US20140142099A1, 2014. (b) X. Xu, Method for preparing tenofovir intermediate (αR)-6-amino-α-methyl-9H-purine-9-ethanol, CN103408547A, 2013. |
| [7] | M.L. Read, M. Braendvang, P.O. Miranda, L.L. Gundersen, Synthesis and biological evaluation of pyrimidine analogs of antimycobacterial purines. Bioorg. Med. Chem. 18 (2010) 3885–3897. DOI:10.1016/j.bmc.2010.04.035 |
| [8] |
(a) P.G. Baraldi, A.U. Broceta, An efficient one-pot synthesis of 6-alkoxy-8, 9-dialkylpurines via reaction of 5-amino-4-chloro-6-alkylaminopyrimidines with N, N-dimethylalkaneamides and alkoxide ions, Tetrahedron 58(2002) 7607-7611; (b) D. Vanda, R. Jorda, B. Lemrova, et al., Synthesis of novel N9-substituted purine derivatives from polymer supported α-amino acids, ACS Comb. Sci. 17(2015) 426-432. |
| [9] |
(a) D. Banerjee, R.V. Jagadeesh, K. Junge, H. Junge, M. Beller, Efficient and convenient palladium-catalyzed amination of allylic alcohols with Nheterocycles, Angew. Chem. Int. Ed. 51(2012) 11556-11560; (b) M. Pompeo, J.L. Farmer, R.D.J. Froese, M.G. Organ, Room-temperature amination of deactivated aniline and aryl halide partners with carbonate base using a Pd-PEPPSI-IPentCl-o-picoline catalyst, Angew. Chem. Int. Ed. 53(2014) 3223-3226; (c) S. Sharif, R.P. Rucker, N. Chandrasoma, et al., Selective monoarylation of primary amines using the Pd-PEPPSI-IPentCl precatalyst, Angew. Chem. Int. Ed. 54(2015) 9507-9511; (d) S. Riedmueller, O. Kaufhold, H. Spreitzer, B.J. Nachtsheim, Synthesis of sterically congested triarylamines by palladium-catalyzed amination, Eur. J. Org. Chem. (2014) 1391-1394; (e) N. Khatun, A. Modi, W. Ali, B.K. Patel, Palladium-catalyzed synthesis of 2-aryl-2H-benzotriazoles from azoarenes and TMSN3, J. Org. Chem. 80(2015) 9662-9670; (f) R.A. Green, J.F. Hartwig, Palladium-catalyzed amination of aryl chlorides and bromides with ammonium salts, Org. Lett. 16(2014) 4388-4391; (g) H.M. Guo, R.Z. Mao, Q.T. Wang, et al., Pd (Ⅱ)-catalyzed one-pot, three-step route for the synthesis of unsymmetrical acridines, Org. Lett. 15(2013) 5460-5463; (h) A. Plas, C. Martin, N. Joubert, M.C. Viaud-Massuard, Palladium-catalyzed amination of N-free 2-chloro-7-azaindole, Org. Lett. 17(2015) 4710-4713. |
| [10] |
(a) D. Maiti, B.P. Fors, J.L. Henderson, Y. Nakamura, S.L. Buchwald, Palladiumcatalyzed coupling of functionalized primary and secondary amines with aryl and heteroaryl halides:two ligands suffice in most cases, Chem. Sci. 2(2011) 57-68; (b) K. Walsh, H.F. Sneddon, C.J. Moody, Amination of heteroaryl chlorides:palladium catalysis or SNAr in green solvents, ChemSusChem 6(2013) 1455-1460. |
| [11] | M. Su, N. Hoshiya, Buchwald, palladium-catalyzed amination of unprotected five-membered heterocyclic bromides. Org. Lett. 16 (2014) 832–835. DOI:10.1021/ol4035947 |
| [12] |
(a) C. Fischer, B. Koenig, Palladium-and copper-mediated N aryl bond formation reactions for the synthesis of biological active compounds, Beilstein. J. Org. Chem. 7(2011) 59-74; (b) M.D. Charles, P. Schultz, S.L. Buchwald, Efficient Pd-catalyzed amination of heteroaryl halides, Org. Lett. 7(2005) 3965-3968; (c) A.A. Trabanco, J.A. Vega, M.A. Fernandez, Fluorous-tagged carbamates for the Pd-catalyzed amination of aryl halides, J. Org. Chem. 72(2007) 8146-8148. |
| [13] |
(a) Y. Sunesson, E. Lime, S.O. Nilsson Lill, et al., Role of the base in Buchwald-Hartwig amination, J. Org. Chem. 79(2014) 11961-11969; (b) A.T. Brusoe, J.F. Hartwig, Palladium-catalyzed arylation of fluoroalkylamines, J. Am. Chem. Soc. 137(2015) 8460-8468; (c) A.S. Guram, R.A. Rennels, S.L. Buchwald, A simple catalytic method for the conversion of aryl bromides to arylamines, Angew. Chem. Int. Ed. Engl. 34(1995) 1348-1350. |
| [14] |
(a) H. Tomori, J.M. Fox, S.L. Buchwald, An improved synthesis of functionalized biphenyl-based phosphine ligands, J. Org. Chem. 65(2000) 5334-5341; (b) N. Kataoka, Q. Shelby, J.P. Stambuli, J.F. Hartwig, Air stable, sterically hindered ferrocenyl dialkylphosphines for palladium-catalyzed C C, CN, and CO bond-forming cross-coupling, J. Org. Chem. 67(2002) 5553-5566; (c) D.S. Surry, S.L. Buchwald, Biaryl phosphane ligands in palladium-catalyzed amination, Angew. Chem. Int. Ed. 47(2008) 6338-6361. |
| [15] |
(a) N. Bilbao, V. Vázquez-González, M.T. Aranda, D. González-Rodríguez, Synthesis of 5-/8-halogenated or ethynylated lipophilic nucleobases as potential synthetic intermediates for supramolecular chemistry, Eur. J. Org. Chem. 2015(2015) 7160-7175; (b) S. Allu, K.C.K. Swamy, Ruthenium-catalyzed oxidative annulation of 6-anilinopurines with alkynes via C-H activation:synthesis of indolesubstituted purines/purine nucleosides, Adv. Synth. Catal. 357(2015) 2665-2680. |
| [16] | X.R. Yu, Y. Tang, W.J. Wang, Discovery and structure-activity analysis of 4-((5-nitropyrimidine-4-yl) amino) benzimidamide derivatives as novel protein arginine methyltransferase 1(PRMT1) inhibitors. Bioorg. Med. Chem. Lett. 25 (2015) 5449–5453. DOI:10.1016/j.bmcl.2015.06.095 |
| [17] |
(a) J. Calderon-Arancibia, C. Espinosa-Bustos, A. Canete-Molina, et al., Synthesis and pharmacophore modelling of 2, 6, 9-trisubstituted purine derivatives and their potential role as apoptosis-inducing agents in cancer cell lines, Molecules 20(2015) 6808-6826; (b) E. Reznickova, A. Popa, T. Gucky, et al., 2, 6, 9-Trisubstituted purines as CRK3 kinase inhibitors with antileishmanial activity in vitro, Bioorg. Med. Chem. Lett. 25(2015) 2298-2301. |
 2017, Vol. 28
2017, Vol. 28 



