Heterocycles are frequently found in a huge number of pharmaceutical drugs, natural products, agrochemicals, functional materials and fine chemicals. The pyridine nucleus is prevalent in numerous natural products and is extremely important in the chemistry of biological systems [1]. Derivatives of pyridine were found to have various biological activities. Amongst these derivatives, fused analogues are often of greater biological interest than the corresponding monocyclic compounds [2]. Furthermore, it should be emphasized that the combination of pyridine with other hetero cycles is a well-known approach for drug-like molecules buildup.
Similarly, pyrimidines represent a class of heterocyclic compounds of great importance in biological chemistry. Moreover, fused pyrimidines, for example, thiazolo[3, 2-a]pyrimidines are known to exhibit versatile biological activity such as anticancer [3], antitumour [4], anti-inflammatory [5], antinociceptive [6], antiviral [7], and antibiofilm properties [8], while the thiazolopyrimidine skeleton is present in drugs proposed as immunomodulators (TEI 3096) [9]. Owing to these remarkably broad pharmacological properties, a variety of synthetic methods have been reported for the preparation of thiazolo[3, 2-a]pyrimidinone derivatives [10-12].
On the other hand, pyridopyrimidines were reported to exhibit antitumour activity which may be attributed to inhibition of cyclin dependent kinase [13], check point kinase [14]. In addition, pyridothienopyrimidines showed antiallergic activity [15].
Recently, we reported the synthesis of novel fused benzofuroand pyridothieno-fused thiazolo[3, 2-a]pyrimidinones via the Pictet-Spengler reaction [16]. In continuation of our interest on the construction of complex thiazolo[3, 2-a]pyrimidine skeletons, herein we report the synthesis of some new fused heterocyclic systems, thieno[30, 20:2, 3]pyrido[4, 5-d]thiazolo[3, 2-a]pyrimidinones via modified Pictet-Spengler reaction (Scheme 1).

|
Download:
|
| Scheme 1. Synthesis of thienolopyrido-fused thiazolopyrimidines. | |
2. Results and discussion
The Pictet-Spengler reaction [17] has become one of the most prominentstrategies forcarbon-carbonbond formationinsynthetic organic chemistry with excellent functional group tolerance, regioand stereo-selectivity. This prompted us to synthesize thieno-[30, 20:2, 3]pyrido[4, 5-d]thiazolo[3, 2-a]pyrimidinones 5 using the modified Pictet-Spengler reaction. It was envisaged that 5 could be obtained by subjecting 7-(3-amino-5-phenylaminothiazolo-2-yl)-5H-thiazolo[3, 2-a]-pyrimidin-5-one 3 to 6-endo cyclization by treating with aromatic aldehydes (Scheme 1).
In this study, the key intermediate amine, 7-(3-amino-5-phenylaminothiazolo-2-yl)-5H-thiazolo[3, 2-a]pyrimidin-5-one (3) was obtained by the condensation of 7-chloromethyl-5Hthiazolo[3, 2-a]pyrimidin-5-one (1) with potassium-(2, 2-dicyano-1-phenylamino ethen-1-yl) thiolate (2) via Thorpe-Ziegler isomerization [18] in 82% yield. Elemental analysis (C17H11N5OS2) and spectral data supported its structure. Its IR spectrum contains absorption peaks at 3435, 3350, 1689cm-1, demonstrating the presence of NH and C=O functions, respectively. Its 1H NMR spectrum (DMSO-d6) shows the presence of a D2O exchangeable broad singlet at δ 7.02 (2H) and 10.32ppm (1H) which can be attributed to the NH2 and NH protons, respectively. The singlet peak at δ 5.61 corresponding to C6-H of the thiazolo[3, 2-a]-pyrimidine nucleus. The multiplet between 7.19-7.92ppm (7H) corresponding to the aromatic protons of benzene and thiazole nucleus.
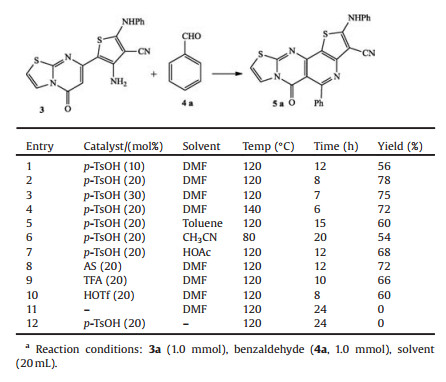
In an initial endeavour, we selected benzaldehyde 4a as model aryl benzaldehyde to react with equimolar amounts of intermediate amine 3 for the preparation of thieno[30, 20:2, 3]pyrido[4, 5-d-thiazolo[3, 2-a]pyrimidin-5-one 5a and investigated the optimal reaction conditions. The screened results were listed in Table 1.
|
|
Table 1 Optimization of reaction conditions on the synthesis of thieno[30, 20:2, 3]pyrido[4, 5-d]thiazolo[3, 2-a]pyrimidin-5-one 5a.a |
To our delight, the good result could be gained when p-TsOH was used as catalyst. The reactions were checked in different solvents, and itwas foundthat thebestyield could be giveninDMF. Later, the loading amount of p-TsOH was investigated in DMF, and the excellentyield could be foundunder theamountof 0.2 mmol p-TsOH (entry 2). Other organic acids such as sulfamic acid (SA), trifluoroacetic acid (TFA) and CF3SO3H (HOTf) were also tested under similar reactions and 72%, 66% and 60% yields were obtained, respectively (entries 8-10). The results indicated that p-TsOH was still the best choice. In addition, the reactions were also checked under catalyst-free (entry 11) and solvent-free (entry 12) conditions, and the results show that the reactions could notbe carried out at all. Finally, best result involving complete conversion was obtained only when reaction was carried out in 20% p-TsOH in DMF at 120 ℃.
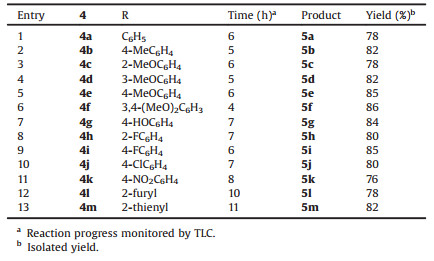
With the optimization of reaction conditions in hand, we then tested the scopes of the substrates carefully. A series of thienopyridine fused thiazolo[3, 2-a]pyrimidin-5-one derivatives could be gained with good yields. The substrate scope of the p-TsOH catalysed coupling of intermediate amine 3 with aromatic aldehydes 4 (Scheme 1) is shown in Table 2 and it was found that this protocol could be applied not only to the aromatic aldehydes with either electron-donating groups (e.g., methyl, methoxy, and hydroxy) (entries 2-7) or electron-withdrawing groups (e.g., fluoro, chloro, and nitro groups) (entries 8-11), but also to heterocyclic aldehydes (entries 12, 13). Therefore, it could be known that the electronic nature of the substituents has no significant effect on this reaction.
|
|
Table 2 Synthesis of thieno[30, 20:2, 3]pyrido[4, 5-d]thiazolo[3, 2-a]pyrimidin-5-ones (5). |
All the products were characterized by IR, 1H NMR, 13C NMR and elemental analysis. And all the data is consistent with the desired structures.
On the basis of these results, a plausible mechanism for the construction of fused thiazolo[3, 2-a]pyrimidinones is proposed (Scheme 2). The formation of ether A occurs through S-alkylation of 7-chloromethyl-5H-thiazolo[3, 2-a]pyrimidin-5-one 1 andpotassium-(2, 2-dicyano-1-phenylaminoethen-1-yl) thiolate (2). Then, the ether A occurred via Thorpe-Ziegler isomerization to generate 7-(3-amino-4-cyano-5-phenylaminothieno-2-yl)-5Hthiazolo[3, 2-a]pyrimidin-5-one (3). Next, the intermediate amine 3 underwent a cationic p-cyclization with aldehyde (4) via Pictet-Spengler reaction to form D, which effects aromatisation to give product 5.

|
Download:
|
| Scheme 2. A proposed mechanism for the formation of 5. | |
3. Conclusion
In summary, we have developed an efficient synthesis of thieno-[30, 20:2, 3]pyrido[4, 5-d]thiazolo[3, 2-a]pyrimidinones in two steps with good yields in the presence of p-TsOH. This method has the advantages of readily available starting materials, mild reaction conditions, andoperationalsimplicity.Furtherstudyisunderwayto the scope of this methodology for some new fused heterocyclic systems.
4. Experimental 4.1. Preparation of 7-(3-amino-4-cyano-5-phenylaminothieno-2-yl)-5H-thiazolo[3, 2-a]pyrimidin-5-one (3)To a solution of 7-chloromethyl-5H-thiazolo[3, 2-a]pyrimidin-5-one 1 [19] (2.01 g, 10.0 mmol) in DMF (25 mL) was added potassium-(2, 2-dicyano-1-phenylaminoethen-1-yl) thiolate 2 [20] (2.39 g, 10.0 mmol) and anhydrous potassium carbonate (2.76 g, 20.0 mmol). The mixture was heated at 100 ℃ for 5 h. After the completion of the reaction judged by TLC analysis, the reaction mixture was cooled to r.t., and then water (50 mL) was added to the mixture and stirred for 20 min. The solid was filtered and recrystallized from HOAc to give 3 (3.0 g, 82%). Yellow crystals; Mp > 300 ℃; IR (KBr, cm-1): v 3435, 3350 (NH), 1689 (C=O); 1H NMR (400 MHz, DMSO-d6): δ 5.61 (s, 1H), 7.02 (s, 2H), 7.19-7.21 (m, 1H), 7.39 (d, 1H, J=4.8 Hz), 7.43-7.45 (m, 4H), 7.92 (d, 1H, J=4.8 Hz), 10.32 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ 183.4, 176.1, 164.7, 160.1, 156.6, 135.1, 130.7, 130.0, 129.6, 123.4, 121.7, 108.7, 105.0, 104.8, 79.4. Anal. Calcd. for C17H11N5OS2: C=.87, H 3.03, N 19.16. Found: C=.93, H 3.12, N 19.24.
4.2. Preparation of 6-aryl-8-cyano-9-phenylamino-5H-thieno[30, 20:2, 3]pyrido[4, 5-d]thiazolo[3, 2-a]pyrimidin-5-one derivativesTo a stirred solution of 7-(3-amino-4-cyano-5-phenylaminothieno-2-yl)-5H-thiazolo[3, 2-a]pyrimidin-5-one (3) (365 mg, 1.0 mmol), aromatic aldehyde (1.0 mmol), and p-TsOH (20 mg, 0.1 mmol) in DMF (20 mL) was added and heated to 120 ℃. After the completion of the reaction judged by TLC analysis, the reaction mixture was cooled to r.t., and then water (20 mL) was added to the mixture. The solid was filtered and recrystallized from DMF to afford the corresponding products (5a-m). Yield of the products is summarized in Table 2, and the physical and spectral data are summarized below:
5a: Mp > 300 ℃. IR (KBr, cm-1): v 3341 (NH), 1679 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 7.27 (d, 1H, J=4.8 Hz), 7.49-7.52 (m, 3H), 7.57-7.69 (m, 7H), 7.71-7.73 (m, 1H), 8.05 (d, 1H, J=4.8 Hz); 13C NMR (100 MHz, CF3CO2D): δ 172.3, 168.9, 159.0, 1=.9, 149.9, 144.6, 136.9, 132, 130.1, 129.2, 129.0, 128.7, 127.7, 123.0, 122.2, 117.1, 113.0, 110.4, 104.7, 76.3. Anal. Calcd. for C24H13N5OS2: C 63.84, H 2.90, N 15.51. Found: C 63.92, H 2.97, N 15.60.
5b: Mp > 300 ℃. IR (KBr, cm-1): v 3338 (NH), 1683 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 2.53 (s, 3H), 7.30 (d, 1H, J=4.8 Hz), 7.45-7.56 (m, 8H), 7.60-7.64 (m, 2H), 8.09 (d, 1H, J=4.8 Hz); 13C NMR (100 MHz, CF3CO2D): δ 172.3, 168.8, 159.4, 156.1, 150.0, 144.6, 144.2, 136.9, 130.1, 129.4, 129.2, 127.8, 127.0, 123.0, 122.3, 116.8, 113.1, 110.0, 104.6, 76.3, 19.5 Anal. Calcd. for C25H15N5OS2: C 64.50, H 3.25, N 15.04. Found: C 64.58, H 3.37, N 15.13.
5c: Mp > 300 ℃. IR (KBr, cm-1): v 3343 (NH), 1680 cm-1 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 3.83 (s, 3H), 7.17-7.22 (m, 2H), 7.29 (d, 1H, J=4.8 Hz), 7.38-7.40 (m, 1H), 7.54-7.62 (m, 6H), 7.67-7.71 (m, 1H), 8.08 (d, 1H, J=4.8 Hz, Arom-H); 13C NMR (100 MHz, CF3CO2D): δ 172.1, 168.7, 156.6, 156.5, 1=.6, 149.6, 145.0, 136.9, 134.0, 130.2, 129.2, 128.8, 123.1, 123.0, 122.3, 121.0, 119.2, 116.7, 113.1, 111.3, 106.0, 76.3, 54.8. Anal. Calcd. for C25H15N5O2S2: C 62.35, H 3.14, N 14.54. Found: C 62.43, H 3.21, N 14.62.
5d: Mp > 300 ℃. IR (KBr, cm-1): v 3345 (NH), 1684 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 4.05 (s, 3H), 7.25-7.30 (m, 2H), 7.31 (d, 1H, J=4.8 Hz), 7.38-7.40 (m, 1H), 7.53-7.66 (m, 7H), 8.09 (d, 1H, J=4.8 Hz); 13C NMR (100 MHz, CF3CO2D): δ 172.3, 168.9, 158.5, 157.9, 1=.8, 149.8, 144.7, 136.9, 131.6, 130.5, 130.2, 130.1, 129.2, 123.0, 122.2, 121.1, 117.3, 116.7, 115.0, 113.1, 104.9, 76.2, =.1. Anal. Calcd. for C25H15N5O2S2: C 62.35, H 3.14, N 14.54. Found: C 62.43, H 3.23, N 14.61.
5e: Mp > 300 ℃. IR (KBr, cm-1): v 3341 (NH), 1686 (C=O); 1H NMR (400 MHz, CF3CO2D): δ 4.05 (s, 3H), 7.22 (d, 2H, J=8.0 Hz), 7.29 (d, 1H, J=4.8 Hz), 7.60-7.63 (m, 8H), 8.08 (d, 1H, J=4.8 Hz); 13C NMR (100 MHz, CF3CO2D): δ 172.3, 168.8, 162.1, 158.7, 156.1, 150.0, 144.6, 137.0, 130.4, 130.2, 129.2, 123.1, 123.0, 122.3, 116.7, 114.5, 113.1, 110.4, 104.6, 76.3, 54.9. Anal. Calcd. for C25H15N5O2S2: C 62.35, H 3.14, N 14.54. Found: C 62.46, H 3.22, N 14.63.
5f: Mp > 300 ℃. IR (KBr, cm-1): v 3337 (NH), 1681 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 4.00 (s, 3H), 4.08 (s, 3H), 7.21-7.23 (m, 2H), 7.31 (d, 1H, J=4.8 Hz), 7.33 (s, 1H), 7.53-7.64 (m, 6H), 8.11 (d, 1H, J=4.8 Hz); 13C NMR (100 MHz, CF3CO2D): δ 172.2, 168.8, 158.1, 1=.9, 151.8, 149.9, 148.3, 144.6, 136.9, 130.1, 129.2, 123.0, 122.9, 122.7, 122.3, 116.9, 113.1, 112.1, 111.3, 110.4, 104.7, 76.3, =.3, =.0. Anal. Calcd. for C26H17N5O3S2: C 61.04, H 3.35, N 13.69. Found: C 61.12, H 3.43, N 13.75.
5g: Mp > 300 ℃. IR (KBr, cm-1): v 3343 (NH), 3335 (OH), 1684 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 4.05 (s, 3H), 7.22-7.24 (m, 3H), 7.33 (d, 1H, J=4.8Hz), 7.54-7.64 (m, 8H), 8.12 (d, 1H, J=4.8Hz); 13C NMR (100MHz, CF3CO2D): d172.3, 168.8, 158.6, 157.8, 156.0, 150.0, 144.6, 136.9, 130.5, 130.1, 129.2, 123.3, 123.0, 122.3, 116.8, 116.1, 115.8, 113.1, 104.6, 76.3. Anal. Calcd. for C24H13N5O2S2: C 61.66, H 2.80, N 14.98. Found: C 61.73, H 2.87, N 15.06.
5h: Mp>300 ℃. IR (KBr, cm-1): v 3347 (NH), 1689 (C=O). 1H NMR (400MHz, CF3CO2D): δ 7.33-7.34 (m, 2H), 7.37 (d, 1H, J=4.8Hz), 7.46-7.67 (m, 7H), 7.78-7.80 (m, 1H), 8.13 (d, 1H, J=4.8Hz); 13CNMR (100MHz, CF3CO2D): δ 172.3, 169.1, 159.1, 1=.6, 152.8, 149.7, 145.2, 136.9, 134.6, 134.5, 130.2, 129.3, 129.1, 124.7, 123.1, 122.3, 117.8, 116.0, 115.8, 113.1, 106.0, 76.4. Anal. Calcd. for C24H14FN5OS2: C 61.39, H 2.58, N 14.92. Found: C 61.46, H 2.65, N 14.98.
5i: Mp>300 ℃. IR (KBr, cm-1): v 3349 (NH), 1686 (C=O). 1H NMR (400MHz, CF3CO2D): δ 7.21-7.25 (m, 3H), 7.42-7.54 (m, 8H), 7.99 (d, 1H, J=4.8Hz); 13C NMR (100MHz, CF3CO2D): d 172.3, 169.0, 157.7, 1=.9, 149.9, 144.7, 136.9, 130.5, 130.2, 129.9, 129.2, 129.0, 126.0, 123.0, 122.7, 122.3, 116.2, 113.1, 104.9, 76.3. Anal. Calcd. for C24H12FN5OS2: C 61.39, H 2.58, N 14.92. Found: C 61.48, H 2.68, N 15.01.
5j: Mp>300 ℃. IR (KBr, cm-1): v 3345 (NH), 1682 (C=O). 1H NMR (400MHz, CF3CO2D): d7.08 (d, 1H, J=4.8Hz), 7.33-7.41 (m, 10H), 7.86 (d, 1H, J=4.8Hz); 13C NMR (100MHz, CF3CO2D): δ 172.5, 169.2, 157.9, 150.2, 156.9, 145.0, 139.8, 139.6, 137.2, 130.4, 129.6, 129.5, 129.3, 128.6, 123.3, 122.5, 117.7, 113.4, 105.1, 76.3. Anal. Calcd. for C24H12ClN5OS2: C 59.32, H 2.49, N 14.41. Found: C 59.39, H 2.56, N 14.48.
5k: Mp>300 ℃. IR (KBr, cm-1): v 3351 (NH), 1689 (C=O). 1H NMR (400MHz, CF3CO2D): d7.16 (d, 1H, J=4.8Hz), 7.43-7.44 (m, 6H), 7.71 (d, 2H, J=6.8Hz), 7.91 (d, 1H, J=4.8Hz), 8.38 (d, 2H, J=6.8Hz); 13C NMR (100MHz, CF3CO2D): δ 171.2, 169.0, 1=.2, 1=.0, 150.0, 149.2, 144.7, 136.9, 136.7, 130.4, 129.7, 129.4, 123.8, 123.1, 123.0, 122.3, 118.4, 113.1, 105.4, 76.3. Anal. Calcd. for C24H12N6O3S2: C 58.06, H 2.44, N 16.93. Found: C 50.13, H 2.=, N 17.01.
5l: Mp>300 ℃. IR (KBr, cm-1): v 3346 (NH), 1680 (C=O); 1H NMR (400MHz, CF3CO2D): δ 6.93-6.94 (m, 1H), 7.34 (d, 1H, J=4.8Hz), 7.53-7.63 (m, 5H), 7.96 (d, 1H, J=4.8Hz), 8.23 (d, 1H, J=4.8Hz), 8.25-8.51 (m, 2H); 13C NMR (100MHz, CF3CO2D): d 172.2, 164.4, 154.9, 149.2, 149.0, 142.7, 136.8, 130.1, 130.0, 129.0, 127.2, 126.6, 123.0, 122.9, 122.8, 122.5, 114.5, 114.3, 102.2, 76.0. Anal. Calcd. for C22H11N5O2S2: C 59.85, H 2.51, N 15.86. Found: C 59.93, H 2.24, N 15.97.
5m: Mp>300 ℃. IR (KBr, cm-1): v 3340 (NH), 1678 (C=O). 1H NMR (400MHz, CF3CO2D): δ 7.38-7.39 (m, 2H), 7.41-7.62 (m, 3H), 7.68-7.72 (m, 4H), 7.95 (d, 1H, J=4.8Hz), 8.20(d, 1H, J=4.8Hz); 13C NMR (100MHz, CF3CO2D): δ 172.4, 168.9, 1=.8, 152.3, 149.9, 144.8, 137.0, 134.2, 132.8, 132.4, 130.2, 129.3, 129.0, 127.7, 123.1, 122.4, 117.6, 113.2, 105.5, 76.3. Anal. Calcd. for C22H11N5OS3: C 57.75, H 2.42, N 15.31. Found: C 57.43, H 2.49, N 15.40.
AcknowledgmentsThis work was partially supported by innovation teamprojectof Liaoning Province Education Department (No. 2015001).
| [1] | G. Bringmann, Y. Reichert, V.V. Kane, The total synthesis of streptonigrin and related antitumor antibiotic natural products. Tetrahedron 60 (2004) 3539–3574. DOI:10.1016/j.tet.2004.02.060 |
| [2] | V.P. Litvinov, V.V. Dotsenko, S.G. Krivokolysko, The chemistry of thienopyridine. Adv. Heterocycl. Chem. 93 (2007) 117–178. DOI:10.1016/S0065-2725(06)93003-7 |
| [3] | E.E. Flefel, M.A. Salama, M. El-Shahat, A novel synthesis of some new pyrimidine and thiazolopyrimidine derivatives for anticancer evaluation. Phosphorus Sulfur Silicon Relat. Elem. 182 (2007) 1739–1756. DOI:10.1080/10426500701313912 |
| [4] | A.A. Abu-Hashem, M.M. Youssef, H.A.R. Hussein, Synthesis, antioxidant, antituomer activities of some new thiazolopyrimidines, pyrrolothiazolo pyrimidines and tria-zolopyrrolothiazolopyrimidines derivatives. J. Chin. Chem. Soc. 58 (2011) 41–48. DOI:10.1002/jccs.201190056 |
| [5] | B. Tozkoparan, M. Ertan, P. Kelicen, R. Demirdamar, Synthesis and anti-inflammatoryactivities of some thiazolo[3, 2-a]pyrimidine derivatives, Ⅱ. Farmaco 54 (1999) 588–593. DOI:10.1016/S0014-827X(99)00068-3 |
| [6] | O. Alam, S.A. Khan, N. Siddiqui, W. Ahsan, Synthesis and pharmacological evaluation of newer thiazolo[3, 2-a]pyrimidines for anti-inflammatory and antinociceptive activity. Med. Chem. Res. 19 (2010) 1245–1258. DOI:10.1007/s00044-009-9267-8 |
| [7] | S.F. Mohamed, E.M. Flefel, A.E. Amra, D.N. Abd El-Shafy, Anti-HSV-1 activity and mechanism of action of some new synthesized substituted pyrimidine, thiopyrimidine and thiazolopyrimidine derivatives. Eur. J. Med. Chem. 45 (2010) 1494–1501. DOI:10.1016/j.ejmech.2009.12.057 |
| [8] | B. Pan, R. Huang, L. Zheng, Thiazolidione derivatives as novel antibiofilm agents:design, synthesis, biological evaluation, and structure-activity relationships. Eur. J. Med. Chem. 46 (2011) 819–824. DOI:10.1016/j.ejmech.2010.12.014 |
| [9] | K. Komoriya, M. Tsuchimoto, T. Naruchi, Immunopharmacological profile of Tei-3096:a new immunomodulator. J. Immunopharmacol. 4 (2008) 285–301. |
| [10] | I.V. Kulakov, Intramolecularcyclization of 4-aryl-3, 4-dihydropyrimidine-(1H)-2-thiones to give bicyclic thiazolo[3, 2-a]pyrimidines. Chem. Heterocycl. Compd. 45 (2009) 1019–1021. DOI:10.1007/s10593-009-0374-8 |
| [11] | A.E. Abbas, Z. Mahdieh, R.F. Ali, An efficient regioselective synthesis of highly functionalized 3-oxo-2, 3-dihydro-5H-thiazolo[3, 2-a] pyrimidines via an isocyanide-based three-component reaction. Tetrahedron Lett. 53 (2012) 1351–1353. DOI:10.1016/j.tetlet.2012.01.005 |
| [12] | E.A. Abd El-Galil, S.S. Maigali, M.M. Abdulla, Synthesis, and analgesic and antiparkinsonian activities of thiopyrimidine, pyrane, pyrazoline, and thiazolopyrimidine derivatives from 2-chloro-6-ethoxy-4-acetylpyridine. Monatsh. Chem. 139 (2008) 1409–1415. DOI:10.1007/s00706-008-0937-x |
| [13] | S.N. VanderWel, P.J. Harvey, D.J. McNamara, Pyrido[2, 3-d]pyrimidin-7-ones as specific inhibitors of cyclindependent kinase 4. J. Med. Chem. 48 (2005) 2371–2387. DOI:10.1021/jm049355+ |
| [14] | B.D. Palmer, J.B. Smaill, G.W. Rewcastle, Structure-activity relationships for 2-anilino-6-phenylpyrido[2, 3-d]pyrimidin-7(8H)-ones as inhibitors of the cellular checkpoint kinase Wee1. Bioorg. Med. Chem. Lett. 15 (2005) 1931–1935. DOI:10.1016/j.bmcl.2005.01.079 |
| [15] | J.M. Quintela, C. Peinador, C. Veiga, Synthesis and antiallergic activity of pyridothienopyrimidines. Bioorg. Med. Chem. Lett. 6 (1998) 1911–1925. DOI:10.1016/S0968-0896(98)00150-3 |
| [16] |
(a) D.L. Wang, D. Wang, L. Yan, et al., Synthesis of novel benzofurano-fused thiazolo[3, 2-a]pyrimidines via Pictet-Spengler reaction, Heterocycles 92(2016) 552-5559; (b) D.L. Wang, D. Wang, L. Yan, et al., An efficient synthesis of novel pyridothieno-fused thiazolo[3, 2-a]pyrimidinones via Pictet-Spengler reaction, Chin. Chem. Lett. 27(2016) 953-956. |
| [17] |
(a) S.W. Youn, The Pictet-Spengler reaction:efficient carboncarbon bond forming reaction in heterocyclic synthesis, Org. Prep. Proced. Int. 38(2006) 505-591; (b) B. Kundu, P.K. Agarwal, S.K. Sharma, et al., Pictet-Spengler reaction revisited:engineering of tetherd biheterocycles into annulated polyheterocycles, Curr. Org. Synth. 9(2012) 357-376. |
| [18] |
(a) A.M. Shestopalov, A.E. Fedorov, P.A. Belyakov, Studyof the orientation of the Thorpe-Ziegler reaction, Chem. Heterocycl. Compd. 36(2000) 609-610; (b) V. Gefenas, Ž. Stankevičūte, A. Malinauskas, Novel method for the synthesis of furo[2, 3-d]pyrimidines by cyclization of 4-(phenacyloxy) pyrimidine-5-carbonitriles, Heterocycl. Compd. 46(2010) 372-374. |
| [19] | S. Djekou, A. Gellisa, H. El-Kashef, P. Vanelle, An efficient synthesis of new thiazolopyrimidinones under microwave irradiation. J. Heterocycl. Chem. 43 (2006) 1225–1229. DOI:10.1002/jhet.v43:5 |
| [20] | M. Bakavoli, H. Beyzaei, M. Rahimizadeh, One-pot synthesis of functionalized tetrahydro-1, 4-thiazepines. Synth. Commun. 41 (2011) 1181–1185. DOI:10.1080/00397911003797908 |
 2017, Vol. 28
2017, Vol. 28