b Key Lab. of Biomass Energy and Material, Jiangsu Province, Nanjing 210042, China;
c National Engineering Lab. for Biomass Chemical Utilization, Nanjing 210042, China;
d Key and Open Lab. on Forest Chemical Engineering, State Forestry Administration, Nanjing 210042, China;
e Institute of Forestry New Technology, Chinese Academy of Forestry, Beijing, 100091, China;
f 2011 Collaborative Innovation Center of Jiangxi Typical Trees Cultivation and Utilization in Jiangxi Agricultural University, Nanchang, 330045, China
With the rapid decrease of the fossil resources, there is an everincreasing interest for the utilization of renewable biomass resources in making more valuable products [1, 2]. Turpentine, obtained by collecting and isolating the oleosus exudates of living pine trees, is the most important and cheapest monoterpene resources all over the world [3]. As the major component of turpentine, β-pinene (1, Fig. 1) contributes to 30% in raw turpentine materials [4]. Due to the presence of a double bond and a strained four-membered ring, β-pinene can be transformed readily to produce a number of useful products for pharmaceutical and other industrial applications [5-7].
|
Download:
|
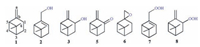
| Figure 1. Structure of β-pinene and its aerobic oxidation products over the catalysis of metallodeuteroporphyrins (MDPs). | |
β-Pinene oxidation products such as myrtenol (2) and pinocarveol (3) are valuable pharmaceuticals, fragrance ingredients or chemical intermediates [8, 9]. Generally, direct allylic oxidation of β-pinene by stoichiometric oxidants such as peracids, SeO2 or H2O2 is the most efficient processes for the production of 2and 3 [10-12]. These procedures have been now rejected because a large amount of toxic waste could be produced in these systems and caused great environmental impacts. In contrast to stoichiometric oxidants, molecular oxygen is an excellent oxidant due to its inexpensive, eco-friendly and easily available characteristics [13]. However, because of the inertness of molecular oxygen and the complex molecular structure of β-pinene, selective aerobic oxidation of β-pinene by molecular oxygen is still amongst the major challenges in academic and industrial research [14].
Metalloporphyrins (MPs) are efficient selective catalysts applied widely for direct aerobic allylic oxidation of hydrocarbons [15-17]. But most are used under the assistance of solvents, reductants and cocatalysts. MPs catalyzed aerobic oxidation of hydrocarbons in absence of solvents and additives has bright industry application prospect since only eco-friendly and readily available molecular oxygen were needed. Aerobic oxidation of simple hydrocarbons catalyzed by MPs in solvent and additive free system has been studied [18, 19]. However, to the best of our knowledge, selective allylic oxidation of complex alkenes in MPs catalyzed aerobic oxidation systems in absence of solvents and additives has not been reported before.
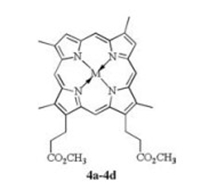
In this work, an efficient metallodeuteroporphyrins (MDPs) catalyzed selective aerobic allylic oxidation method of β-pinene (Fig. 1, containing one C=C bond and six different C--H bonds in a molecule) was established. Allylic hydroxylation products could be obtained with high selectivity under the catalyzing of metallodeuteroporphyrin dimethyl esters (Fig. 2, MDPDMEs, 4a-4d) in absence of solvents and additives because of the high reactivity of allylic C--H bonds of β-pinene in this aerobic oxidation system. The effects of reactionparameters and metal nuclei of MDPDMEs on this reaction were investigated. The possible reaction mechanism and the role of MDPDMEs in this procedure were initially discussed.
|
Download:
|
| Figure 2. Formula of MDPDMEs mentioned in the text. M=FeCl (4a), Co (4b), MnCl (4c), Cu (4d). | |
2. Results and discussion 2.1. Aerobic oxidation of β-pinene under the catalysis of MDPs
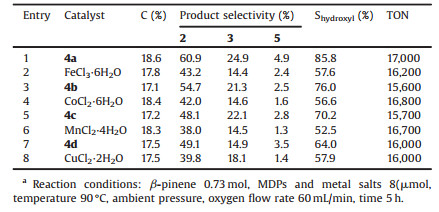
According to GC, GC-MS and chemical analysis data, the oxidation products consisted of 2, 3, pinocarvone (5) and trace amount of 2, 10-epoxypinane (6) and hydroperoxides (HPs). HPs are proved to be myrtenyl-hydroperoxide (7) and pinocarvyl-hydroperoxide (8) [4]. Products 2, 3 and 5 belonged to the oxidation of allylic C--H bonds. Product 6 was attributed to the epoxidation of p-bond. Products 7 and 8 belonged to the hydroperoxidation of allylic C--H bonds. β-Pinene could be oxidized by dioxygen in the absence of any catalysts under similar parameters (Table 1). But the selectivity (S) of allylic hydroxylation products is much lower than the values under the catalysis of MDPs, indicating that MDPs acted as critical regioselective catalysts in this reaction. The accumulation of HPs was detected at the initial of this reaction. But HPs could be decomposed under the catalysis of MPs [19]. When this reaction proceeded for 5 h, the yield of HPs decreased to a stable value of about 0.5% while the conversion (C) value of β-pinene reached 18.6%, indicating a completely decomposition of HPs in this protocol.
|
|
Table 1 Comparison for aerobic hydroxylation oxidation of β-pinene at different reaction temperatures. |
2.2. Optimization of the working conditions
MPs are important biomimetic catalysts with bright industry application prospect. In order to obtain the optimal reaction conditions, FeClDPDME was selected as a model to investigate the influence of various working conditions on this reaction. Table 1 listed the C values of β-pinene and the S values of various oxidation products of this reaction at different temperatures. The results showed thatthe C valuesincreased withreaction temperatureat the temperaturerange from70 -Cto110 -C.The total selectivity of allylic hydroxylation products 2 and 3 reached a maximal value of 85.8% at 90 -C. It has been reported that only high-spin unsteady state perfluorinated or poly-halogenated MPs can activate molecular oxygen under ambient pressure in this solvent and additive free system [18]. The reductive potentials of simple MPs such as metallotetraphenylporphyrines (MTPPs) and MDPs are so low that they can be easily reduced to their high-spin unsteady state by thermal decomposition reduction [18, 19]. Thus, the catalytic of MDPs on aerobic oxidation of β-pinene at low reaction temperatures plays a trivial role, which leads to the decrease of S values of hydroxylation products 2 and 3. When the reaction temperature was higher than 100 -C, the selectivity of 2 and 3 decreased dramatically due to the over-oxidation of initial oxidation products.
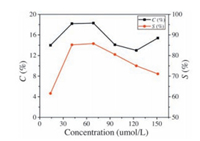
In our previous research, catalyst concentration was found to be a critical factor in MPs biomimetic catalyzed reactions. Fig. 3 showed the conversion of β-pinene and the selectivity of various oxidation products over the catalysis of different concentration MDPs from 13.7 umol/L to 151.2 umol/L. The results indicated that the conversion of β-pinene and the selectivity of allylic C-H oxidation products increased with the increasing of FeClDPDME concentration when the catalyst concentration was lower than 68.7 umol/L. Higher concentration of FeClDPDME would lead to the formation of inactive μ-oxo metalloporphyrin dimers [20, 21]. Therefore, although more catalyst was added, the efficient concentration of active catalyst reduced and caused the decrease of C and S values in this reaction.
|
Download:
|
| Figure 3. Effects of catalyst concentration C and S values of this reaction. Reaction conditions: temperature 90 (℃), ambient pressure and time 5 (h). | |
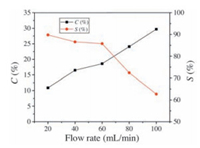
The effect of oxygen flow rate on conversion and product selectivity were also investigated. As illustrated in Fig. 4, the conversion of β-pinene increased with the increase of the flow rate of oxygen at flow rate less than 60 mL/min. If the oxygen flow rate was higher than 80 mL/min, the selectivity of product 2 and 3 decreased evidently with the increase of oxygen flow rate. This phenomenon might be explained as follows: The dissolved oxygen in liquid phase increased with the increase of the oxygen flow rate. Thus, the production of 2 and 3 increased with the increase of oxygen flow rate. However, the higher flow rate of oxygen would accelerate the over-oxidation of 2 and 3 to other by-products.
|
Download:
|
| Figure 4. Effects of oxygen flow rate on C and S values of this reaction. Reaction conditions: β-pinene 0.73 mol, FeClDPDME 8 μmol, temperature 90 ℃, ambient pressure and time 5 h. | |
2.3. Effect of central metal of MDPs
As reported in our previous work, MPs with different central metal possess different catalytic activities. Table 2 summarized the data obtained from the aerobic oxidation of β-pinene under the catalysis of various MDPs. The results showed that the S values of allylic hydroxylation products varied dramatically with the change of central metal nuclei. Under the catalysis of various corresponding metal salts, the S values were much lower, indicating this variation is mainly caused by the different catalytic abilities of MDPs, which follows a sequence of FeClDPDME > CoDPDME > MnClDPDME > CuDPDME. It has been reported that the catalytic activity of MPs can be influenced by the stability and redox potential of central metal nuclei, resulting to the higher selectivity for allylic hydroxylation products in our procedure [19, 20]. This phenomenon might be attributed to the different redox potential of various MDPs.
|
|
Table 2 Comparison for aerobic oxidation of β-pinene under the catalysis of different MDPs.a |
2.4. Probable reaction mechanism
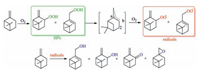
Aerobic oxidation of hydrocarbons in presence and in absence of catalysts has been widely investigated in previous researches. It is widely accepted that these processes are initiated by different active radical intermediates, which generated from the decomposition of HPs [4, 22]. These radicals remove hydrogen atoms in substrate molecules, yielding corresponding resonance-stabilised alkyl radicals. As it is mentioned above, β-pinene could be oxidized by oxygen in or in absence of MPs. Aerobic oxidation of β-pinene in absence of catalysts has been explicitly investigated by Hermans and coworkers and found to be propagated by different peroxyl radicals [4]. These radicals abstract weakly bonded a-hydrogen atoms in the substrate, yielding the corresponding hydroperoxides and resonance-stabilised alkyl radicals (Scheme 1). O2 adds to these allyl radicals and generates allylic hydroperoxidation, allylic hydroxylation and epoxidation porducts via complex approaches.
|
Download:
|
| Scheme 1. Aerobic oxidation of β-pinene in absence of catalysts. | |
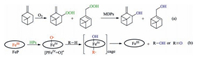
In MPs biomimetic catalyzed system, β-pinene might be oxidized in a different procedure. Under the catalyst of MPs, HPs could decomposed rapidly by MPs to corresponding hydroxylation products (Scheme 2a). Meanwhile, MPs transformed to highvalence MP radicals (e.g. ferric (Ⅳ) porphyrin radicals, [FePⅣ=O+], Scheme 2b) [19]. These radicals are regarded as the active species to initiate this reaction, which removes hydrogen atoms in alkyls and yields caged pair of alkyl radicals and high-valence MPs. The caged pair collapses to alcohol or over-oxidized ketone products via a complex oxygen transfer process [19, 23]. Perhaps, this is the main reason for the high selectivity of allylic hydroxylation products in this catalytic oxidation system. Aerobic oxidation of bpinene in absence of catalysts might occur as a competing reaction in this system. Therefore, the increasement of the flow rate increased the selectivity of HPs and other over-oxidation products. MPs with different central metal nuclei possess different activities in this biomimetic catalytic process. Higher reactive catalysts could promote the decomposing of HPs and the propagating of MPs catalyzed process, resulting to the different selectivity for allylic hydroxylation products.
|
Download:
|
| Scheme 2. Aerobic oxidation of β-pinene under the catalysis of FeP. | |
3. Conclusion
Aerobic oxidation of β-pinene catalyzed by MDPs in absence of solvents and additives at ambient pressure was studied. The optimal reaction conditions of this protocol were evaluated to be 90 -C, 68.7 umol/L and 60 mL/min. The catalyst active of MDPs with different central metal nuclei followed a sequence FeClDPDME > CoDPDME > MnClDPDME > CuDPDME. This catalytic system has bright application prospect since only eco-friendly and readily available dioxygen were needed.
4. Experimental1H NMR spectra were determined on a Bruker Avance Ⅲ 500 MHz spectrometer (Bruker, German). IR spectra were characterized by a Thermo Nicolet IS10 IR instrument (Thermo, USA). ESIMS/MS spectra were recorded on a Finnigan TSQ Quantum Ultra AM mass spectrometer (Finnigan, USA). GC detection were performed through a Shimadzu GC-2014AF (Shimadzu, Japan) GC instrument. GC-MS analysis was performed on an Agilent 6890N/5973N GC-MS instrument (Agilent, USA).
Hemin (purity > 98.5%) was obtained from Tianjin Institute of Life Sciences Applications (China). β-pinene obtained from Zhuzhou Sonbon Forest Chemical Co. was redistilled and purified to over 98.5% before use. Other chemicals were of analytical grade obtained commercially and used without further purification. Organic solvents were dried before use. MDPDMEs and their intermediates were synthesized from hemin according to the literatures published in our previous work [19, 24, 25] and identified by 1H NMR, IR and ESI+-MS.
Aerobic oxidation of β-pinene was carried out in a 250 mL fournecked glass flask containing a reflux condenser, a thermometer and a breather pipe. β-Pinene (100 g, 0.73 mol) and a certain amount of MDPs were added. When the flask was heated to a certain temperature (60-110 -C), oxygen was fed into the mixture with a flow rate of 20-100 mL/min. The reaction was sampled every one-hour and analyzed by GC using n-nonane as an inert internal standard. Hydroperoxides were determined according to the method reported in our previous work [19]. Other components were identified by GC-MS.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at 10.1016/j.cclet.2016.11.020.
AcknowledgmentsThis work was funded by the Fundamental Research Funds for Jiangsu Key Lab of Biomass Energy and Material (No. JSBEM-S- 201605), the National Natural Science Foundation of China (No. 31600466) and the Fundamental Research Funds for the Central Non-profit Research Institution of CAF (No. CAFYBB2014QA022).
| [1] | D.M. Carari, M.J. da Silva, Fe (NO3)3-catalyzed monoterpene oxidation by hydrogen peroxide:an inexpensive and environmentally benign oxidative process. Catal. Lett. 144 (2014) 615–622. DOI:10.1007/s10562-013-1189-x |
| [2] | C. Fang, J. Dai, H. Xu, Q. Guo, Y. Fu, Iron-catalyzed selective oxidation of 5-hydroxylmethylfurfural in air:a facile synthesis of 2, 5-diformylfuran at room temperature. Chin. Chem. Lett. 26 (2015) 1265–1268. DOI:10.1016/j.cclet.2015.07.001 |
| [3] | R. Rachwalik, M. Hunger, B. Sulikowski, Transformations of monoterpene hydrocarbons on ferrierite type zeolites. Appl. Catal. A 427 (2012) 98–105. |
| [4] | U. Neuenschwander, E. Meier, I. Hermans, Peculiarities of b-pinene autoxidation. ChemSusChem 4 (2011) 1613–1621. DOI:10.1002/cssc.v4.11 |
| [5] | F.G.F. de Paula, L. Berllini, M.J. da Silva, A novel Fe (Ⅲ) salt-catalyzed monoterpene aerobic oxidation in methyl alcohol. Catal. Commun. 42 (2013) 129–133. DOI:10.1016/j.catcom.2013.08.018 |
| [6] | M.J. da Silva, P. Robles-Dutenhefner, L. Menini, E.V. Gusevskaya, Cobalt catalyzed autoxidation of monoterpenes in acetic acid and acetonitrile solutions. J. Mol. Catal. A 201 (2003) 71–77. DOI:10.1016/S1381-1169(03)00180-8 |
| [7] | S.V. Jadhav, K.M. Jinka, H.C. Bajaj, Nanosized sulfated zinc ferrite as catalyst forthe synthesis of nopol and other fine chemicals. Catal. Today 198 (2012) 98–105. DOI:10.1016/j.cattod.2012.01.028 |
| [8] | M. Skočibušić, N. Bezić, V. Dunkić, Phytochemical composition and antimicrobial activities of the essential oils from Satureja subspicata Vis. growing in Croatia. Food Chem. 96 (2006) 20–28. DOI:10.1016/j.foodchem.2005.01.051 |
| [9] | F. Bakkali, S. Averbeck, D. Averbeck, M. Idaomar, Biological effects of essential oils-a review. Food Chem. Toxicol. 46 (2008) 446–475. DOI:10.1016/j.fct.2007.09.106 |
| [10] | L.M. Joshel, S. Palkin, The oxidation of & pinene with selenium dioxide. J. Am. Chem. Soc. 64 (1942) 1008–1009. DOI:10.1021/ja01256a503 |
| [11] | L. Menini, M.J. da Silva, M.F.F. Lelis, Novel solvent free liquid-phase oxidation of b-pinene over heterogeneous catalysts based on Fe3-XMXO4(M=Co and Mn). Appl. Catal. A 269 (2004) 117–121. DOI:10.1016/j.apcata.2004.04.005 |
| [12] | J. Jin, M. Shen, Progress in oxidation of b-pinene. Guangzhou Chem. 31 (2006) 51–56. |
| [13] | S. Yi, M. Li, X. Hu, W. Mo, Z. Shen, An efficient and convenient method for the preparation of disulfides from thiols using oxygen as oxidant catalyzed by tertbutyl nitrite. Chin. Chem. Lett. 27 (2016) 1505–1508. DOI:10.1016/j.cclet.2016.03.016 |
| [14] | N.T. Thao, H.H. Trung, Selective oxidation of styrene over Mg-Co-Al hydrotalcite like-catalysts using air as oxidant. Catal. Commun. 45 (2014) 153–157. DOI:10.1016/j.catcom.2013.11.004 |
| [15] | B. Meunier, Metalloporphyrins as versatile catalysts for oxidation reactions and oxidative DNA cleavage. Chem. Rev. 92 (1992) 1411–1456. DOI:10.1021/cr00014a008 |
| [16] | C.M. Che, V.K.Y. Lo, C.Y. Zhou, J.S. Huang, Selective functionalisation of saturated C H bonds with metalloporphyrin catalysts. Chem. Soc. Rev. 40 (2011) 1950–1975. DOI:10.1039/c0cs00142b |
| [17] | X.D. Li, Y.C. Zhu, L.J. Yang, Crown ether-appended Fe (Ⅲ) porphyrin:synthesis, characterization and catalytic oxidation of cyclohexene with molecular oxygen. Chin. Chem. Lett. 23 (2012) 375–378. DOI:10.1016/j.cclet.2011.12.011 |
| [18] | Q. Liu, C.C. Guo, Theoretical studies and industrial applications of oxidative activation of inert C-H bond by metalloporphyrin-based biomimetic catalysis. Sci. China Ser. B Chem. 55 (2012) 2036–2053. DOI:10.1007/s11426-012-4739-y |
| [19] | S.C. Xu, Z.D. Zhao, L.W. Bi, Selective aerobic hydroxylation of p-menthane to dihydroterpineols catalyzed by metallopor-phyrins in solvent and additive free system. Catal. Commun. 59 (2015) 26–29. DOI:10.1016/j.catcom.2014.09.039 |
| [20] | W.Y. Zhou, B.C. Hu, Z.L. Liu, Metallo-deuteroporphyrin complexes derived from heme:a homogeneous catalyst for cyclohexane oxidation. Appl. Catal. A 358 (2009) 136–140. DOI:10.1016/j.apcata.2009.02.003 |
| [21] | C.C. Guo, W.J. Yang, Y.L. Miao, Selectively aerobic oxidation of C=C and allylic C-H bonds in (a-pinene over simple metalloporphyrins. J. Mol. Catal. A 226 (2005) 279–284. DOI:10.1016/j.molcata.2004.10.049 |
| [22] | U. Neuenschwander, F. Guignard, I. Hermans, Mechanism of the aerobic oxidation of a-pinene. ChemSusChem 3 (2010) 75–84. DOI:10.1002/cssc.v3:1 |
| [23] | R.D. Bach, O. Dmitrenko, The somersault mechanism for the P-450 hydroxylation of hydrocarbons The intervention of transient inverted metastable hydroperoxides. J. Am. Chem. Soc. 128 (2006) 1474–1488. DOI:10.1021/ja052111+ |
| [24] | S.C. Xu, W.W. Liu, B.C. Hu, W. Cao, Z.L. Liu, Biomimetic enhanced chemiluminescence of luminol-H2O2 system by manganese (Ⅲ) deuteroporphyrin and its application in flow injection determination of phenol at trace level. J. Photochem. Photobiol. A Chem. 227 (2012) 32–37. DOI:10.1016/j.jphotochem.2011.10.021 |
| [25] | C.G. Sun, W.Y. Zhou, B.C. Hu, S.C. Xu, Z.L. Liu, A facile synthesis of 2, 7, 12, 18-tetramethyl-13, 17-di (3-hydroxypropyl) porphyrin. Fine Chem. 26 (2009) 919–922, 927. |
 2017, Vol. 28
2017, Vol. 28