Histatins are a family of natural occurring cationic peptides found in human saliva [1]. These peptides have received many attentions recently because of the diverse antibacterial and antifungal activities [2]. For example, Histatin 5 is a histidinerich peptide (DSHAKRHHGYKRKFHEKHHSHRGY), which is known to display the most potent antifungal activity against Candida albicans [3]. P-113, also named as Demegen, is a fragment of Histatin 5 containing 12 amino acids with a sequence of AKRHHGYKRKFH. Previous studies have demonstrated that P-113 exhibited broad spectrum of antibacterial properties [4]. In particular, P-113 has been developed as a drug candidate for the treatment of oral candidiasis infection, such as C. albicans, Candida glabrata, Candida parapsilosis, and Candida tropicalis [4, 5]. However, the obvious disadvantages of using linear peptides as therapeutic reagents are short plasma life time and sensitive to enzymatic degradation, which restrict their further application in practice [6]. Cyclization of the parent peptides is one of the popular strategies to improve the metabolic stability and binding specificity in drug discovery [7]. In addition, other essential properties required for drugs, such as membrane permeability, bioavailability and enhanced biological activity, are observed to improve after cyclization as well [8]. Thus, design and synthesis of cyclized P-113 could potentially not only generate new antibacterial reagent, but also enhance the metabolic stability and other drug like properties.
Sortase A is a transpeptidase original from gram positive bacterial Staphylococcus aureus, which is responsible for covalent anchoring surface protein onto cell surface by a cell wall sorting reaction [9]. It recognizes a signal peptide LPXTG (X standing for any amino acids except cysteine) near the C-terminus of protein, breaks the amide bond of T and G to form an enzyme-protein thioester intermediate; then couple with acceptor containing oligo-glycine residues to generate ligation product [10]. This technology has been widely used in site specific protein modification [11], protein to protein fusion [12], immobilization of biocatalyst onto solid support [13] and preparing complex glycoconjugates [14]. We and others have applied this method to realize protein and peptides head to tail cyclization in model to excellent yields [15]. Therefore, in this study, a bifunctional P-113 peptide 2 analogue was designed; where the N-and C-terminus was equipped with Sortase A recognized signal peptide tri-glycine and LPETGGS, respectively. In addition, another three P-113 peptide analogues 3, 4 and 5 were designed as well, where the N-terminal natural glycine was replaced by glycine analogs, namely hydrazinecarboxylic acid, β-hydrazinyl acetic acid and β-alanine (Fig. 1). By replacing the α-CH moiety of glycine in peptide chain with nitrogen atom, or one carbon extension, this modification has been shown to induce peptide geometry change due to intramolecular hydrogen bonds [16]. Therefore, it is expecting that the introducing of these non-natural linkages could further enhance the metabolic stability and generate more potent biological activities relative to parent peptide P-113. In another hands, using glycine analogues to mimic glycine will incorporate LPETG* (G*=G1-G3) structure into the products, which are no longer substrates of Sortase A and thus will inhibit the reversible reaction and hydrolysis reaction [17].

|
Download:
|
| Figure 1. List of peptide P-113 and its bifunctional linear analogues as substrates of Sortase A. | |
2. Results and discussion
Peptides 1 and 2 were synthesized by automatic peptide synthesizer with the assistant of microwave irradiation. To access C-terminus amidated peptides, rink amide resin was used as the solid support. After the amino acids were assembled on resin by Fmoc chemistry, peptides were cleaved by treatment with TFA/iPr3SiH/H2O (95:2.5:2.5, v/v/v) followed by cold ether precipitation. Peptide 3, 4 and 5 are analogues of peptide 2, where the last N-terminus glycine residue was replaced with hydrazinecarboxylic acid, β-hydrazinyl acetic acid and β-alanine, respectively. To synthesize peptide 3, Fmoc protetected hydrazine was activated with CDI; then coupled with 6 at room temperature in the presence of DIPEA. After washing off the excess reagent and removing the Fmoc group, peptide 3 was released by cocktail solution. For peptide 4, resin 6 was coupled with bromoacetic acid, then a SN2 substitute reaction was used to replace bromo group with Fmoc protected hydrazine. After deprotection and cleavage, peptide 4 was afforded. Peptide 5 was obtained by direct coupling of resin 6 with Fmoc protecting β-alanine using DIC as condensation reagent in the presence of DIPEA followed by deprotection and cleavage (Scheme 1). Peptides 1-5 were characterized by Maldi Tof MS, which gave expected mass peak. HPLC analysis showed that the final products were >95% pure, which are good enough to use directly.
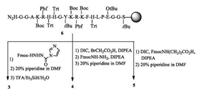
|
Download:
|
| Scheme 1. Solid phase synthesis of peptides 3-5. | |
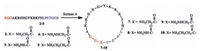
With peptides 2-5 in hand, we explored the Sortase A-mediated head totail cyclization. Reactionwas performed at 37 ℃ in0.3 mol/L Tris-HCl buffer (pH 7.5) containing 0.15 mol/L NaCl, 5 μmol/L CaCl2, and 2 mmol/L mercaptoethanol in the presence of 20 umol/L Sortase A. The concentrations of peptides 2-5 were set up at 0.2 mol/L according to our previous results [15c] (Scheme 2).
|
Download:
|
| Scheme 2. Sortase A-mediated ligation for the synthesis of cyclic analogues of P-113. | |
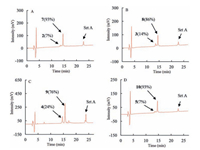
Reaction progress was monitored by HPLC and Maldi-Tof MS. Therefore, reaction aliquots after quenched with 0.1% TFA were obtained at various time points in 12 h; then analyzed by HPLC. For peptide 2 (Fig. 2A), which contained Sortase A recognized triglycine and LPETGGS motif at the N-and C-terminus, the HPLC analysis showed that the reaction proceeded very smoothly and reached the equilibrium in 6 h. The starting peptide 2 peak at retention time of 13.5 min was converting to a new peak with retention time at 14.6 min. The new peak was identified to be head to tail cyclization product 7 [MS (m/z): calcd. 2158.4, found 2259.1 (M+H)+] by Maldi-Tof MS. The conversion yield was 93% after incubation for 12 h. For the peptides 3, 4 and 5 with glycine analogues at the N-terminus, the reactions displayed similar profiles. Majority of the starting peptides were rapidly consumed in 6 hand new product peaks were generated with retention time at 14.5, 15.7, 14.6 min, respectively, as shown in Fig. 2B-D. MaldiTof MS analysis indicated that all the new peaks were corresponding to their head to tail cyclization products 8 [MS (m/z): calcd. 2159.4, found 2160.0 (M+H)+], 9 [MS (m/z): calcd. 2173.4, found 2174.3 (M+H)+] and 10 [MS (m/z): calcd. 2172.4, found 2173.0 (M+H)+]. The reaction yields were determined to be 86%, 76% and 93% based on HPLC, respectively. Hydrazine derivatives are good mimics of gycine structure as nucleophiles in Sortase A-mediated ligation, which has been applied in intermolecular ligation successfully; methylene amine is also a demonstrated Sortase A recognizing structure but with varied nucleophilic capability to react with enzyme-peptide or protein thioester intermediate [14a, 17, 18]. Our results demonstrated that both hydrazine derivatives and methylene amine structure were good mimics of glycine motif, which can be recognized by Sortase A and reacted with peptides-enzyme thioester intermediate to generate ligation products in high efficiency. And this is the first demonstration of using hydrazine derivatives and methylene amine as mimics of glycine in Sortase A-mediated peptides head to tail cyclization.
|
Download:
|
| Figure 2. HPLC profile of Sortase A-mediated head to tail cyclization for the synthesis of cyclic analogues of P-113. (A) peptide 2; (B) peptide 3; (C) peptide 4; (D) peptide 5. | |
To explore the conformation of cyclic peptides 7-10, the Sortase A-mediated cyclization was scale up to obtain milligram of products. Previous studies have demonstrated that the antibacterial activity of P-113 is strongly related to the existence of α-helix conformation [4, 19]. Generally, low percentage of or breaking the α-helix structure of P-113 usually decrease the potency of its biological activities. Therefore, the conformation behavior of linear peptides 2-5 and cyclic analogues 7-10 were briefly studied by circular dichroism (CD) spectrum in aqueous buffer and in different percentage of trifluroethanol (TFE), respectively. The conformation insight of peptides in TFE solution, which is a mimic of hydrophobic membrane environment, plays an important role for its biological activities. As shown in Fig. 3A-D, linear peptides 2-5 displayed a positive band in 220 nm in 100% aqueous buffer indicating a polypeptide random coil characteristic. On the contrary, the conformation profile of these peptides were varied dramatically as negative absorption at 206 to 208 nm and 220 nm were produced gradually with the increasing concentration of TFE from 10% to 90%, suggesting α-helix structure was formed in hydrophobic environment. Subsequent CD analyses of cyclic analogues 7-10 showed that their behaviors followed the same conformation transformation as those of linear peptides 2-5, as presented in Fig. 3E-H. These results also suggested that the propensity of forming α-helix is in-dependent of the cyclization. By comparing with the CD spectrum of P-113 (Fig. S13 in Supporting information), all of the P-113 analogues including linear and cyclic peptides exhibited the similar structure change, which reveal that these P-113 analogues probably conserve the corresponding biological activities [4, 5, 5c].

|
Download:
|
| Figure 3. CD spectrum of linear peptides and cyclic peptides in 10 mmol/L phosphate buffer (pH 7.0, black line), 10% of TFE (red line), 50% of TFE (blue line) and 90% TEF (purple line). (A-D) peptide 2-5; (E-H) peptide 7-10. | |
3. Conclusion
Bifunctional P-113 peptides were designed as Sortase A substrates and synthesized by solid support peptide synthesis, where the N-terminuses were equipped with glycine and its analogues, and C-terminus were extended with LPETGGS, respectively. Cyclized peptides of P-113 analogues were prepared by Sortase A-mediated head to tail ligation in yields from 76% to 93%. The conformation studies by circular dichroism in aqueous buffers and in trifluroethanol (TFE) suggested that a-helix structures were produced progressively in hydrophobic environment independent of the cyclization, which displayed the similar behavior as parent peptide P-113. The detailed conformation information of these peptides in the presence of Zn (Ⅱ), Cu (Ⅱ) ion, and biological activities are undergoing in our lab and will report in full paper in future.
4. ExperimentalChemical reagents and solvents were purchased from Titan Scientific Lab. (Shanghai, China) and used without further purification. Fmoc-protected amino acids and peptide synthesis reagents were purchased from Changzhou Kanglong Biotech Ltd. (Changzhou, China). Analytical RP-HPLC was performed on Waters E2695 or Beijing ChuangXinTongHeng LC3000 (analytic) instrument with a C18 column (5 μm, 4.6 mm × 250 mm) at 40 ℃. Analysis HPLC conditions: (A) 10% ACN in H2O (both containing 0.1% TFA) to 40% ACN in H2O in 50 min; flow rate: 1 mL/min; column: C-18 (5 μm, 4.6 mm × 250 mm); (B) HPLC conditions: 10% ACN in H2O (both containing 0.1% TFA) to 40% ACN in H2O in 30 min; flow rate: 1 mL/min; column: C18 (5 μm, 4.6 mm × 250 mm); Preparative HPLC was performed on a Waters 1525 with a preparative C18 column (5 μm, 10.0 mm × 250 mm). The column was eluted with a suitable gradient of aqueous acetonitrile containing 0.1% TFA at a flow rate of 3 mL/min. Solid-phase peptide synthesis was performed on a CEM Liberty Blue peptide synthesizer. Peptides were characterized by MALDI-TOF Mass (UltrafleXtreme, Bruker Daltonics; Bremen, Germany).
Solid-phase peptide synthesis of peptides 1 and 2: The peptides were synthesized on an automatic CEM Liberty Blue peptide synthesizer by Fmoc-chemistry using Fmoc-protected amino acid derivatives. A Rink Amide resin (loading 0.317 mmol/g) was used as the solid support. To construct the full length of peptides: 5.0 equiv. of amino acids were used in each cycle with the microwave assisted irradiation at 90 ℃, and TBTU (0.5 mol/L in DMF), DIPEA (1.0 mol/L in DMF) (1:1, v/v) were used as the coupling reagent; deprotection of the Fmoc group was carried out using 20% ofpiperidine in DMF. After the peptides elongation was completed, the peptides were cleaved from the resin by treatment with TFA/i-Pr3SiH/H2O (95:2.5:2.5, v/v/v) followed by precipitation with cold ether. The crude peptide was dissolved in water and lyophilized. The residue was subject to HPLC and Maldi-Tof MS analysis to give AKRHHGYKRKFH-NH2 (1) and GGGAKRHHGYKRKFHLPETGGS-NH2 (2). For peptide 1: analytic HPLC (condition A): Rt=8.3 min; Maldi-Tof MS: calcd. for C71H110N28O13: 1563.8, observed 1565.3 [M+H]+; for peptide 2: analytic HPLC (condition A): Rt=18.8 min; Maldi-Tof MS: calcd. for C104H162N38O27: 2376.6, observed 2377.5 [M+H]+.
Solid-phase peptide synthesis of peptide 3: Fmoc-NH-NH2 (0.5 μmol) and CarbonyldiiMidazole (CDI) (0.5 μmol) was dissolved in 5 mL DMF and stirring at room temperature for 3 h; then the solutionwas transferredtoa glass reactor containing 0.05 μmol Rink amide Resin with full protected sequence GGAKRHHGYKRKFHLPETGGS synthesized according to the above protocol. After the reaction was shaken at room temperature for 2 h, Fmoc protecting group removed with 20% of piperidine in DMF; then washed with DMF and DCM. Peptide was cleaved off with TFA/iPr3SiH/H2O (95:2.5:2.5, v/v/v) followed by precipitation with cold ether. The crudepeptidewas dissolved inwaterand lyophilized.The residue was subject to HPLC and Maldi-Tof MS analysis to give 3. Analytic HPLC (condition A): Rt=18.8 min; Maldi-Tof MS: calcd. for C103H161N39O27: 2377.6, observed 2378.3 [M + H]+.
Solid-phase peptide synthesis of peptide 4: 0.05 μmol Rink amide Resin with full protected sequence GGAKRHHGYKRKFHLPETGGS, bromoacetic acid (20 equiv., 1 mmol) and DIC (24 equiv., 1.2 mmol) was reacted in microwave condition at 75 ℃ from 5 min; then washed with DMF and DCM. The resin was transferred to glass reactor containing FmocNH-NH2 (20 equiv., 1.5 μmol), DIPEA (40 equiv., 3 mmol) in 2 mL DMF. The reaction was continued at room temperature for 6 h and Fmoc was deprotected with 20% piperidine. After washing with DMF and DCM, peptide was cleaved off with TFA/i-Pr3SiH/H2O (95:2.5:2.5, v/v/v) followed by precipitation with cold ether. The crude peptide was dissolved in water and lyophilized. The residue was subject to HPLC and Maldi-Tof MS analysis to give 4. Analytic HPLC (condition A): Rt=19.2 min; Maldi-Tof MS: calcd. for C104H163N39O27: 2391.6, observed 2392.5 [M+H]+.
Solid-phase peptide synthesis of peptide 5: 0.05 μmol Rink amide Resin with full protected sequence GGAKRHHGYKRKFHLPETGGS, Fmoc protected β-alanine (5.0 equiv., 0.25 μmol), DIC (5.0 equiv., 0.25 μmol) and HOBt (5.0 equiv., 0.25 μmol) was stirred at room temperature for 2 h, then washed with DMF and DCM. After Fmoc was deprotected with 20% piperidine, resin was washed with DMF and DCM. Peptide was cleaved off with TFA/i-Pr3SiH/H2O (95:2.5:2.5, v/v/v) followed by precipitation with cold ether. The crude peptide was dissolved in water and lyophilized. The residue was subject to HPLC and Maldi-Tof MS analysis to give 5. Analytic HPLC (condition A): Rt=18.7 min; Maldi-Tof MS: calcd. for C105H164N38O27: 2390.6, observed 2391.6 [M+H]+.
Sortase A mediated ligation for the synthesis of cyclic P-113 analogs: the reaction was performed in 200 μL volume with 40 uL of 100 umol/L SrtA in the presence of peptides 2, 3, 4 or 5. The final concentration was set up as bellow: 0.2 mmol/L peptide, 0.3 mol/L Tris-HCl buffers (pH 7.5), 0.15 mol/L NaCl, 5 μmol/L CaCl2, 2 mmol/L 2-mercaptoethanol. The reaction was incubated in 37 ℃ for 12 h. 20 μL of reaction mixture was quenched with same volume of 0.1% of TFA. The aliquots were analyzed by analytic HPLC using RP C18 column (condition B) and fractions were analyzed by MALDI-TOF MS.
CD Measurements: CD spectrum were acquired on a Jasco Spectropolarimeter at 25 ℃ between 195 nm and 250 nm at a scan rate of 100 nm/min with bandwidth set to 1 nm and the response set to 0.5 s. Peptide concentration was 50 mmol/L in phosphate buffer (25 μmol/L, pH 7.0) or in 10-90% of TFE. The final spectrum was the accumulated average of 3 scans. A blank spectrum for the solution without peptide was collected for each sample and subtracted in the final analysis.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.11.001.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21472070), the Project for Jiangsu Scientific and Technological Innovation Team, Fund for Jiangsu Distinguished Professorship Program, Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the 111 Project (No. 111-2-06), and the Jiangsu province "Collaborative Innovation Center for Advanced Industrial Fermentation" industry development program. We appreciated the help of Professor Fei Xu and Dr. Hongning Zheng in Jiangnan University for the CD spectrum.
| [1] | F.G. Oppenheim, Y.C. Yang, R.D. Diamond, The primary structure and functional characterization of the neutral histidine-rich polypeptide from human parotid secretion. J. Biol. Chem. 261 (1986) 1177–1182. |
| [2] |
(a) J.J. Pollock, J. Shoda, T.F. McNamara, et al., In vitro and in vivo studies of cellular lysis of oral bacteria by a lysozyme-protease-inorganic monovalent anion antibacterial system, Infect. Immun. 45(1984) 610-617; (b) E.J. Helmerhorst, I.M. Reijnders, W. van't Hof, et al., Amphotericin B-and fluconazole-resistant Candida spp., Aspergillus fumigatus, and other newly emerging pathogenic fungi are susceptible to basic antifungal peptides, Antimicrob. Agents Chemother. 43(1999) 702-704; (c) I. Čipáková, E. Hostinová, Mammalian antimicrobial peptides, Biologia 58(2003) 335-341; (d) M.D. Seo, H.S. Won, J.H. Kim, et al., Antimicrobial peptides for therapeutic applications:a review, Molecules 17(2012) 12276-12286. |
| [3] |
(a) S. Melino, C. Santone, P. Di Nardo, et al., Histatins:salivary peptides with copper (Ⅱ)-and zinc (Ⅱ)-binding motifs:perspectives for biomedical applications, FEBS J. 281(2014) 657-672; (b) S. Puri, R. Li, D. Ruszaj, et al., Iron binding modulates candidacidal properties of salivary histatin 5, J. Dent. Res. 94(2015) 201-208. |
| [4] | A. Di Giampaolo, C. Luzi, B. Casciaro, P-113 peptide:new experimental evidences on its biological activity and conformational insights from molecular dynamics simulations. Biopolymers 102 (2014) 159–167. DOI:10.1002/bip.v102.2 |
| [5] |
(a) P. Spacciapoli, L. Tran, F.D. Roberts, et al., Characterization of the antimicrobial spectrum of the histatin peptide P-113, J. Dent. Res. 76(1997) 2736; (b) L.T. Tran, P. Spacciapoli, P.M. Friden, et al., The antimicrobial peptide P-113 has potent activity against Candida species, J. Dent. Res. 79(2000) 152; (c) D.M. Rothstein, P. Spacciapoli, L.T. Tran, et al., Anticandida activity is retained in P-113, a 12-amino-acid fragment of histatin 5, Antimicrob. Agents Chemother. 45(2001) 1367-1373; (d) K. Kulon, D. Valensin, W. Kamysz, et al., The his-his sequence of the antimicrobial peptide demegen P-113 makes it very attractive ligand for Cu2+, J. Inorg. Biochem. 102(2008) 960-972; (e) W.C. Cheng, G. Lin, H. Chen, et al., Development of P-113-derived peptides as novel inhibitors for drug-resistant Candida spp. and biofilm formation, Mycoses 58(2015) 81-82. |
| [6] |
(a) P.R. Chaturvedi, C.J. Decker, A. Odinecs, Prediction of pharmacokinetic properties using experimental approaches during early drug discovery, Curr. Opin. Chem. Biol. 5(2001) 452-463; (b) O. Pelkonen, M. Turpeinen, J. Uusitalo, et al., Prediction of drug metabolism and interactions on the basis of in vitro investigations, Basic Clin. Pharmacol. 96(2005) 167-175. |
| [7] |
(a) M. Werle, A. Bernkop-Schnurch, Strategies to improve plasma half life time of peptide and protein drugs, Amino Acids 30(2006) 351-367; (b) A. Bhat, L.R. Roberts, J.J. Dwyer, Lead discovery and optimization strategies for peptide macrocycles, Eur. J. Med. Chem. 94(2015) 471-479; (c) C.M. Zhang, J.X. Guo, L. Wang, et al., Total synthesis of cyclic heptapeptide euryjanicin B, Chin. Chem. Lett. 22(2011) 631-634. |
| [8] |
(a) T.A. Hill, N.E. Shepherd, F. Diness, et al., Constraining cyclic peptides to mimic protein structure motifs, Angew. Chem. Int. Ed. 53(2014) 13020-13041; (b) H. Wahyudi, S.R. McAlpine, Predicting the unpredictable:recent structureactivity studies on peptide-based macrocycles, Bioorg. Chem. 60(2015) 74-97; (c) H. Karatas, S.Y. Lee, E.C. Townsend, et al., Structure-based design of conformationally constrained cyclic peptidomimetics to target the MLL1-WDR5 protein-protein interaction as inhibitors of the MLL1 methyltransferase activity, Chin. Chem. Lett. 26(2015) 455-458. |
| [9] | S.K. Mazmanian, G. Liu, H. Ton-That, Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285 (1999) 760–763. DOI:10.1126/science.285.5428.760 |
| [10] | H. Ton-That, S.K. Mazmanian, L. Alksne, Anchoring of surface proteins to the cell wall of Staphylococcus aureus. Cysteine 184 and histidine 120 of sortase form a thiolate-imidazolium ion pair for catalysis. J. Biol. Chem. 277 (2002) 7447–7452. DOI:10.1074/jbc.M109945200 |
| [11] |
(a) H.Y. Mao, S.A. Hart, A. Schink, et al., Sortase-mediated protein ligation:a new method for protein engineering, J. Am. Chem. Soc. 126(2004) 2670-2671; (b) J.M. Antos, G.M. Miller, G.M. Grotenbreg, et al., Lipid modification of proteins through Sortase-catalyzed transpeptidation, J. Am. Chem. Soc. 130(2008) 16338-16343. |
| [12] | J.M. Antos, G.L. Chew, C.P. Guimaraes, Site-specific N-and C-terminal labeling of a single polypeptide using Sortases of different specificity. J. Am. Chem. Soc. 131 (2009) 10800–10802. DOI:10.1021/ja902681k |
| [13] | T. Ito, R. Sadamoto, K. Naruchi, Highly oriented recombinant glycosyltransferases:site-specific immobilization of unstable membrane proteins by using Staphylococcus aureus Sortase A. Biochemistry 49 (2010) 2604–2614. DOI:10.1021/bi100094g |
| [14] |
(a) S. Samantaray, U. Marathe, S. Dasgupta, et al., Peptide-sugar ligation catalyzed by transpeptidase sortase:a facile approach to neoglycoconjugate synthesis, J. Am. Chem. Soc. 130(2008) 2132-2133; (b) Z.M. Wu, X.Q. Guo, Q.L. Wang, et al., Sortase A-catalyzed transpeptidation of glycosylphosphatidylinositol derivatives for chemoenzymatic synthesis of GPI-anchored proteins, J. Am. Chem. Soc. 132(2010) 1567-1571; (c) Z.M. Wu, X.Q. Guo, J. Gao, et al., Sortase A-mediated chemoenzymatic synthesis of complex glycosylphosphatidylinositol-anchored protein, Chem. Commun. 49(2013) 11689-11691; (d) Z.M. Wu, X.Q. Guo, G.F. Gu, et al., Chemoenzymatic synthesis of the human CD52 and CD24 antigen analogues, Org. Lett. 15(2013) 5906-5908. |
| [15] |
(a) J.M. Antos, M.W.L. Popp, R. Ernst, et al., A Straight path to circular proteins, J. Biol. Chem. 284(2009) 16028-16036; (b) J.G.M. Bolscher, M.J. Oudhoff, K. Nazmi, et al., Sortase A as a tool for highyield histatin cyclization, Faseb J. 25(2011) 2650-2658; (c) Z.M. Wu, X.Q. Guo, Z.W. Guo, Sortase A-catalyzed peptide cyclization for the synthesis of macrocyclic peptides and glycopeptides, Chem. Commun. 47(2011) 9218-9220; (d) X.Y. Jia, S. Kwon, C.I.A. Wang, et al., Semienzymatic cyclization of disulfiderich peptides using Sortase A, J. Biol. Chem. 289(2014) 6627-6638; (e) K. Stanger, T. Maurer, H. Kaluarachchi, et al., Backbone cyclization of a recombinant cystine-knot peptide by engineered Sortase A, Febs Lett. 588(2014) 4487-4496; (f) W. van't Hof, S.H. Manaskova, E.C.I. Veerman, et al., Sortase-mediated backbone cyclization of proteins and peptides, Biol. Chem. 396(2015) 283-293. |
| [16] |
(a) J. Bondebjerg, H. Fuglsang, K.R. Valeur, et al., Novel semicarbazide-derived inhibitors of human dipeptidyl peptidase I (hDPPI), Bioorg. Med. Chem. 13(2005) 4408-4424; (b) N. Ollivier, S. Besret, A. Blanpain, et al., Silver-catalyzed azaGly ligation. application to the synthesis of azapeptides and of lipid-peptide conjugates, Bioconjug. Chem. 20(2009) 1397-1403; (c) J. Gante, Peptide and azapeptide synthesis by means of a new N-activated amino acid derivative, Chem. Ber. 99(1966) 1576-1579; (d) R.E. Melendez, W.D. Lubell, Aza-amino acid scan for rapid identification of secondary structure based on the application of N-boc-aza-dipeptides in peptide synthesis, J. Am. Chem. Soc. 126(2004) 6759-6764. |
| [17] | Y.M. Li, Y.T. Li, M. Pan, Irreversible site-specific hydrazinolysis of proteins by use of sortase. Angew. Chem. Int. Ed. 53 (2014) 2198–2202. DOI:10.1002/anie.201310010 |
| [18] | S. Baer, J. Nigro, M.P. Madej, Comparison of alternative nucleophiles for Sortase A-mediated bioconjugation and application in neuronal cell labelling. Org. Biomol. Chem. 12 (2014) 2675–2685. DOI:10.1039/c3ob42325e |
| [19] |
(a) E. Porciatti, M. Milenkovic, E. Gaggelli, et al., Structural characterization and antimicrobial activity of the Zn (Ⅱ) complex with P113(Demegen), a derivative of histatin 5, Inorg. Chem. 49(2010) 8690-8698; (b) E. Kurowska, A. Bonna, G. Goch, W. Bal, Salivary histatin-5, a physiologically relevant ligand for Ni (Ⅱ) ions, J. Inorg. Biochem. 105(2011) 1220-1225. |
 2017, Vol. 28
2017, Vol. 28 


