b Kosar University of Bojnord, Department of Applied Chemistry, North Khorasan, Iran
Current environmental friendly motor gasoline has forced severe controls for reducing the aromatic hydrocarbons. These compounds are harmful to the environment and human health [1]. To avoid reducing of the gasoline octane number with a decrease in aromatic hydrocarbons, n-alkanes isomerization was introduced as a desired reaction. An effective bifunctional catalyst is needed for ideal performance of the isomerization reaction. The hydrogenating ability of these bifunctional catalysts encourages the hydrogenation of unsaturated hydrocarbons and their acid properties provide the selective isomerization of n-alkanes presented in feedstock into high-octane isomers [2]. Different catalysts were used for this reaction. According to literatures, solid acids [3-6] have shown favorable advantages for isomerization reaction, such as high catalytic activity at low temperature, no corrosion in the reactor and no environmental problems in disposing of the used catalysts [7, 8]. Among these super acids, WO3/ZrO2 supported catalysts have more stable conversion and the absence of leaching of any catalyst component into the products; however, these catalysts are less active compared to SO42-/ZrO2 supported materials [8, 9]. Therefore, in the present study we focused on this support (WO3/ZrO2) but with some changes. To increase the surface area of the metal oxide catalysts these oxides can be introduced into mesoporous supports that have a high surface area [10]. Considering that WO3/ZrO2 has a good acidity and on the other hand, HMS has a suitable surface area, we predicted that WO3/ZrO2-HMS, especially when promoted with Pt and in the presence of hydrogen stream in the feed, would be of interest to explore the catalytic isomerization performance. In the current work, n-heptane isomerization on a series of Pt-WO3/ZrO2-HMS catalysts was studied and compared with that on Pt/ZrO2-HMS. The effects of WO3 addition and Si/Zr molar ratio on the catalytic isomerization reaction were examined. The reasons for the improvement on activity, selectivity, stability, coke deposition, octane-number and kinetics of these catalysts in n-heptane isomerization reaction were discussed at the temperature range of 200-350 ℃.
2. Results and discussion 2.1. StructureIn our previous work [10], Pt/Zr (x)-HMS catalysts were evaluated. These catalysts are the reference catalysts for the prepared catalysts in present work (Pt-W/Zr (x)-HMS).
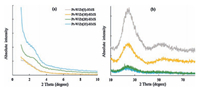
To identify HMS, zirconia and tungsten oxide phases, X-ray diffraction was used. Fig. 1 presents the wide-angle range XRD patterns of the calcined Pt-W/Zr (x)-HMS samples with a different loading of ZrO2. In all patterns, a strong {100} X-ray reflection at low angle (2θ~2.3°), arising from the hexagonal array of silica tubes, and a broad diffraction line (2θ=20°-30°) for the shapeless part of HMS [10] are observed. Meanwhile, XRD patterns show no signals of Pt, ZrO2 and WO3 particles, suggesting that these metallic phases were highly dispersed in prepared solids, in well agreement with the H2 chemisorption and UV-vis DRS results. After incorporating ZrO2 and WO3 into the HMS framework, the d100 peak shifts to lower angles and broadened, suggesting a lattice expansion during the incorporation and a decrease in the dimensions of the scattering domain.
|
Download:
|
| Figure 1. X-ray diffraction patterns of Pt-W/Zr (x)-HMS catalysts in (a) low angle (1-10°) and (b) high angle (10-80°) regions. | |
Table 1 shows the d-spacing (d100), unit cell parameter (a0) and wall thickness (wt) values that have been calculated from XRD data. These values increase with increasing ZrO2 content.
|
|
Table 1 Composition and physico-chemical properties of tungstated zirconia-HMS samples. |
Fig. 2 explains the FT-IR spectra at the room temperature for the HMS doped Pt-W/Zr catalysts in the range of 4000-400 cm-1. As expected, these spectra are same together, so we show one of them (Pt-W/Zr (10)-HMS catalyst).

|
Download:
|
| Figure 2. FT-IR spectra of powder calcined Pt-W/Zr (10)-HMS catalyst. | |
In comparison to the reported IR spectra for Zr (x)-HMS catalysts [10], Pt-W/Zr (x)-HMS catalysts show some added bands. All bands underwent a red shift towards lower wave numbers and intensified with entering tungsten and increasing zirconium amounts. According to literatures [11, 12], these red shifts (especially for Si--O--Si band) are related to the decaying silica framework of HMS after insertion of Zr and W atoms. It can be observed that two new bands with same intensities are emerged at 2900 and 1532 cm-1 in Pt-W/Zr (x)-HMS catalysts. These bands can be related to the vibrations of (Wx+)nOH disturbed by the support or a bridged W-OH-Zr species and W=O, respectively [11, 12].
To decide the chemical nature and coordination states of tungsten and zirconium, UV-vis DRS of catalysts have been obtained and shown in Fig. 3. To comparison, the UV-vis analysis of the Zr (x)-HMS catalysts is also included as reference. All UV-vis DRS spectra of Zr-HMS samples show the absorption band with maxima about 200 nm. This band is attributable to the chargetransfer transition from the valence band of an oxygen ion (2p character) to the conduction band of isolated Zr (Ⅳ) ion (4d character) in tetrahedral arrangement [13, 14]. An absorption band with low intensity is also seen at 240 nm, which is normally assigned to Zr (Ⅳ) species in monoclinic ZrO2 phase [13, 14]. A band with a maximum around 300 nm and a band roughly at 375 nm are observed in all spectra. These bands agree to isolated Zr4+ with low coordination and oligomer zirconium species, respectively [15-17]. The results show that Zr ions in structure of catalysts have different states and are not as a separate ZrO2 phase. These evidences show that zirconium incorporated well in the framework of the mesoporous catalysts. After incorporating tungsten, the bands of isolated [WO4]2- species, low-condensed oligomer tungsten oxide species and WO3 crystallites are detected as a strong adsorption band at 210-375 nm [15]. As can be seen, first band has low intensity and last band is as a weak shoulder. The low intensity of these bands shows the low presence of these species in our catalysts. Increasing the zirconium concentration increases the intensity of the bands and shifts the maximum of these bands to low wavelength. The UV-vis characterization of the Pt/Zr (x)-HMS and Pt-W/Zr (x)-HMS catalysts is in good agreement with the results earned from XRD patterns.

|
Download:
|
| Figure 3. Diffuse reflectance UV-vis absorption spectra of Zr (x)-HMS catalysts ((a) with and (b) without introducing tungsten). | |
2.2. Morphology and elements distribution
Typical morphology of Pt-W/Zr (x)-HMS catalysts is observed through SEM photos (not shown here). SEM photographs show the catalysts after loading the metals lose their morphology and do not show the remarked structure for platinated catalyst [10].
For checking the Pt, W, Zr and Al contents, the catalysts were examined by XRF technique. The earned results are close to the expected theoretical ones for all catalysts.
H2 chemisorption method was used for discovering the metal dispersion. The results of H2 chemisorption (assuming H/metals=1) for the Pt-W/Zr (x)-HMS catalysts were reported in Table 1. The results show the addition of WO3 to Pt/Zr (x)-HMS catalysts [10] decreases the H2 adsorption ability and platinum dispersion on the catalysts surfaces.
2.3. Pore propertiesThe physical properties of our porous silica catalysts were measured from nitrogen adsorption-desorption isotherms and tabulated in Table 1. The physical properties of the parent HMS and Zr (x)-HMS samples have been reported in our previous work [10]. After supporting ZrO2 and finally WO3 (in this work) on HMS, the BET surface area and pore volume were found to decrease with the increase in oxide loading. About the HMS and Zr (x)-HMS samples, the surface area and pore volume of the Zr (x)-HMS samples loaded with WO3 decreased almost significantly, inferring the supported oxide should be dispersed onto the internal surfaces of these mesoporous catalysts especially in their micro pores (Table 1). The lower pore volume on Pt-W/Zr (5)-HMS supported catalyst presents that WO3/ZrO2 mixed oxides in compared with other catalysts may seriously block its mesopores.
2.4. AcidityA key feature in the catalytic isomerization is the catalyst acidity, and therefore the total acidity of our catalysts has been evaluated by NH3-TPD technique (Fig. 4 and Table 1). NH3-TPD technique has been extensively used to analyze the amount and strength of acid sites for solid catalysts. The presence of platinum on the samples does not significantly change the TPD profiles. Therefore, only the profiles of the platinated samples are shown here. The NH3-TPD results for Pt/Zr (x)-HMS catalysts were reported in Reference [10]. Although Zr4+ ions with low coordination create a notable acidity in the HMS support, these values are considerably enhanced after introducing WO3 species. The Pt-W/Zr (x)-HMS catalysts have a broad desorption band covering 150-500 ℃ and center at 170 ℃ (weak acid sites) and 260 ℃ (medium acid sites), implying the acid strength of these catalysts is widely distributed on their surfaces.

|
Download:
|
| Figure 4. NH3-TPD profiles for Pt-W/Zr (x)-HMS catalysts. | |
No direct correlation was found between the acid amount and the oxide loading. However, the samples with the fixed weight percent of WO3 (12 wt%) and lower Si/Zr molar ratios contained more acid sites. Among these catalysts, Pt-W/Zr (5)-HMS catalyst shows the lowest acid sites and its acid property does not follow the order of decreasing molar ratio. Since the higher loading of mixed oxide because of the lower surface area, it seems the good dispersion of this mixed oxide is necessary to create many acid sites.
Another key feature in catalytic isomerization is the nature of acid sites (Brönsted and Lewis). Therefore, the FT-IR spectra of pyridine adsorbed on Pt-W/Zr (x)-HMS catalysts were prepared to study these sites. The amounts of both types of acid sites have been estimated from the integrated absorption at room temperature. The IR vibration spectra of these catalysts are included the characteristic bands of pyridine coordinated to Lewis acid sites at ca. 1450 and 1609 cm-1, as well as the band at ca. 1550 cm-1 that is assigned to pyridinium ions adsorbed on protonic acid sites (Brönsted acid sites); whereas the band at 1480 cm-1 is a combined band originated from pyridine bonded to both Brönsted and Lewis acid sites [10, 15]. The results show the intensity of the Py-IR bands for both acid sites decreases when Si/Zr molar ratio increases.
According to literatures [20, 21], W=O terminal groups and W--(OH)--W (or W--OH) bonds have been ascribed to the Lewis and Brönsted acid sites, respectively. Also, Zr-WOx nanoclusters are responsible for the generation of strong Brönsted acid sites in WOx/ ZrO2 supported catalysts. Also, the results of previous researches show the formation of Brönsted acid sites depends on the coverage of support surface [15, 20, 21]. The conclusions of these researches allow us explaining our observed results of WZHM-x catalysts about the type and number of acid sites.
Judging from the area ratio, one result can decide the number of Lewis acid sites is rather more than that of Brönsted ones. The B/L ratio and the number of Brönsted acid sites for Pt-W/Zr (x)-HMS catalysts were tabulated in Table 1. It seems Pt-W/Zr (10)-HMS catalyst has a better interaction between Zr-WOx species. As a result, a good B/L ratio is observed for this catalyst.
2.5. Catalytic performanceThe n-C7 isomerization was tested over a series of catalysts that varies depending on the Si/Zr molar ratio, which were prepared with the same procedure simply by sol-gel method and impregnation in a solution of H2PtCl6·6H2O. The conversion and isomerization selectivity results of the n-C7 reactant at 200-350 ℃ temperature range, atmospheric pressure, 40 mL/min H2 gas flow, 2 mL/h n-C7 flow and 60 min of time on stream (TOS) using 1 g loading of each catalyst were expressed in Table 2. From the conversion results, it is clearly seen that W-containing materials are obviously more active than W-Free Pt/Zr (x)-HMS catalysts and the conversion of these catalysts increases with raising temperature. Also after incorporating W the activities of Pt-W/Zr (x)-HMS catalysts generally increase with decreasing the zirconium content (or with increasing Si/Zr ratio). The observed catalytic activity does not correlate well with the catalyst acidity, because the Pt-W/Zr (35)-HMS catalyst has the lowest number of Brönsted acid sites, however, its catalytic activity is high. It seems that a complex of reasons including surface properties, structural regularity, metal function and distribution, geometry, strength and type of acid sites should also be considered.
|
|
Table 2 Catalytic activity (Conv.), selectivity, coke amounts (C) and RON at different temperatures over prepared catalysts. |
About the isomerization selectivity and surface acidity, it is easy to find a relationship between them. Looking into results shows that at low conversion or low reaction temperature, the selectivity to isoheptanes (i-C7) for all the catalysts is high. By increasing the n-C7 conversion or the reaction temperature, the isomerization selectivity gradually reduces. Considering the catalytic activity and the selectivity to various products (especially the isomerized products), the ideal reaction temperature is 200 ℃ and the Pt-W/Zr (10)-HMS catalyst seems to perform this process better than other catalysts. In all, the total isomerization selectivity for all catalysts follows the order of Pt-W/Zr (10)-HMS > Pt-W/Zr (20)-HMS > Pt-W/ Zr (35)-HMS > Pt-W/Zr (5)-HMS.
Also, the further results of the selectivity to mono-branched (MOB) and multi-branched (MUB) isomers were shown in Table 2. It is clear the selectivity to these branched isomers almost follows the same order as that of the total selectivity for our catalysts. The selectivity behaviors of these catalysts are in general related to their properties such as acidity, metal dispersion, surface and pore properties.
The ratio of MUB to MOB isomers (R), within the temperature range, varies between 0.5 and near 1.0. In low temperature, R is very close to the thermodynamic equilibrium value (1.0). Since the molecular size of the MUB isomers is larger than that of the MOB ones and to diffuse these isomers easily, the size of pores and channels in the catalysts is very important in forming these types of isomers. It is well known that the smaller size of the channels prevents the easy diffusion of the primary reaction products; as a result, these products undergo successive reactions leading to cracking. This is because of the smaller size of MOB isomers is in favor of these pores. According to literature [22], the mechanism of n-paraffins isomerization and cracking involves the formation of isomers that are afterward cracked on the acid sites. With the increasing strength of acidic sites, the possibility of cracking alkanes adsorbed on the acidic sites increases.
The Pt-W/Zr (10)-HMS catalyst with proper mesoporous channels allows the good diffusion of MUB isomers through the pores before their cracking than other catalysts. Thus this catalyst has the best selectivity to MUB isomers and the R value.
The first catalyst (Pt-W/Zr (5)-HMS) has the highest cracking activity and low isomerization selectivity because of the strong B acidity nature and metals dispersion. Pt-W/Zr (10)-HMS is the second prepared catalyst that improves isomerization selectivity without significant loss of activity. This catalyst has the best selectivity to i-C7 products such as MOB, MUB and total isomers and the least selectivity to cracking products. These results are because of the high original strong B acidity, despite the low dispersion of metals. Third catalyst is Pt-W/Zr (20)-HMS. This catalyst after Pt-W/Zr (10)-HMS improves greatly isomerization selectivity and activity because of the good strong B acidity. The last catalyst is the Pt-W/Zr (35)-HMS with slightly improved isomerization selectivity because of the nearly loss of strong B acidity. This catalyst has the best activity and good dispersion of metals. According to behavior of catalysts and obtained results, it seems the strength and type of acidic sites more effective compared to other properties of catalysts for this process.
To justify the stability of Pt-W/Zr (x)-HMS catalysts in n-C7 isomerization at 300 ℃ after 72 h of time on stream, the thermal stability of carbonaceous formation on the surfaces of the spent catalysts has been analyzed by thermal gravimetric analysis (TGA). The weight percentages of coke as a function of catalysts nature were summarized in Table 2. Obviously, the coke amounts of Pt-W/ Zr (x)-HMS catalysts are lower than Pt/Zr (x)-HMS catalysts. Among tungstate catalysts, Pt-W/Zr (35)-HMS has the lowest deactivation with 16.7% coke amount. The results suggest that these catalysts are not deactivated significantly during 72 h TOS.
We also studied the effect of Pt-W/Zr (x)-HMS catalysts on research octane number (RON) of the gained products during each reactor test (Table 2). To calculate RON, we used the method described in our reported work [10]. Results show that Pt-W/Zr (10)-HMS at 350 ℃ compared to other catalysts provides higher RON, because of molecules with higher RONi and as expected from the isomerization and other products.
2.6. Kinetics studyBefore kinetics study, the Koros-Nowak and Madon-Boudart [10] tests were used to ensure the catalytic activity itself is the only reason affecting on the conversion (kinetic regime) and transport phenomena does not limit it (diffusion regime). The results show the rate and weight of catalyst change proportionally. Thus the isomerization reaction over these catalysts is controlled by kinetic regime.
The kinetic studies were carried out at various temperatures ranging from 200 to 350 ℃ with the hydrogen pressure in the range of 2.6-5.9 Pa and n-C7 pressure in the range of 0.002-0.009 Pa. The reaction rate is defined as follows:

|
(1) |
Another form of reaction rate, according to a power law is:

|
(2) |
In this reaction, k is the rate constant and is determined by below equation.
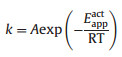
|
(3) |
where A is Arrhenius constant.
Linear fits from double-log plots of the kinetic data measured by varying the partial pressures of H2 (2.6-5.9 Pa) and n-C7 (0.002-0.009 Pa) at different temperatures were performed to decide the orders of H2 and n-C7 and these data were shown in Fig. 5. For deciding the order of n-C7 and H2 at different temperatures, the hydrogen and n-C7 pressures are kept constant with varying pressure of n-C7 and H2, respectively. The earned slopes from depicting logarithmic graphs of the isomerization reaction rates against the various pressures of any parts of H2 or n-C7 are expressed as the partial order of reaction. The results show the change of temperature has no significant effect on the partial order of isomerization reaction over each of these catalysts and the order amounts (nH2 and mC7 respectively) for Pt-W/Zr (5)-HMS are -0.1 and 0.8 and for other catalysts are -0.1 and 0.9. Negative amounts of nH2 suggest the inhibition effect of hydrogen (as expected [23, 24]) on the isomerization reaction.

|
Download:
|
| Figure 5. (a) Arrhenius plot and (b, c) double-log plots of the n-C7 isomerization reaction rate versus the partial pressures of hydrogen and n-C7 at different temperatures, respectively. | |
The apparent activation energy (Eappact) for isomerization reactions has been determined graphically from an Arrhenius plot (Fig. 5a) in 200 ℃ to 350 ℃ and at a constant PH2 and Pn-C7 for conversion levels below 10% where there is the best linear correlation between the logarithmic isomerization rate constant (ln k) and the inverse temperature (1/T). The Eappact values for Pt-W/Zr (5)-HMS, Pt-W/Zr (10)-HMS, Pt-W/Zr (20)-HMS and Pt-W/Zr (35)-HMS are 17.8, 8.7, 9.7 and 13.1 kJ/mol, respectively. These values of Eappact are lower than the reported values (~100-135 kJ/mol) in literatures [23, 24] that confirm the better reaction rate for our prepared catalysts. About our catalysts, Pt-W/Zr (10)-HMS shows the lowest activation energy for n-C7 isomerization reaction that presents this reaction will be easy on this catalyst. So i-C7 conversion for this catalyst will be better than others.
A simplified mechanism of bifunctional catalytic isomerization was proposed as a kinetic modeling according to the earned isomerized products for this reaction (Scheme 1).

|
Download:
|
| Scheme 1. Proposed kinetics model for Pt-W/Zr (x)-HMS catalysts in n-C7 isomerization reaction. | |
In this mechanism, the metallic functions create and hydrogenate the olefinic intermediates and the neighboring acidic functions catalyze the isomerization products. It is assumed the transport of olefins from the metallic sites to the acidic sites (step 4) is slow and the rate-determining step (RDS) [8]. In the low conversion, the reverse reaction in step (4) can be neglected. Thus a rate equation (r) for this step will be as follows.

|
(4) |
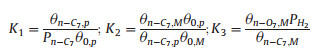
|
(5) |
where r is the reaction rate (mol/g/s); k4 is the rate constant of step 4; Ki is the adsorption equilibrium constant for step i (Ki=ki/k-i); Pn-C7 is the partial pressure of n-C7 (Pa); PH2 is the partial pressure of H2 (Pa); θn-O7, M is the coverage of metallic sites by n-olefins; θn-C7, M is the coverage of metallic sites by n-C7; θn-C7, p is the coverage of support mesopore sites by n-C7; θ0, a is the empty fraction of acid sites; θ0, p is the empty fraction of support mesopore sites; θ0, M is the empty fraction of metallic sites. Also, the summation of free and covered fractions of different sites is one.
According to these equations and the proposed mechanism and with using Langmuir adsorption isotherm, the following rate expression can be written.

|
(6) |
This equation confirms the inhibiting effect of hydrogen in this process.
3. ConclusionW-containing Pt/Zr-HMS type materials with various ZrO2 loadings (Si/Zr=5, 10, 20 & 35) without great degradation of the early HMS pore structure were prepared by co-impregnation method. For these mesoporous silica, tungstated/zirconia was generally dispersed inside the mesoporous channels of HMS, and the surface area and pore volume decreased with loading of these mixed oxides. Acidity results showed that these catalysts had both Lewis and Brönsted acid sites and the strength of Lewis acid sites was more than that of the Brönsted ones. The prepared catalysts had efficient conversion in catalyzing n-C7 isomerization with a high selectivity of i-C7. The catalytic activity did not directly correlate to the acid amounts. Loading tungstate in Pt/Zr (x)-HMS played an important role in stabilizing the Pt-W/Zr (x)-HMS catalysts because of its effect on acidic properties of supports. The ideal activity, selectivity, stability, RON and kinetic results were observed on HMS supported with 0.6%Pt/12%WO3/ZrO2 (x=10%). Based on this analysis the major conclusion should have been that Pt-W/Zr (10)-HMS shows the best performance in this reaction.
4. Experimental 4.1. MaterialsEthanol, tetraethyl orthosilicate, dodecyl amine and hydrochloric acid were purchased from Merck Company and zirconyl (Ⅳ) nitrate hydrate and ammonium meta tungstate hydrate were purchased from Sigma-Aldrich company. All materials were used without any pretreatment.
4.2. Catalyst developmentThe Zr-HMS materials with various Si/Zr molar ratios were synthesized by using a sol-gel method according to our reported work [10]. In these samples, zirconyl (Ⅳ) nitrate hydrate, dodecyl amine and tetraethyl orthosilicate were used as zirconium, surfactant and silica sources, respectively. W/Zr-HMS catalysts with W=12 wt% and Si/Zr=5, 10, 20 & 35 molar ratios were prepared by co-impregnation of separate solution of zirconyl (Ⅳ) nitrate hydrate and ammonium meta tungstate hydrate. The solid products were filtered and then dried at 110 ℃ overnight and calcined at 600 ℃ for 6 h in air.
The resultant calcined samples were also impregnated with suitable concentration of hexa chloro platinic acid (0.6 wt% of each support) and dried overnight at 110 ℃. This process was followed by calcination in air at 300 ℃ for 4 h. The prepared catalysts were named Pt/Zr (x)-HMS and Pt-W/Zr (x)-HMS that x is various molar ratios of Si/Zr.
4.3. Catalyst characterizationThe prepared catalysts were characterized by conventional techniques that will be explained briefly. The chemical compositions of the catalysts were evaluated by X-ray fluorescence (XRF) with an XRF-8410 Rh apparatus and a voltage of 60 kV. X-ray diffraction (XRD) patterns were recorded from 2θ 1° to 80° at 45 kV and 50 mA with a 0.06° 2θ-step and 1 s per step by an X-PERT diffractometer with Ni filter, graphite monochromator and Cu ka radiation (0.15406 nm) as an X-ray source. For measuring the metals dispersion, hydrogen chemisorption by a TPD/TPR analyzer (2900 Micromeritics) equipped with a thermal conductivity detector (TCD) was used. Fourier transform infrared (FT-IR) spectra were recorded by a BOMEM FT-IR spectrophotometer model AridZone TM, MB series in a spectral region from 4000 cm-1 to 400 cm-1. UV-vis diffuse reflectance (UV-vis DRS) was investigated by a Shimadzu UV-2100 spectrophotometer equipped with a diffuse reflectance attachment with an integrating sphere coated with BaSO4 as a reference in a 200-800 nm range. N2adsorption-desorption isotherms were recorded using an ASAP-2010 Micromeritics (USA) instrument at -196 ℃. Temperature programmed desorption of NH3 (NH3-TPD) was carried out on a TPD/TPR analyzer (2900 Micromeritics) equipped with a TCD. Fourier transform infrared of pyridine (Py-IR) was performed by a Nicolet-170 SX FT-IR spectrophotometer. Field emission scanning electron microscope (FESEM) with a HITACHI S-4160 instrument operating at an accelerating voltage of 30 kV was used for determining the morphology and thermogravimetric/differential thermal analysis (TG/DTA) was performed by a STA503 M instrument under air atmosphere in a range of 25-800 ℃ with a heating rate of 10 ℃/min.
4.4. Catalytic testsBefore measuring the catalytic activity, 1 g of each calcined catalyst was pretreated in a fixed bed reactor by hydrogen (2 h, 400 ℃). H2 flow (40 mL/min), n-heptane flow (2 mL/h) and liquid hourly space velocity (1.0 h-1) earned as optimized conditions. The products were analyzed on-line by gas chromatography (Agilent Technologies 7890A) equipped with a flame ionization detector (FID). From these chromatographic data, conversion, selectivity and RON for mixture of products were calculated by applying the method that was described in Reference [10].
| [1] | V.A. Shkurenok, M.D. Smolikov, S.S. Yablokova, Pt/WO3/ZrO2 catalysts for n-heptane isomerization. Procedia Eng. 113 (2015) 62–67. DOI:10.1016/j.proeng.2015.07.291 |
| [2] | L.N. Kuznetsova, A.V. Kazbanova, P.N. Kuznetsov, L.S. Tarasova, Activity of the Pt/WO42-/ZrO2 catalyst in hydroisomerization reaction of n-heptane-benzene mixture. Petrol. Chem. 55 (2015) 57–62. DOI:10.1134/S0965544115010090 |
| [3] | A. Miyaji, T. Echizen, K. Nagata, Y. Yoshinaga, T. Okuhara, Selective hydroisomerization of n-pentane to isopentane over highly dispersed Pd-H4SiW12O40/SiO2. J. Mol. Catal. A:Chem. 201 (2003) 145–153. DOI:10.1016/S1381-1169(03)00129-8 |
| [4] | A. Miyaji, R. Ohnishi, T. Okuhara, Skeletal isomerization of n-heptane over Pd-H4SiW12O40 supported on SiO2:comparative study with typical bifunctional catalysts. Appl. Catal. A:Gen. 262 (2004) 143–148. DOI:10.1016/j.apcata.2003.11.041 |
| [5] | K. Föttinger, K. Zorn, H. Vinek, Influence of the sulfate content on the activity of Pt containing sulfated zirconia. Appl. Catal. A:Gen. 284 (2005) 69–75. DOI:10.1016/j.apcata.2005.01.019 |
| [6] | Y.C. Yang, H.S. Weng, Al-promoted Pt/SO42-/ZrO2 with low sulfate content for n-heptane isomerization. Appl. Catal. A:Gen. 384 (2010) 94–100. DOI:10.1016/j.apcata.2010.06.010 |
| [7] | Y. Nie, S. Shang, X. Xu, In2O3-doped Pt/WO3/ZrO2 as a novel efficient catalyst for hydroisomerization of n-heptane. Appl. Catal. A:Gen. 433 (2012) 69–74. |
| [8] | S. Kuba, B.C. Gates, R.K. Grasselli, H. Knözinger, An active and selective alkane isomerization catalyst:iron-and platinum-promoted tungstated zirconia. Chem. Commun. 4 (2001) 321–322. |
| [9] | D. Kaucký, B. Wichterlová, J. Dedecek, Z. Sobalik, I. Jakubec, Effect of the particle size and surface area of tungstated zirconia on the WOx nuclearity and n-heptane isomerization over Pt/WO3-ZrO2. Appl. Catal. A:Gen. 397 (2011) 82–93. DOI:10.1016/j.apcata.2011.02.020 |
| [10] | M.H. Peyrovi, N. Parsafard, P. Peyrovi, Influence of zirconium addition in platinum-hexagonal mesoporous silica (Pt-HMS) catalysts for reforming of n-heptane. Ind. Eng. Chem. Res. 53 (2014) 14253–14262. DOI:10.1021/ie5024244 |
| [11] | S. Lecarpentier, K. van Gestel, K. Thomas, M. Houalla, Study of Ir/WO3/ZrO2-SiO2 ring opening catalysts:part I:characterization. J. Catal. 245 (2007) 45–54. DOI:10.1016/j.jcat.2006.09.018 |
| [12] | A.H. Karim, S. Triwahyono, A.A. Jalil, H. Hattori, WO3 monolayer loaded on ZrO2:property-activity relationship in n-butane isomerization evidenced by hydrogen adsorption and IR studies. Appl. Catal. A:Gen. 433 (2012) 49–57. |
| [13] | J.A. Wang, L.F. Chen, L.E. Norena, Mesoporous structure, surface acidity and catalytic properties of Pt/Zr-MCM-41 catalysts promoted with 12-tungstophosphoric acid. Micropor. Mesopor. Mater. 112 (2008) 61–76. DOI:10.1016/j.micromeso.2007.09.015 |
| [14] | O.Y. Gutiérrez, G.A. Fuentes, C. Salcedo, T. Klimova, SBA-15 supports modified by Ti and Zr grafting for NiMo hydrodesulfurization catalysts. Catal. Today 116 (2006) 485–497. DOI:10.1016/j.cattod.2006.06.035 |
| [15] | J.A. Cecilia, C. García-Sancho, J.M. Mérida-Robles, WO3 supported on Zr doped mesoporous SBA-15 silica for glycerol dehydration to acrolein. Appl. Catal. A:Gen. 516 (2016) 30–40. DOI:10.1016/j.apcata.2016.02.016 |
| [16] | E. Rodrıguez-Castellón, A. Jiménez-López, P. Maireles-Torres, Textural and structural properties and surface acidity characterization of mesoporous silica-zirconia molecular sieves. J. Solid State Chem. 175 (2003) 159–169. DOI:10.1016/S0022-4596(03)00218-4 |
| [17] | Y. Du, S. Liu, Y. Zhang, Urea-assisted synthesis of hydrothermally stable Zr-SBA-15 and catalytic properties over their sulfated samples. Micropor. Mesopor. Mater. 121 (2009) 185–193. DOI:10.1016/j.micromeso.2009.01.030 |
| [18] | T.A. Zepeda, A. Infantes-Molina, J.D. de León, Hydrodesulfurization enhancement of heavy and light S-hydrocarbons on NiMo/HMS catalysts modified with Al and P. Appl. Catal. A:Gen. 484 (2014) 108–121. DOI:10.1016/j.apcata.2014.06.033 |
| [19] | B. Pawelec, T. Halachev, A. Olivas, T.A. Zepeda, Impact of preparation method and support modification on the activity of mesoporous hydrotreating CoMo catalysts. Appl. Catal. A:Gen. 348 (2008) 30–41. DOI:10.1016/j.apcata.2008.06.014 |
| [20] | M. Massa, A. Andersson, E. Finocchio, Performance of ZrO2-supported Nb-and W-oxide in the gas-phase dehydration of glycerol to acrolein. J. Catal. 297 (2013) 93–109. DOI:10.1016/j.jcat.2012.09.021 |
| [21] | T. Kitano, T. Hayashi, T. Uesaka, Effect of high-temperature calcination on the generation of Brönsted acid sites on WO3/Al2O3. ChemCatChem 6 (2014) 2011–2020. DOI:10.1002/cctc.v6.7 |
| [22] | J.M. Grau, C.R. Vera, V.M. Benitez, J.C. Yori, Optimization of Pt/WOx-ZrO2 catalysts for the production of reformulated fuels by isomerization-cracking of medium length C8-C12 paraffins. Energy Fuel 22 (2008) 1680–1686. DOI:10.1021/ef700711q |
| [23] | A. Holló, J. Hancsok, D. Kalló, Kinetics of hydroisomerization of C5-C7 alkanes and their mixtures over platinum containing mordenite. Appl. Catal. A:Gen. 229 (2002) 93–102. DOI:10.1016/S0926-860X(02)00018-2 |
| [24] | Ü.B. Demirci, F. Garin, Kinetic study of n-heptane conversion on sulfated zirconia-supported platinum catalyst:the metal-proton adduct is the active site. J. Mol. Catal. A:Chem. 188 (2002) 233–243. DOI:10.1016/S1381-1169(02)00337-0 |
 2017, Vol. 28
2017, Vol. 28 




