b Department of Chemistry, State Key Laboratory of Molecular Engineering of Polymers, and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Collaborative Innovation Center of Chemistry for Energy Materials (iChEM), Fudan University, Shanghai 200433, China;
c State Key Lab of Transducer Technology, Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Sciences, Shanghai 200050, China;
d Materials Science and Technology Program, College of Arts and Sciences, Qatar Department of Chemistry, Qatar University, PO Box 2713, Doha, Qatar;
e College of Science, King Saud University, Riyadh 11451, Saudi Arabia
Pancreatic cancer is the sixth leading cause of cancer-associated deaths in China with about 90, 100 estimated new cases and 79, 400 estimated deaths by the end of 2015 [1]. It is projected that by 2030, pancreatic cancer will become the second leading cause of cancer-related deaths in United States [2]. Radical resection is still the sole curative option, but about 80% of pancreatic cancers are diagnosed at locally advanced or/and metastatic stages, and curative surgical resection is not possible [3]. Therefore, chemotherapy remains the main treatment for most patients with pancreatic cancer to alleviate the symptom and prolong the survival.
Gemcitabine (Gem, 2', 2'-difluoro-2'-deoxycytidine, dFdC) has been currently recommended as the first-line chemotherapeutic drug available for treatment of pancreatic cancer, but it merely extends the median survival of patients for several months [4]. The therapeutic efficacy of Gem is disappointing as a result of a short half-life and poor cell membrane permeability. Following intravenous administration, most of the Gem undergoes rapid metabolism into inactive dFdU (2', 2'-difluoro-2'-deoxyuridine) by cytidine deaminase in the blood, making Gem half-life as short as 8-17 min in human plasma and 9 min in murine plasma [5, 6]. Moreover, Gem is a prodrug that requires cellular uptake by the hENT1 (human equilibrative nucleoside transporter 1) receptors on the cancer cells surface. Unfortunately, more than 65% of patients lack this receptor, further limiting the therapeutic efficacy of the Gem [7].
In order to overcome these limitations, Gem has been delivered by employing various drug delivery vehicles, such as targeted liposomes [8], micelles [9], and polymeric nanoparticulate [10], mesoporous silica nanoparticles [11, 12]. Among various drug delivery systems [13-16], vesicles are promising candidates due to their high loading capacity with a hollow structure. Hollow polymeric nanocapsules have been used extensively to improve the effectiveness of therapeutic agents by increasing their bio availability and promoting accumulated release within tumor tissues [17]. For example, Paolino et al. used a double-emulsion to produce Gem loaded-PLA nanocapsules of~200nm with~90% encapsulation efficiency (EE) and~1.33% loading efficiency [18]. Mesoporous silica vesicles (MSVs) which are hollow spheres with tunable pores and thin walls have gathered remarkable attention because of their special characteristics such as tunable surface hydrophilicity [19], mechanical robustness [20], large cavity for drug loading [21], efficient intracellular delivery [22], and so on. Zhang et al. developed a two-step approach to synthesis silica vesicles used a tri-block polymer EO39BO47EO39 [23]. Although the samples theyobtained were uniform, the process was complex and expensive, and thus limiting their practical application. Therefore, a simple, convenient, cheap approach to synthesis vesicles is highly desired as efficient drug carriers.
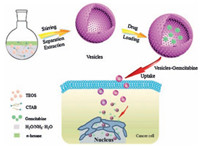
In this study, we synthesized water dispersible mesoporous silica vesicles (MSVs) with a large pore size (6.7nm), large pore volume (1.57cm3/g), and perpendicular mesoporous channels directly connected with thecavity for effective delivery of Gem into pancreatic cancer cells through a biliquid-interface co-assembly process (Scheme 1). Encapsulation of hydrophilic anti-cancer drugs Gem in the MSVs was achieved via nanocasting approach, achieving a loading efficiency of 92.7% and encapsulation efficiency of 8.33%. The confocal laser scanning microscopy demonstrated fluorescein isothiocyanate (FITC) labeled MSVs could be easily taken up by the pancreatic cancer cells. These FITC labeled MSVs could even reach the nuclei of the cancer cells. The Gem-MSVs show pH responsive release behavior, discharging Gem more quickly from cavity through mesopore channels in acidic condition (pH 5.0). The Gem-MSVs showed enhanced anticancer effect compared with free Gem. These findings indicate the potential of the MSVs in therapy of pancreatic cancer.
|
Download:
|
| Scheme 1. Illustration for the synthesis of mesoporous silica vesicles using CTAB as a structure-directing agent, TEOS as silica source in the n-hexane/water biliquid system, and the loading of Gem into MSVs via the "nanocasting" method of Gem loading into MSVs, and finally the cellular internalization process of Gem-MSVs. | |
2. Results and discussion 2.1. Synthesis and characterization of the mesoporous silica vesicles
Gem is the most widely used anticancer drug for pancreatic cancer. However, poor cell membrane permeability and short halflife lead to its discouraging therapeutic efficacy [24]. Being a nucleoside analog, Gem is rapidly converted into its inactive metabolite dFdU (2', 2'-difluorodeoxyuridine) by cytidine deaminase after systemic administration [25]. In addition, Gem is too hydrophilic to cross the cell membrane, which must rely on specialized nucleoside transporters, such as human equilibrative nucleoside transporter 1 and human equilibrative nucleoside transporter 2 [26], while only one-third of pancreatic cancers express such a transporter. So it is highly important to design drug delivery systems, which can protect the loaded drug from enzymatic metabolism, penetrate cell membrane in transporter independent pathway, and release the cargo in a controllable and sustained manner, so as to improve the Gem efficacy. It has been reported that gemcitabine-loaded lipid nanocapsules can be taken up by endocytosis [27], while polymersomes encapsulated with gemcitabine and doxorubicin showed significant cytotoxicity to breast and pancreatic cancer cells [28].
In the present study, we attempted to synthesize and administrate MSVs as a novel therapeutic delivery system for Gem in treatment of pancreatic cancer. The synthesized MSVs have diameter of 0.6-2.4 mm and exhibit a nearly spherical shape with a hollow structure and 50nm thick porous walls, as observed by transmission electron microscopy (Fig. 1a, b). High magnification TEM image reveals radially aligned mesopore channels in the walls of MSVs, which is beneficial for cargo storage and delivery (Fig. 1b, insert). Scanning electron microscopy (SEM) image of MSVs also shows a spherical shape and hollow structure (Fig. 1c). The confocal laser scanning image clearly indicate excellent dispensability of MSVs and confirmed the MSVs were successfully labeled with FTTC without destroying the morphology of MSVs, and its three dimension reconstructions imagine also showed their hollow characteristics, in good agreement with the TEM results (Fig. 1d). The nitrogen adsorption measurements indicated that the MSVs have large mesopores of 6.7nm in the walls, high surface area of 728m2/g and large pore volume of 1.57cm3/g (Fig. 2). The large pore size is favorable for efficient drug loading, while the large pore volume can guarantee a large drug-loading capacity. Under an optimized weight ratio of MSVs and Gem at 12:1, the encapsulation efficiency of Gem-MSVs was calculated to be about 92.7%, and the drug-loading efficiency was about 8.33%.

|
Download:
|
| Figure 1. Characteristics of synthesized MSVs. (a) and (b) TEM images of the mesoporous silica vesicles with different magnifications, the inset in panel b shows the radially aligned mesopore channels in the walls of MSVs. (c), SEM image of MSVs. (d) Laser scanning confocal image of the FITC-labeled MSVs, and the inset in panel d is the 3-dimensional reconstruction imagine of one FITC-MSV. | |

|
Download:
|
| Figure 2. (a) Nitrogen adsorption-desorption isotherms recorded at 77 K and (b) pore size distribution curves calculated from the adsorption branches by using the Barrett-Joyner-Halanda (BJH) method. | |
2.2. Drug release study
The perpendicularly aligned mesoporous walls of MSVs not only serves as a protective barrier to prevent the loaded Gem from enzymatic catalyst, but also provides lots of nanochannels for sustained release of Gem from the MSVs cavity. In this study, the drug release behavior of Gem-MSVs at 37 ℃ was investigated in different phosphate buffered saline (pH 7.4) and acetate buffered saline (pH 5.0), respectively. No initial burst release pattern was observed in either pH conditions indicating that Gem was mainly encapsulated in the cavity and mesopores of the MSVs. The Gem-MSVs exhibit sustained release features in both pH condition. In case of pH 5.0, Gem-MSVs release Gem continuously at rate of 0.31 mg/h within 24h, which is faster than that in pH 7.4 (0.07mg/h). By 24h, 62.8% of the Gem was released in the acetate buffered saline, while only 15.7% of the Gem was released in the phosphate buffered saline. These results clearly reveal an accelerated drug release of Gem-MSVs at pH 5.0 as compared to pH 7.4 conditions. Such an enhanced release is due to the protonation of the Gem molecules under acidic condition, which improves its water solubility (Fig. 3b). This interesting pH responsive release behavior of Gem-MSVs greatly benefits the Gem release for cancer therapy, due to the acidic tumor microenvironments. That will eventually improve the chemotherapeutic efficiency of Gem, and reduce its side effects [29].

|
Download:
|
| Figure 3. (a) The cumulative release curve of Gem from MSVs at different pH conditions. (b) Scheme illustration of the protonation of Gem at lower pH condition due to the improved solubility which accelerates its release from the MSVs. | |
2.3. Cellular uptake analysis
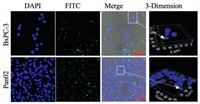
Fluorescently labeling the MSVs makes them to be easily tracked using fluorescent microcopy. The internalization of MSVs by pancreatic cancer cell Pan02 and BxPC-3 cells were confirmed under laser scanning confocal microscopy. Most of Pan02 cells and majority of BxPC-3 cells took up the FITC-labeled MSVs after co-cultured for 4 h and the vesicles were mainly localized in the cytoplasmic region of the cells (Fig. 4). The high uptake of vesicles by pancreatic cancer cell lines suggests that MSVs may interact with plasma membranes of pancreatic cancer cells and enter cells through endocytosis effectively [30, 31]. The excellent cellular uptake performance suggested the MSVs should be ideal nano-carriers for drug delivery. Furthermore, by using a novel three-dimensional reconstruction technique for the laser scanning confocal image, we found that a few FITC-labeled MSVs reached the nuclei of both human and mouse pancreatic cancer cells, i.e. the site that the Gem takes effect (Fig. 4). These results strongly indicate that our MSVs could deliver Gem molecules directly into their target site. Consequently, our MSVs are promising vector for Gem to avoid the multi-drug resistance mechanism of the pancreatic cancer cell to chemotherapeutic agents [32].
|
Download:
|
| Figure 4. Fluorescent confocal microscopy images of pancreatic cancer cells taking up the FITC-mesoporous silica vesicles, nuclei of cells were counterstained using DAPI (blue). | |
2.4. Cell viability assay
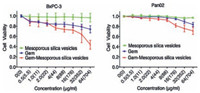
The cytotoxic effects of Gem, MSVs, and Gem-MSVs were evaluated in both human and mouse pancreatic cancer cells using the CCK-8 assay. As summarized in Fig. 5, pure MSVs show little toxic effect on pancreatic cancer cells within the used gradient concentrations (5.5-704 mg/mL). Both Gem and Gem-MSVs inhibited the growth of BxPC-3 and Pan02 in a dose dependent manner. Importantly, it also showed that Gem-MSVs exhibited higher cytotoxicity than free Gem. It is due to our MSVs protect Gem from inactivation, its excellent cellular uptake performance and its ability to directly reach the nuclei of the cancer cells. The findings provide strong evidence in support of a promising prospect for therapeutic application of MSVs as Gem delivery system for effective treatment of pancreatic cancer.
|
Download:
|
| Figure 5. In vitro cytotoxicity assay of Gem-mesoporous silica vesicles on human pancreatic cancer cell line BxPC-3 (a) andmouse pancreatic cancer cell line (b). The horizontal is the concentration of Gem, and MSVs (in parentheses) used. | |
3. Conclusion
In conclusion, hydrophilic mesoporous silica vesicles (MSVs) with large mesopores that are perpendicularly aligned and connected with the cavity have been synthesized through a biliquid-interface co-assembly process. Due to their unique pore orientation and large pore volume, MSVs show good performance in loading Gem and exhibit pH responsive release behavior. As a result of their excellent biocompatibility, the obtained MSVs can be easily taken up by the pancreatic cancer cells and even reach thenuclei of the cancer cells, exhibiting enhanced anticancer effect compared with free Gem. The results presented in this study provideda rationale for future further development of gemcitabine-loaded vesicles, containing single or combined drugs, to be used for the treatment of pancreatic cancer.
4. Experimental 4.1. MaterialsTetraethyl orthosilicate (TEOS), n-hexane, concentrated ammonia solution (28wt%), and cetyltrimethyl ammonium bromide (CTAB) were of analytical grade (Shanghai Chemical Corp.) Fluorescein isothiocyanate (FITC) and 3-aminopropyltriethoxysilane (APTES) were purchased from Sigma-Aldrich. Gemcitabine was purchased from MedChemExpress. Human pancreatic cancer cell line BxPC-3 was obtained from Type Culture Collection Cell Bank of Chinese Academy of Sciences (Shanghai, China), murine pancreatic adenocarcinoma cell line Pan02 was purchased from the Frederick National Laboratory for Cancer Research (Maryland, USA). RPMI 1640 and fetal bovine serum (FBS) were purchased from Gibco. CCK-8 was purchased from Dojindo. DAPI mounting reagent was purchased from Thermo fisher.
4.2. Methods 4.2.1. Synthesis of FITC-labeled mesoporous silica vesiclesMesoporous silica vesicles were synthesized using CTAB as the structure-directing agent, tetraethoxysilane (TEOS) as silica source in n-hexane/water biliquid system according to our previous report with some modification [33]. Briefly, CTAB (0.3g) was dissolved in deionized water (30mL) containing 0.8mL of concentrated ammonia aqueous solution (25wt%) under ultrasonication. Into the obtained solution, 30mL of n-hexane containing 1.5mL of TEOS was added with stirring, resulting in a white emulsion. The reactionwas allowed to proceed for 8h with continuous mechanical stirring (160rpm) at 35 ℃. After reaction, the sample was collected by centrifugation at 3000rpm and washed sequentially with ethanol and deionized water three times. The obtained sample was dispersed in 100mL acetone and refluxed at 70 ℃ for 12 h to remove CTAB templates, and washed sequentially with ethanol and deionized water three times. The sample was finally vacuum dried at 30 ℃ overnight. In order to localize the MSVs within pancreatic cancer cells, FITC was grafted onto the MSVs according to our previous report [34].
4.2.2. Preparation of gemcitabine-loaded MSVsEncapsulation of hydrophilic anticancer drugs Gem in MSVs was achieved via a "nanocasting" strategy through which Gem molecules can be deposited inside the vesicles during the evaporation of solvent from the casting ethanol/Gem solution. Briefly, purified MSVs (0.32 mg) were first mixed with 25 mL of ethanol solution of gemcitabine (1.08 mg/mL). After evaporation of solvent, we used cool water to wash samples 3 times for washing down the drug GEM adhere to the surface of MSVs. Then we used ultraviolet-visible (UV-vis) spectrophotometer to measure the collected eluent to calculate the concentration of not-loaded drug GEM. The standard curve of GEM is y=0.036χ-0.012, R2=0.99. Accordingly, we can calculate the concentration of loaded drug GEM in MSVs. The Fourier transform infrared (FI-TR) was employed to prove the GEM was successful loading in MSVs (S1~3). The encapsulation efficiency and loading efficiency were calculated using the formulas below:

|
(1) |

|
(2) |
To measure gemcitabine release, freshly prepared Gem-MSVs dispersed in neutral medium (PBS, pH 7.4) or acidic system (Na2HPO4/NaH2PO4 aq., pH 5.0) were placed in a flask with stirring at 37.0 ℃. Samples were taken at time points and analyzed by UV-vis spectrophotometer as described above. The amount of gemcitabine released from Gem-MSVs was expressed as a percentage of total Gem and plotted.
4.2.4. Cell culturePan02 and BxPC-3 pancreatic cancer cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum and streptomycin (100 mg/mL) and penicillin (100 units/mL), and maintained in humidified air containing 5% CO2 at 37 ℃.
4.2.5. Cell uptake assayPan02 and BxPC-3 cells were seeded in a 6-well plate containing a sterilized cover slip. After overnight incubation to bring the cells adheres to the cover slips, the culture medium was replaced with 2 mL of complete medium containing FITC-MSVs (100 mg/mL). After incubation for another 4 h, the cells were washed with PBS three times and fixed in 100% acetone for 5 min. Before observation under confocal laser scanning microscopy (Leica-SP8), the nucleus of cells was counterstained with DAPI.
4.2.6. In vitro cell viability assayThe in vitro antitumor efficacy of Gem-MSVs was assessed using a standard CCK-8 assay in both two pancreatic cancer cell lines. Briefly, pancreatic cancer cells BxPC-3 and Pan02 were seeded at 20, 000 cells/well in 100 mL of media in 96-well plates and allowed to grow until 90% confluent. Pancreatic cancer cells were treated with gradient concentrations of free Gem, empty MSVs, Gem-MSVs, and incubated for 24 h. Untreated cells were considered as positive control. After 24 h incubation, 10 mL of CCK-8 was added to each well and incubated for another 3 h. The absorbance of each well at 450 nm was measured using a Bio-Tek Synergy 2 (Bio-Tek, USA). Mean and standard deviation for 6 parallel wells for each sample were reported. The cell viability values were determined in relation to control cells cultured in drug-free media.
4.2.7. Measurements and characterizationTransmission electron microscopy (TEM) images were observed on a JEOL 2011 microscope (Japan) operated at 200 kV. Scanning electron microscopy (SEM) images were collected with a Philips XL30 electron microscope operated at 20 kV. The preparing samples were knocked by pestle during SEM experiment process. The Brunauer-Emmett-Teller (BET) method was utilized to calculate the specific surface areas using adsorption data in a relative pressure range from 0.005 to 0.35. Using the Barrett-Joyner-Halenda (BJH) model, the pore volumes and pore size distributions were derived from the adsorption branches of isotherms, and the total pore volumes (Vt) were estimated from the adsorbed amount at a relative pressure P/P0 of 0.992. UV-vis spectra were obtained by using a Shimadzu (UV-2401 PC) UV-vis recording spectrophotometer. Fluorescence microscopy observation was carried out by means of laser scanning confocal microscope (Leica TCS SP8, Germany) using a 480 nm excitation source with a 515-565 nm band pass filter and a transmitted light detector. The absorbance of each well at 450 nm was measured using a Bio-Tek Synergy 2 (Bio-Tek, USA).
AcknowledgmentsThis work was supported by National Natural Science Foundation of China (Nos. 51372041, 51422202), the Shanghai Committee of Science and Technology (No. 13140902401), the "ShuGuang" Project (No. 13SG02) of Shanghai Municipal Education Commission, Shanghai Municipal Science and Technology Commission (No. 13140902401), National Youth Top-notch Talent Support Program in China, and Qatar University (No. QUUG-CAS-DMST-1516-18). The authors extend their sincere appreciations to the Deanship of Scientific Research at King Saud University for its funding this Prolific Research group (No. PRG-1437-32).
| [1] | W. Chen, R. Zheng, P.D. Baade, Cancer statistics in China, 2015. CA:Cancer J. Clin. 66 (2016) 115–132. DOI:10.3322/caac.21338 |
| [2] | L. Rahib, B.D. Smith, R. Aizenberg, Projecting cancer incidence and deaths to 2030:the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74 (2014) 2913–2921. DOI:10.1158/0008-5472.CAN-14-0155 |
| [3] | R.D. Peixoto, M. Ho, D.J. Renouf, Eligibility of metastatic pancreatic cancer patients for first-line palliative intent nab-paclitaxel plus gemcitabine versus FOLFIRINOX. Am. J. Clin. Oncol. (2015) . DOI:10.1097/coc.0000000000000193 |
| [4] | V. Heinemann, M. Reni, M. Ychou, Tumour-stroma interactions in pancreatic ductal adenocarcinoma:rationale and current evidence for new therapeutic strategies. Cancer Treat. Rev. 40 (2014) 118–128. DOI:10.1016/j.ctrv.2013.04.004 |
| [5] | J.M. Reid, W. Qu, S.L. Safgren, Phase Ⅰ trial and pharmacokinetics of gemcitabine in children with advanced solid tumors. J. Clin. Oncol. 22 (2004) 2445–2451. DOI:10.1200/JCO.2004.10.142 |
| [6] | R. Moog, A.M. Burger, M. Brandl, Change in pharmacokinetic and pharmacodynamic behavior of gemcitabine in human tumor xenografts upon entrapment in vesicular phospholipid gels. Cancer Chemother. Pharmacol. 49 (2002) 356–366. DOI:10.1007/s00280-002-0428-4 |
| [7] | J.J. Farrell, H. Elsaleh, M. Garcia, Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology 136 (2009) 187–195. DOI:10.1053/j.gastro.2008.09.067 |
| [8] | E. DallaPozza, C. Lerda, C. Costanzo, Targeting gemcitabine containing liposomes to CD44 expressing pancreatic adenocarcinoma cells causes an increase in the antitumoral activity. Biochim. Biophys. Acta 1828 (2013) 1396–1404. DOI:10.1016/j.bbamem.2013.01.020 |
| [9] | S.J. Zhu, P. Wonganan, P.D. Lansakara, The effect of the acid-sensitivity of 4-(N)-stearoyl gemcitabine-loaded micelles on drug resistance caused by RRM1 overexpression. Biomaterials 34 (2013) 2327–2339. DOI:10.1016/j.biomaterials.2012.11.053 |
| [10] | J.L. Arias, L.H. Reddy, P. Couvreur, Polymeric nanoparticulate system augmented the anticancer therapeutic efficacy of gemcitabine. J. Drug Target. 17 (2009) 586–598. DOI:10.1080/10611860903105739 |
| [11] | A. Malfanti, I. Miletto, E. Bottinelli, Delivery of gemcitabine prodrugs employing mesoporous silica nanoparticles. Molecules 21 (2016) 522. DOI:10.3390/molecules21040522 |
| [12] | H. Meng, M.Y. Wang, H.Y. Liu, Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano 9 (2015) 3540–3557. DOI:10.1021/acsnano.5b00510 |
| [13] | J.X. Shao, M.J. Xuan, T.Y. Si, L.R. Dai, Q. He, Biointerfacing polymeric microcapsules for in vivo near-infrared light-triggered drug release. Nanoscale 7 (2015) 19092–19098. DOI:10.1039/C5NR06350G |
| [14] | J.X. Shao, M.J. Xuan, L.R. Dai, Near-infrared-activated nanocalorifiers in microcapsules:vapor bubble generation for in vivo enhanced cancer therapy. Angew. Chem. Int. Ed. Engl. 54 (2015) 12782–12787. DOI:10.1002/anie.201506115 |
| [15] | M.J. Xuan, J.X. Shao, L.R. Dai, Q. He, J.B. Li, Macrophage cell membrane camouflaged mesoporous silica nanocapsulesfor in vivo cancer therapy. Adv. Healthc. Mater. 4 (2015) 1645–1652. DOI:10.1002/adhm.v4.11 |
| [16] | M.J. Xuan, J.X. Shao, L.R. Dai, J.B. Li, Q. He, Macrophage cell membrane camouflaged au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl. Mater. Interfaces 8 (2016) 9610–9618. DOI:10.1021/acsami.6b00853 |
| [17] | C.E. Mora-Huertas, H. Fessi, A. Elaissari, Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 385 (2010) 113–142. DOI:10.1016/j.ijpharm.2009.10.018 |
| [18] | D. Paolino, D. Cosco, M. Celano, Gemcitabine-loaded biocompatible nanocapsules for the effective treatment of human cancer. Nanomedicine (Lond.) 8 (2013) 193–201. DOI:10.2217/nnm.12.101 |
| [19] | C.B. Field, M.J. Behrenfeld, J.T. Randerson, P. Falkowski, Primary production of the biosphere:integrating terrestrial and oceanic components. Science 281 (1998) 237–240. DOI:10.1126/science.281.5374.237 |
| [20] | F.H. Meng, Z.Y. Zhong, J. Feijen, Stimuli-responsive polymersomes for programmed drug delivery. Biomacromolecules 10 (2009) 197–209. DOI:10.1021/bm801127d |
| [21] | F.Q. Tang, L.L. Li, D. Chen, Mesoporous silica nanoparticles:synthesis, biocompatibility and drug delivery. Adv. Mater. 24 (2012) 1504–1534. DOI:10.1002/adma.201104763 |
| [22] | A. Sood, R. Panchagnula, Peroral route:an opportunity for protein and peptide drug delivery. Chem. Rev. 101 (2001) 3275–3303. DOI:10.1021/cr000700m |
| [23] | J. Zhang, S. Karmakar, M.H. Yu, Synthesis of silica vesicles with controlled entrance size for high loading, sustained release, and cellular delivery of therapeutical proteins. Small 10 (2014) 5068–5076. |
| [24] | M.L. Immordino, P. Brusa, F. Rocco, Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing lipophilic gemcitabine prodrugs. J. Control. Release 100 (2004) 331–346. DOI:10.1016/j.jconrel.2004.09.001 |
| [25] | D. Paolino, D. Cosco, L. Racanicchi, Gemcitabine-loaded PEGylatedunilamellar liposomes vs GEMZAR:biodistribution, pharmacokinetic features and in vivo antitumoractivity. J. Control. Release 144 (2010) 144–150. DOI:10.1016/j.jconrel.2010.02.021 |
| [26] | X.M. Tao, J.C. Wang, J.B. Wang, Enhanced anticancer activity of gemcitabine coupling with conjugated linoleic acid against human breast cancer in vitro and in vivo. Eur. J. Pharm. Biopharm. 82 (2012) 401–409. DOI:10.1016/j.ejpb.2012.06.007 |
| [27] | E. Garcion, A. Lamprecht, B. Heurtault, A new generation of anticancer drug-loaded, colloidal vectors reverses multidrug resistance in glioma and reduces tumor progression in rats. Mol. Cancer Ther. 5 (2006) 1710–1722. DOI:10.1158/1535-7163.MCT-06-0289 |
| [28] | R. Nahire, M.K. Haldar, S. Paul, Multifunctional polymersomes for cytosolic delivery of gemcitabine and doxorubicin to cancer cells. Biomaterials 35 (2014) 6482–6497. DOI:10.1016/j.biomaterials.2014.04.026 |
| [29] | K. Ulbrich, V. Subr, Polymeric anticancer drugs with pH-controlled activation. Adv. Drug Deliv. Rev. 56 (2004) 1023–1050. DOI:10.1016/j.addr.2003.10.040 |
| [30] | J. Panyam, W.Z. Zhou, S. Prabha, S.K. Sahoo, V. Labhasetwar, Rapid endolysosomal escape of poly (DL-lactide-co-glycolide) nanoparticles:implications for drug and gene delivery. FASEB J. 16 (2002) 1217–1226. DOI:10.1096/fj.02-0088com |
| [31] | J. Rejman, V. Oberle, I.S. Zuhorn, D. Hoekstra, Size-dependent internalizationof particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 377 (2004) 159–169. DOI:10.1042/bj20031253 |
| [32] | M. Tanaka, T. Okazaki, H. Suzuki, J.L. Abbruzzese, D.H. Li, Association of multidrug resistance gene polymorphisms with pancreatic cancer outcome. Cancer 117 (2011) 744–751. DOI:10.1002/cncr.v117.4 |
| [33] | Y. Zhang, Q. Yue, Y.J. Jiang, A facile biliquid-interface co-assembly synthesis of mesoporous vesicles with large pore sizes. CrystEngComm 18 (2016) 4343–4348. DOI:10.1039/C5CE02592C |
| [34] | Y.H. Deng, C.C. Wang, X.Z. Shen, Preparation, characterization, and application of multistimuli-responsive microspheres with fluorescencelabeled magnetic cores and thermoresponsive shells. Chemistry 11 (2005) 6006–6013. DOI:10.1002/(ISSN)1521-3765 |
 2017, Vol. 28
2017, Vol. 28 


