b Key Laboratory of Drug-Targeting and Drug Delivery System of the Ministry of Education, West China School of Pharmacy, Sichuan University, Chengdu 610041, China;
c University of Chinese Academy of Sciences, Beijing 100049, China
The polycyclic indole or indoline skeletons are widely distributed in both terrestrial and marine organisms, and a number of synthetic indole derivatives also show interesting biological and pharmacological properties [1]. As a result, the development of efficient methods for the construction of such frameworks triggers increasing interest in organic and medicinal chemistry [2]. In general, the 2, 3-C¼C bond of indole substances is an enamine-type functional group, and exhibits nucleophilic feature in a variety of Friedel-Crafts reactions and cycloaddition reactions [3]. Nevertheless, it was also established that the indoles bearing electron-withdrawing substitutions at both N1-and C3-positions could perform as a class of electron-deficient alkene reagents, thus a few dearomatic cycloaddition reactions have been reported to access polycyclic indoline architectures [4]. Moreover, in 2014, the Arai group first uncovered the asymmetric [3+2] dipolar cycloaddition reaction with N-Ts-3-nitroindoles and azomethine imines catalyzed by a PyBidine/Cu complex [5]. Subsequently, Zhao et al. developed a highly stereoselective asymmetric Michael/cyclization cascade reaction of 3-isothiocyanatooxindoles and 3-nitroindoles [6]. Therefore, the development of other types of dearomatic reactions of 3-nitroindoles, including the potential asymmetric versions, to construct libraries with more structural diversity, is still in demand. On the other hand, we noticed that 3-nitro-7-azaindoles, a type of potentially more promising electrophilic alkenes than the indole analogues owing to the electron-withdrawing effect of pyridine moiety [7], have been barely explored in this field. Thus, in our continuing efforts to expand the synthetic utility of Morita-Baylis-Hillman (MBH) carbonates [8], here we would like to present the previously unexplored dearomatic [3+2] annulation reaction of 3-nitro-7-azaindoles and MBH carbonates from isatins, delivering a spectrum of complex polycyclic spirooxindoles containing fused azaindoline architectures and vicinal quaternary centers.
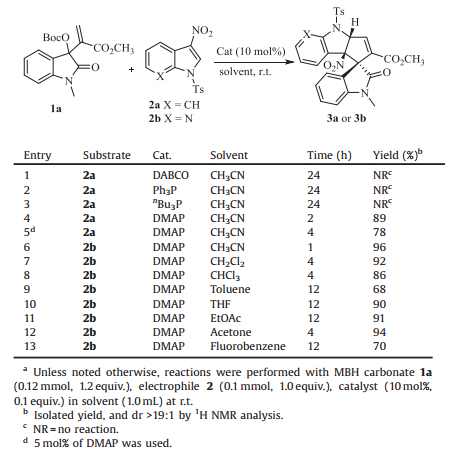
2. Results and discussionThe initial investigation was conducted with MBH carbonate 1a and readily available N-Ts-3-nitroindole 2a in acetonitrile in the presence of 10 mol% of 1, 4-diaza-bicyclo[2.2.2]octane (DABCO), but no reaction was observed (Table 1, entry 1). The reaction also did not occur catalyzed by either Ph3P or nBu3P even at a higher temperature (Table 1, entries 2 and 3). To our delight, the reaction proceeded smoothly at room temperature when 4-dimethylaminopyridine (DMAP) was used, and the α-regioselective [3+2] annulation product 3a was produced in 89% yield with exclusive diastereoselectivity (Table 1, entry 4) [9]. The yield was significantly decreased by employing less amounts of catalyst (Table 1, entry 5). Pleasingly, N-Ts-3-nitro-7-azaindole 2b showed higher reactivity as expected, and the corresponding product 3b was obtained in a higher yield (96%) for 1 h (Table 1, entry 6). On the other hand, a number of solvents were further examined, while inferior results were generally observed (Table 1, entries 7-13).
|
|
Table 1 Screening conditions of [3+2] annulation reactions with MBH carbonate 1a and electrophiles 2a or 2b.a |
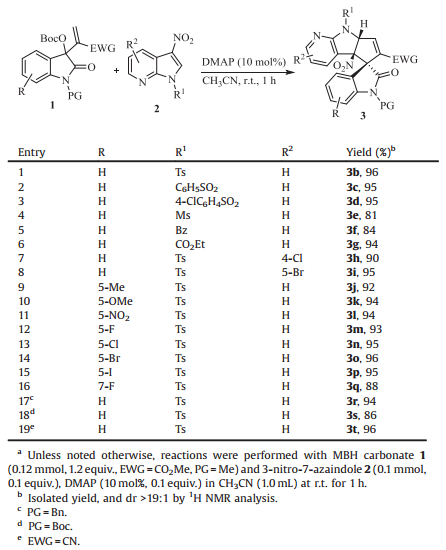
With the optimized conditions in hand, we next set out to examine the scope and limitations of this dearomatic [3+2] annulation reaction by the catalysis of DMAP. The results are summarized in Table 2. At first, a series of 3-nitro-7-azaindoles 2 bearing different N-protecting groups were explored in the reactions with MBH carbonate 1a. It was found that all the reactions proceeded smoothly to produce the corresponding a-regioselective products 3c-3g in excellent yields and diastereoselectivities (Table 2, entries 2-6). It should be noted that the reactions did not occur when the N-protecting group was replaced by either H or Me, or the 3-nitro group was substituted by other functional groups, demonstrating that both the electron-withdrawing group on nitrogen and the nitro group on C3 position of 7-azaindoles played a key role in this [3+2] annulation reaction. High yields also were obtained by introducing some substitutions on the 7-azaindole ring (Table 2, entries 7 and 8). On the other hand, we tested the reactions of 3-nitro-7-azaindole 2b and MBH carbonates derived from various isatins, and found that the MBH adducts bearing both electron-withdrawing and -donating substituents could afford the corresponding [3+2] annulation products 3j-3q in excellent yields (91%-96%) and exclusive diastereoselectivities (>19:1) (Table 2, entries 9-16). Moreover, the annulation products 3r and 3s were attained with excellent results when the protective group on nitrogen of MBH carbonates was benzyl or Boc, respectively (Table 2, entries 17 and 18). It is worth mentioning that the MBH carbonate derived from isatin and acrylonitrile also provided the annulation product 3t in 96% yield with remarkable diastereoselectivity (Table 2, entry 19).
|
|
Table 2 Substrate scope of DMAP-catalyzed dearomatic [3+2] annulation.a |
We further explored the reaction of MBH carbonate 1a and N-Ts-2-nitroindole 4. DMAP exhibited high catalytic activity under the same conditions, while other types of Lewis bases also failed to catalyse the reaction. Nevertheless, as outlined in Scheme 1, the dearomtic [3+2] annulation reaction afforded the different g-regioselective product 5 with excellent diastereoselectivity. The absolute configuration of product 5 was confirmed by X-ray analysis (Fig. 1a). The yield was modest because the substance 6 was also produced after elimination of HNO2 according to our previous report [10]. The switched γ-regioselectivity might be ascribed to the steric effect in both addition and annulation steps, avoiding the formation of highly congested vicinal quaternary centres.

|
Download:
|
| Scheme 1. γ-Regioselective [3+2] annulation reaction of N-Ts-2-nitroindole 4. | |

|
Download:
|
| Figure 1. X-ray structures of 5 (a) and enantiopure 3b (b). | |
On the other hand, we paid much attention on the asymmetric version of this new reaction. Although a number of chiral tertiary amines and phosphines failed to catalyse the reaction, we found that our previously reported chiral DMAP-type catalysts could promote the desired annulation [11], albeit in much lower activity in comparison with simple DMAP. After extensive screenings, it showed that moderate ee value and yield could be obtained for chiral product 3b when catalyst C1 was applied in dioxane at room temperature for 24h (Scheme 2). The absolute configuration of chiral product 3b was unequivocally established by X-ray analysis (Fig. 1b). In addition, both yield and enantioselectivity were reduced when 3-nitroindole 2a was used.

|
Download:
|
| Scheme 2. Asymmetric [3+2] annulations catalysed by chiral DMAP-type catalyst C1. | |
To furtherexpand the potentials of this methodology, adduct 3b could be transformed to other synthetically or biologically useful spirocyclic oxindole compounds. As illustrated in Scheme 3, the nitro group of 3b was efficiently reduced by zinc powder and 1mol/L HCl [12], affording product 7 in 96% yield. In addition, the denitronation process also could be conducted with 3b in the presence of DBU, and product 8 was attained in a quantitative yield [10].
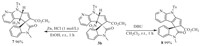
|
Download:
|
| Scheme 3. Transformations of product 3b to other polycyclic spirocyclic oxindoles. | |
3. Conclusion
We have investigated the dearomatic [3+2] annulation reaction of 3-nitro-7-azaindoles and Morita-Baylis-Hillman carbonates from isatins. The reaction proceeded effectively under the mild catalysis of 4-dimethylaminopyridine, and an array of complex polycyclic spirooxindole substances containing fused azaindoline skeletons and vicinal quaternary centres were constructed in excellent yields and diastereoselectivities. In addition, the attempts to developing an enantioselective version of this reaction were conducted, and moderate enantioselectivity was achieved by employing a chiral DMAP-type substance. Further exploration of these multifunctional and drug-like compounds in organic synthesis and biological studies is under way in our laboratory.
4. ExperimentalGeneral procedure for dearomatic [3+2] annulation reaction of 3-nitro-7-azaindoles and Morita-Baylis-Hillman carbonates from isatins: 3-Nitro-7-azaindole 2 (0.1mmol, 1.0equiv.) was dissolved in acetonitrile (1mL). To this solution was added DMAP (0.01mmol, 0.1equiv.) and MBH carbonate 1 (0.12mmol, 1.2equiv.). The solution was stirred at room temperature for 1h. After completion, purification by chromatography (petroleum ether/ethyl acetate=5:1-2:1, silica gel) afforded the desired product 3.
The characterization data of some representative products 3 and derivatives arelisted as follows and the othersaresummarized in the Supporting information.
3b, 52.4mg, 96% yield, white solid; 1H NMR (400MHz, CDCl3): δ 8.41 (d, 1H, J=4.0Hz), 8.00 (d, 2H, J=8.0Hz), 7.76 (d, 1H, J=7.2Hz), 7.38 (t, 1H, J=7.6Hz), 7.26-7.22 (m, 3H), 7.05 (t, 1H, J=7.2Hz), 7.01-6.97 (m, 2H), 6.92 (d, 1H, J=7.6Hz), 6.71 (d, 1H, J=2.0Hz), 3.60 (s, 3H), 3.37 (s, 3H), 2.36 (s, 3H). 13C NMR (150MHz, CDCl3): δ 172.1, 161.3, 153.7, 152.5, 152.3, 145.0, 144.2, 139.7, 138.6, 137.2, 135.2, 130.7, 129.7, 128.1, 126.5, 125.9, 122.9, 122.8, 122.7, 118.4, 115.8, 108.9, 102.0, 70.7, 63.1, 52.4, 27.1, 21.6. ESI-HRMS: calcd. for C27H22N4O7S+Na+ 569.1101, found 569.1107.
3f, 47.5mg, 84% yield, white solid; 1H NMR (400MHz, CDCl3): δ 8.09 (t, 1H, J=7.6Hz), 7.90 (d, 1H, J=7.6Hz), 7.60 (d, 2H, J=7.6Hz), 7.54 (t, 1H, J=7.2Hz), 7.44-7.36 (m, 4H), 7.10-7.05 (m, 2H), 7.00-6.94 (m, 2H), 6.85 (d, 1H, J=1.2Hz), 3.59 (s, 3H), 3.39 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 172.3, 168.7, 161.5, 153.4, 151.4, 144.1, 139.6, 138.5, 137.6, 134.7, 133.5, 131.5, 130.7, 130.1, 128.7, 128.4, 127.8, 125.9, 123.1, 122.9, 118.7, 116.4, 108.9, 100.6, 69.7, 63.8, 52.4, 27.1. ESI-HRMS: calcd. for C27H20N4O6+Na+ 519.1275, found 519.1274.
3j, 51.5 mg, 92% yield, white solid; 1H NMR (400 MHz, CDCl3): δ 8.40 (dd, 1H, J=4.8, 1.2 Hz), 8.01 (d, 2H, J=8.4 Hz), 7.77 (dd, 1H, J=9.6, 1.2 Hz), 7.27-7.25 (m, 2H), 7.21 (d, 1H, J=2.0 Hz), 7.16 (d, 1H, J=7.2 Hz), 6.97 (dd, 1H, J=7.6, 4.8 Hz), 6.80 (d, 2H, J=7.6 Hz), 6.73 (d, 1H, J=1.6 Hz), 3.60 (s, 3H), 3.35 (s, 3H), 2.36 (s, 3H), 2.31 (s, 3H). 13C NMR (150 MHz, CDCl3): δ 172.0, 161.3, 153.6, 152.4, 145.0, 141.8, 139.5, 138.7, 137.3, 135.2, 132.6, 131.1, 129.64, 129.60, 128.1, 125.9, 123.5, 118.3, 115.9, 108.6, 101.9, 70.7, 63.1, 52.4, 27.1, 21.6, 21.0. ESIHRMS: calcd. for C28H24N4O7S+Na+ 583.1258, found 583.1260.
3r, 58.5 mg, 94% yield, white solid; 1H NMR (400 MHz, CDCl3): δ 8.39 (d, 1H, J=4.8 Hz), 7.99 (d, 2H, J=8.0 Hz), 7.63 (d, 1H, J=8.0 Hz), 7.47 (d, 2H, J=7.6 Hz), 7.37 (t, 2H, J=7.2 Hz), 7.31 (d, 1H, J=7.2 Hz), 7.25 (d, 4H, J=7.2 Hz), 7.02 (d, 2H, J=4.0 Hz), 6.90 (dd, 1H, J=7.6, 5.2 Hz), 6.77 (d, 1H, J=7.6 Hz), 6.73 (s, 1H), 5.22 (d, 1H, J=16.0 Hz), 4.93 (d, 1H, J=16.0 Hz), 3.57 (s, 3H), 2.35 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 172.1, 161.3, 153.6, 152.4, 145.0, 143.5, 139.9, 138.7, 137.1, 135.3, 135.1, 130.7, 129.6, 128.9, 128.0, 127.7, 127.0, 125.8, 122.9, 122.8, 118.4, 115.8, 109.8, 102.2, 70.6, 63.2, 52.4, 44.5, 21.6. ESIHRMS: calcd. for C33H26N4O7S+Na+ 645.1414, found 645.1414.
3t, 49.2 mg, 96% yield, white solid; 1H NMR (400 MHz, CDCl3): δ 8.44 (d, 1H, J=4.4 Hz), 8.00 (d, 2H, J=8.0 Hz), 7.72 (d, 1H, J=7.6 Hz), 7.46 (t, 1H, J=7.6 Hz), 7.28 (d, 3H, J=10.0 Hz), 7.16-7.13 (m, 2H), 7.07 (d, 1H, J=7.2 Hz), 7.01 (dd, 1H, J=8.0, 5.2 Hz), 6.96 (d, 1H, J=8.0 Hz), 6.69 (d, 1H, J=2.0 Hz), 3.35 (s, 3H), 2.38 (s, 3H). 13C NMR (150 MHz, CDCl3): δ 170.0, 153.6, 152.9, 145.4, 144.5, 143.6, 138.2, 134.9, 131.8, 129.80, 129.75, 128.1, 123.5, 118.6, 118.4, 115.1, 111.5, 109.5, 101.2, 70.8, 65.1, 27.2, 21.7. ESI-HRMS: calcd. for C26H19N5O5S+Na+ 536.0999, found 536.0995.
5, 30.0 mg, 55% yield, white solid; 1H NMR (400 MHz, CDCl3): δ 7.93 (d, 2H, J=8.4 Hz), 7.72 (s, 1H), 7.39 (t, 1H, J=8.0 Hz), 7.33 (t, 3H, J=8.8 Hz), 7.24-7.13 (m, 3H), 6.93-6.89 (m, 2H), 6.65 (d, 1H, J=7.6 Hz), 4.54 (s, 1H), 3.66 (s, 3H), 3.05 (s, 3H), 2.39 (s, 3H). 13C NMR (150 MHz, CDCl3): δ 172.9, 161.7, 145.4, 145.1, 143.6, 141.8, 138.7, 135.3, 130.1, 130.04, 129.95, 129.8, 127.7, 124.5, 124.2, 123.7, 123.5, 123.0, 113.1, 112.8, 108.5, 64.2, 63.8, 52.5, 26.6, 21.6. ESI-HRMS: calcd. for C28H23N3O7S+Na+ 568.1149, found 568.1147.
6, 19.9 mg, 40% yield, white solid; 1H NMR (400 MHz, CDCl3): d 8.26 (s, 1H), 8.00 (d, 1H, J=8.8 Hz), 7.80 (d, 2H, J=8.4 Hz), 7.33 (t, 1H, J=7.6 Hz), 7.27-7.22 (m, 3H), 7.04 (dd, 2H, J=13.2, 7.6 Hz), 6.90 (t, 1H, J=7.6 Hz), 6.78 (d, 1H, J=8.0 Hz), 6.62 (d, 1H, J=7.6 Hz), 3.68 (s, 3H), 3.39 (s, 3H), 2.35 (s, 3H). 13C NMR (150 MHz, CDCl3): δ 171.9, 162.4, 145.5, 145.1, 144.0, 142.0, 140.2, 136.2, 134.6, 133.5, 130.1, 129.0, 126.9, 125.5, 125.3, 124.3, 124.1, 122.9, 122.7, 118.8, 114.8, 108.8, 60.0, 51.8, 27.3, 21.6. ESI-HRMS: calcd. for C28H22N2O5S+Na+ 521.1142, found 521.1145.
7, 49.5 mg, 96% yield, white solid; 1H NMR (400 MHz, CDCl3): δ 8.29 (d, 1H, J=4.8 Hz), 7.99 (d, 2H, J=8.4 Hz), 7.40 (dd, 2H, J=16.0, 7.6 Hz), 7.26-7.23 (m, 3H), 7.17 (d, 1H, J=7.2 Hz), 7.10 (t, 1H, J=7.6 Hz), 6.93-6.88 (m, 2H), 5.52 (s, 1H), 5.03 (br, 1H), 3.70 (br, 1H), 3.56 (s, 3H), 3.26 (s, 3H), 2.37 (s, 3H). 13C NMR (100 MHz, d6-DMSO): δ 175.0, 162.5, 155.0, 148.6, 145.2, 144.7, 142.9, 139.0, 136.2, 135.9, 129.9, 129.4, 128.4, 127.1, 125.8, 124.6, 122.0, 118.5, 109.0, 77.6, 72.4, 66.0, 52.5, 26.9, 21.5. ESI-HRMS: calcd. for C27H24N4O5S+Na+ 539.1360, found 539.1365.
8, 49.4 mg, 99% yield, white solid; 1H NMR (400 MHz, CDCl3): δ 8.33 (d, 2H, J=7.6 Hz), 8.14 (d, 2H, J=8.0 Hz), 7.37-7.30 (m, 3H), 7.16 (d, 1H, J=7.6 Hz), 7.04-7.01 (m, 2H), 6.94 (t, 1H, J=7.6 Hz), 6.69 (d, 1H, J=7.2 Hz), 3.69 (s, 3H), 3.40 (s, 3H), 2.39 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 171.8, 162.2, 151.1, 145.7, 145.0, 144.9, 143.6, 142.5, 136.3, 134.9, 129.8, 129.2, 128.7, 128.2, 126.7, 125.0, 123.0, 122.7, 119.6, 116.9, 108.9, 60.4, 51.9, 27.3, 21.7. ESI-HRMS: calcd. for C27H21N3O5S+Na+ 522.1094, found 522.1096.
AcknowledgmentWe are grateful for the financial support from the NSFC (21572135 and 21321061).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.11.003.
| [1] |
(a) P.M. Barbour, L.J. Marholz, L. Chang, W.Q. Xu, X. Wang, Gold approaches to polycyclic indole alkaloids, Chem. Lett. 43(2014) 572-578; (b) K. Walton, J. Berry, Indole alkaloids of the stigonematales (cyanophyta):chemical diversity, biosynthesis and biological activity, Mar. Drugs 14(2016) 73-100; (c) W. Zi, Z. Zuo, D. Ma, Intramolecular dearomative oxidative coupling of indoles:a unified strategy for the total synthesis of indoline alkaloids, Acc. Chem. Res. 48(2015) 702-711; (d) A. Steven, L.E. Overman, Total synthesis of complex cyclotryptamine alkaloids:stereocontrolled construction of quaternary carbon stereocenters, Angew. Chem. Int. Ed. 46(2007) 5488-5508. |
| [2] |
(a) Y.N. Gao, Q. Xu, M. Shi, Enantioselective synthesis of polycyclic indole derivatives based on aza-Morita-Baylis-Hillman reaction, ACS Catal. 5(2015) 6608-6614; (b) J.X. Liu, M. Shen, Y.D. Zhang, et al., A new entry to polycyclic indole skeletons via palladium-catalyzed intramolecular heteroannulation, Org. Lett. 8(2006) 3573-3575; (c) T. Wang, S. Shi, D. Pflasterer, et al., Synthesis of polycyclic indole skeletons by a gold (Ⅰ)-catalyzed cascade reaction, Chem. Eur. J. 20(2014) 292-296; (d) S.P. Wong, K.W. Chong, K.H. Lim, et al., Arborisidine and Arbornamine, two monoterpenoid indole alkaloids with new polycyclic carbon-nitrogen skeletons derived from a common pericine precursor, Org. Lett. 18(2016) 1618-1621; (e) J.M. Yang, P.H. Li, Y. Wei, X.Y. Tang, M. Shi, Gold (Ⅰ)-catalyzed highly stereoselective synthesis of polycyclic indolines:the construction of four contiguous stereocenters, Chem. Commun. 52(2016) 346-349. |
| [3] |
(a) G. Bartoli, G. Bencivenni, R. Dalpozzo, Organocatalytic strategies for the asymmetric functionalization of indoles, Chem. Soc. Rev. 39(2010) 4449-4465; (b) T.B. Poulsen, K.A. Jørgensen, Catalytic asymmetric Friedel-Crafts alkylation reactions-copper showed the way, Chem. Rev. 108(2008) 2903-2915; (c) S.-L. You, Q. Cai, M. Zeng, Chiral Bronsted acid catalyzed Friedel-Crafts alkylation reactions, Chem. Soc. Rev. 38(2009) 2190-2201; (d) M. Bandini, A. Eichholzer, Catalytic functionalization of indoles in a new dimension, Angew. Chem. Int. Ed. 48(2009) 9608-9644; (e) C.C.J. Loh, D. Enders, Exploiting the electrophilic properties of indole intermediates:new options in designing asymmetric reactions, Angew. Chem. Int. Ed. 51(2012) 46-48. |
| [4] |
(a) M. Bandini, Electrophilicity:the "dark-side" of indole chemistry, Org. Biomol. Chem. 11(2013) 5206-5212; (b) G.W. Gribble, E.T. Pelkey, W.M. Simon, H.A. Trujillo, Regioselective 1, 3-dipolar cycloaddition reactions of unsymmetrical munchnones (1, 3-oxazolium-5-olates) with 2-and 3-nitroindoles. A new synthesis of pyrrolo 3, 4-b indoles, Tetrahedron 56(2000) 10133-10140; (c) B. Biolatto, M. Kneeteman, E. Paredes, P.M.E. Mancini, Reactions of 1-tosyl-3-substituted indoles with conjugated dienes under thermal and/or highpressure conditions, J. Org. Chem. 66(2001) 3906-3912; (d) T.L.S. Kishbaugh, G.W. Gribble, Diels-Alder reactions of 2-and 3-nitroindoles. A simple hydroxycarbazole synthesis, Tetrahedron Lett. 42(2001) 4783-4785; (e) I. Chataigner, S.R. Piettre, Multicomponent domino[4+2]/[3+2] cycloadditions of nitroheteroaromatics:an efficient synthesis of fused nitrogenated polycycles, Org. Lett. 9(2007) 4159-4162; (f) S. Roy, T.L.S. Kishbaugh, J.P. Jasinski, G.W. Gribble, 1, 3-Dipolar cycloaddition of 2-and 3-nitroindoles with azomethine ylides. A new approach to pyrrolo 3, 4-b indoles, Tetrahedron Lett. 48(2007) 1313-1316; (g) M. Victoria Gomez, A.I. Aranda, A. Moreno, et al., Microwave-assisted reactions of nitroheterocycles with dienes. Diels-Alder and tandem hetero Diels-Alder/[3, 3] sigmatropic shift, Tetrahedron 65(2009) 5328-5336; (h) S. Lee, S. Diab, P. Queval, et al., Aromatic C=C bonds as dipolarophiles:facile reactions of uncomplexed electron-deficient benzene derivatives and other aromatic rings with a non-stabilized azomethine ylide, Chem. Eur. J. 19(2013) 7181-7192; (i) M. Andreini, M. De Paolis, I. Chataigner, Thiourea-catalyzed dearomatizing[4+2] cycloadditions of 3-nitroindole, Catal. Commun. 63(2015) 15-20. |
| [5] | A. Awata, T. Arai, PyBidine/copper catalyst. Asymmetric exo0-selective[3+2] cycloaddition using imino ester and electrophilic indole. Angew. Chem. Int. Ed. 53 (2014) 10462–10465. DOI:10.1002/anie.201405223 |
| [6] |
(a) J.Q. Zhao, Z.J. Wu, M.Q. Zhou, et al., Zn-catalyzed diastereo-and enantioselective cascade reaction of 3-isothiocyanato oxindoles and 3-nitroindoles:stereocontrolled syntheses of polycyclic spirooxindoles, Org. Lett. 17(2015) 5020-5023; (b) J.Q. Zhao, M.Q. Zhou, Z.J. Wu, et al., Asymmetric Michael/cyclization cascade reaction of 3-isothiocyanato oxindoles and 3-nitroindoles with amino-thiocarbamate catalysts:enantioselective synthesis of polycyclic spirooxindoles, Org. Lett. 17(2015) 2238-2241. |
| [7] |
(a) B. Dayde-Cazals, B. Fauvel, M. Singer, et al., Rational design, synthesis, and biological evaluation of 7-azaindole derivatives as potent focused multitargeted kinase inhibitors, J. Med. Chem. 59(2016) 3886-3905; (b) S.B. Dongare, H.V. Chavan, D.N. Surwase, et al., Indium trichloride (InCl3) catalyzed synthesis of fused 7-azaindole derivatives using domino Knoevenagel-Michael reaction, J. Chin. Chem. Soc. 63(2016) 323-330; (c) Q. He, G. Zhan, W. Du, Y.C. Chen, Application of 7-azaisatins in enantioselective Morita-Baylis-Hillman reaction, Beilstein J. Org. Chem. 12(2016) 309-313; (d) S.S. Li, H. Lin, C.F. Liu, et al., Rhodium-catalyzed tandem annulation reactions of 7-azaindoles with electron-deficient olefins via double C-H activation, Adv. Synth. Catal. 358(2016) 1595-1601; (e) B. Liu, R.D. Li, W. Zhan, et al., Rh (Ⅲ)-catalyzed C-H oxidative orthoolefination of arenes using 7-azaindole as a directing group and utilization in the construction of new tetracyclic heterocycles containing a 7-azaindole skeleton, RSC Adv. 6(2016) 48205-48211; (f) S. Rekulapally, R. Jarapula, K. Gangarapu, S. Manda, J.R. Vaidya, In silico and in vitro studies of novel 7-azaindole and 7-azaisatin derivatives as potent anticancer agents, Med. Chem. Res. 24(2015) 3412-3422; (g) E.R. Wood, L. Kuyper, K.G. Petrov, et al., Discovery and in vitro evaluation of potent TrkA kinase inhibitors:oxindole and aza-oxindoles, Bioorg. Med. Chem. Lett. 14(2004) 953-957; (h) N.M. Barl, E. Sansiaume-Dagousset, K. Karaghiosoff, P. Knochel, Full functionalization of the 7-azaindole scaffold by selective metalation and sulfoxide/magnesium exchange, Angew. Chem. Int. Ed. 52(2013) 10093-10096. |
| [8] |
(a) P. Xie, Y. Huang, Morita-Baylis-Hillman adduct derivatives (MBHADs):versatile reactivity in lewis base-promoted annulation, Org. Biomol. Chem. 13(2015) 8578-8595; (b) Y. Wei, M. Shi, Recent advances in organocatalytic asymmetric Morita-Baylis-Hillman/aza-Morita-Baylis-Hillman reactions, Chem. Rev. 113(2013) 6659-6690; (c) R. Rios, Organocatalytic enantioselective methodologies using Morita-Baylis-Hillman carbonates and acetates, Catal. Sci. Technol. 2(2012) 267-278; (d) T.Y. Liu, M. Xie, Y.C. Chen, Organocatalytic asymmetric transformations of modified Morita-Baylis-Hillman adducts, Chem. Soc. Rev. 41(2012) 4101-4112. |
| [9] |
(a) G.Y. Ran, P. Wang, W. Du, Y.C. Chen, α-Regioselective[3+2] annulations with Morita-Baylis-Hillman carbonates of isatins and 2-nitro-1, 3-enynes, Org. Chem. Front. 3(2016) 861-864; (b) K.K. Wang, T. Jin, X. Huang, et al., α-Regioselective asymmetric[3+2] annulations of Morita-Baylis-Hillman carbonates with cyclic 1-azadienes and mechanism elucidation, Org. Lett. 18(2016) 872-875. |
| [10] | J. Peng, G.Y. Ran, W. Du, Y.C. Chen, Tertiary-amine-catalyzed asymmetric[3+2] annulations of Morita-Baylis-Hillman carbonates of isatins with nitroolefins to construct spirooxindoles. Synthesis 47 (2015) 2538–2544. DOI:10.1055/s-00000084 |
| [11] | G. Zhan, M.L. Shi, Q. He, Catalyst-controlled switch in chemo-and diastereoselectivities:annulations of Morita-Baylis-Hillman carbonates from isatins. Angew. Chem. Int. Ed. 55 (2016) 2147–2151. DOI:10.1002/anie.201510825 |
| [12] | X.Y. Guan, Y. Wei, M. Shi, Phosphine-catalyzed tandem reaction of allenoates with nitroalkenes. Org. Lett. 12 (2010) 5024–5027. DOI:10.1021/ol102191p |
 2017, Vol. 28
2017, Vol. 28