Solar flux is the major source of renewable and sustainable energy on earth, which can be converted into electricity by the expenditure of photovoltaic effect of light-absorbing semiconductors. First and second generation photovoltaics utilized silicon and cadmium telluride (CdTe) or copper indium gallium selenide/ sulfide (CIGS) as absorbers. They are efficient, but made through the high-temperature and/or high-vacuum production process that require high energy inputs. In recent decades, solutionprocessable thin film photovoltaics represent transformative technology, for instance, organic solar cells (OSCs) [1, 2], dyesensitized solar cells (DSSCs) [3] and the emerging perovskite solar cells (PVSCs) acquired intensive consideration from researchers in academia and industry. It is because these light weight, flexible and low-cost power sources potentially can be made with the highthroughput solution fabrication techniques. The state-of-the-art DSSC and OPV have already reached the power conversion efficiencies (PCEs) of 13% and 11%, respectively [4-7]. As a rising star, PVSC was just deployed at 2009. At that time, Kojima et al. incorporated organolead halide, CH3NH3PbI3 (MAPbI3) and CH3NH3PbBr3 (MAPbBr3) as sensitizers into DSSC [8], delivering the 3.8% and 3.1% PCE, respectively. Investigation sustained by Im et al., effectivelyenhanced the PCE up to 6.5% for a liquid electrolyte based DSSC with perovskite sensitizers [9]. After that, breakthroughs were made by Kim et al. who introduced spiro-OMeTAD as a solid hole transport material (HTM) atop of perovskite absorbers to gain 10.9% and 9.7% PCEs for MAPbI3-xClx and MAPbI3 [10]. In the past seven years, the rapid progresses in PVSCs have been achieved to reach over 22% PCE [11], demonstrating the rivalry in efficiency to that of 60 years old silicon solar cells. The progress in PCEs for PVSCs is given in the following Fig. 1.

|
Download:
|
| Figure 1. (a) Illustrative crystal structure of an ABX3 perovskite. In the thus far studied materials, a typical combination of A is methyl ammonium, B is Pb, and X is a halide ion [12]. (b) The progress in PCEs of PVSCs: 3.8% [8], 6.5% [9], 9.7% [10], 10.9% [13], 12.3% [14], 15% [15], 15.4% [16], 16.7% [17], 17.9% [18], 19.3% [19], 20.2% [20], 22.1% [11]. | |
Despite the significant progresses were made, there are still a number of barriers ranging from fundamental to practical need to overcome for further advancing PVSC as a prolific clean energy solution. Especially, it is well acknowledged that the nature of electrical and electronic contact of perovskite/electrode interfaces significantly affect the resulting photovoltaic performance of PVSCs, including open-circuit voltage (VOC), short-circuit current (Jsc), and fill factor (FF) [21-24]. Organic lead halide perovskite itself exhibits excellent optoelectronic properties such as strong optical absorption coefficient, long carrier diffusion length over 175 mm and high electron/hole mobility (~100 cm2 V-1 s-1) in single crystal state [25]. This value is even much better than those organics in the single crystalline state [26-28]. Most importantly, the excited organic-inorganic lead halide perovskites generate loosely bonded Wannier exciton with bonding energy as low as~25 meV [29], which easily enables free carrier generation within the perovskite by small driving force. To match with such efficient photoabsorbers, new generation of electron and hole-transport layers (ETL and HTL) are strongly needed that can promote the optimal contact between perovskite and the corresponding electrodes. Depending on the device architectures, PVSCs can be catalogued into mesoscopic and planar heterojunction structures (Fig. 2). The planar structure can be further divided into two categories depending on which selective contact is used on the bottom, that is, regular (n-i-p) and inverted (p-i-n). Recently, the efficiency of the planar structure was pushed over 19%, which is close to those of mesoscopic devices.

|
Download:
|
| Figure 2. Device architectures of mesoporous, regular (n-i-p) and inverted (p-i-n) [30]. | |
In the following parts of this review article, we first introduce some key features for the buffer layers discovered recently that enable efficient PVSCs. It is then followed by highlighting several representative organic π-functional materials as buffer layers for constructing efficient PVSCs. In the last section, we provide the brief perspective on emphasizing interfacial material development for improving PVSC.
2. Buffer layers in PVSCsIn this section, we first outline some key roles and features for the desired buffer layers in PVSCs that can be used to enhance perovskite crystal growth and the related device performance (Fig. 3). Basically, the free carriers are generated within the photonexcited perovskite, of which holes are collected by anode while electrons are extracted to cathode with the assistance of the builtin field between the asymmetric electrodes. Consequently, the electrical contact at perovskite/electrode interface is critical to determine the photovoltaic performance [24]. First of all, the effective buffer layers need to have proper energy level alignment with that of perovskite, in the meanwhile, are able to tune the work function of electrode. So that it can result in minimizing the interfacial energy barriers at the corresponding interface that is credited for achieving simultaneously enhanced charge collection efficiency and VOC of devices [21, 31-34]. Secondly, it has been recently shown that the suitable surface energy from the bottom buffer layer can strongly affect the crystal growth of perovskite. For instance, the relatively hydrophobic (non-wetting) HTL show advantages to achieve large-aspect-ratio perovskite grains, comparing with those of hydrophilic PEDOT:PSS [35, 36]. Bi et al. suggested that the non-wetting HTL increases the nucleus spacing by effectively suppressing heterogeneous nucleation and facilitate grain boundary migration in grain growth by imposing less drag force. It is there promote large-aspect-ratio perovskite grains, and by the reduction in grain boundary area will show increase in crystallinity which ultimately reduce the charge recombination [36]. Thirdly, the charge transport of p-and n-buffer layers need to be sufficiently high to reduce device series resistance and hence maximize charge-extraction. It was previously estimated by Werner et al. that Ohmic loss at the interface of a solar cell is negligible if the conductivity of the charge transport layers is higher than 10-3 S m-1 [37]. Additional requirement is to enhance the charge selectivity of the corresponding buffer layer, i.e. electron transporting and hole-blocking for ETL, and hole-transporting and electron-blocking for HTL, by preventing unfavorable interfacial charge recombination at electrodes to improve FF and the rectification of the device characteristics. Finally, it is also the desired feature for new HTL/ETL has the capabilities to passivate the surface defeats and prevent ion-migration of the perovskite. Besides of the crystal engineering of perovskite, the buffer layer with proper functionalities should be ideal candidate to passivate the defeats, charge traps and ion-migration channels presenting at the perovskite grain boundary [38]. For example, the Lewis basic heteroatom, show potentially passivate perovskite surface, i.e. by coordinating with lead ion via halide vacancies [36, 39-41], which have positive effect to both reduce the surface recombination and device hysteresis. In the comparison with the traditional metal oxide based buffer layers (TiO2, SnOx, CuO, NiOx etc.), organic buffer materials (small molecule and polymer) can avoid harsh processing condition, like high temperature sintering for TiO2, yet exhibit additional merits, like wide range of functional tunabilities due to rich chemistry tools are available and good compatibility with flexible plastic substrates [42, 43]. This flexibility of the organic buffer materials will allow researchers in the innovation of low cost, portable devices with reduced dimensions. Besides, organic buffer materials can also provide additional properties such as stabilize mobile ion between perovskite grain boundaries and provide barrier protection to halide perovskites form moisture to enhance solar cell stability [44].
|
Download:
|
| Figure 3. Outlines for the design features of organic π-functional buffer layers for PVSCs. | |
3. Hole-transport layer for efficient perovskite solar cells
In this section, some of the effective HTLs and their applications in high-performance PVSCs will be discussed. The desired optical properties of HTL require being transparent in the visible and near infrared region, which allow maximizing the photon utilization of perovskite absorbers. The energetic requirements prefer to align HTL HOMO with the valence band of perovskite, while remaining sufficient higher LUMO than perovskite conduction band. This will generate appropriate energy diagram for hole-extraction and electron-blocking to avoid charge recombination at cathode, whereas to retain high build-in potential of devices. In addition, the solution-deposition of HTL atop of perovskite needs to avoid the usage of polar and protic solvents, or remain good resistance to tolerate the perovskite layer deposition when used as bottom layer. This could fulfill the orthogonal solvent-processability between perovskite and HTL. Till now, a number of studies have been conducted to incorporate the organic π-functional HTLs into mesoporous and planner PVSCs to achieve high performances.
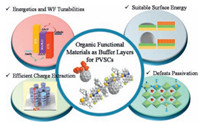
Mesoporous PVSCs generally consist of FTO glass, mesoporous TiO2 or Al2O3, a perovskite absorber, HTL and metal electrode. The 2, 20, 7, 70-tetrakis[N, N-di (4-methoxyphenyl) amino]-9, 90-spirobifluorene (spiro-OMeTAD) (Fig. 4) is one of the most prominent HTL molecule utilized in mesoporous PVSCs. With the combined efforts in improving device and perovskite film, mesoporous PVSCs boosted the PCEs to 22% [16, 19, 45-48]. Saliba et al. investigated cesium containing ternary cation perovskites, i.e. Csx(MA0.17FA0.83)(100-x)Pb (I0.83Br0.17)3 and achieved high efficiency perovskite solar cells with a stabilized PCE at 21.17%, which effectively suppresses yellow phase impurities and produces uniform perovskite grains extending from the electron to the hole collecting layer consistent with seed-assisted crystal growth [49]. However, the HTL made with spiro-OMeTAD usually requires additional additive doping to improve the conductivity for holetransport. Besides of that, spiro-OMETAD is also quite costly to be made. These are drawbacks adding to the complicated process and overall expense of the solar cell. Therefore, there are a significant amount of efforts being devoted to develop new HTL. Jeon et al. replaced the spiro-fluorene core with a pyrene, and installed OMeTAD at 1, 3, 6, 8, position of pyrene, which reported HOMO of -5.11 eV for Py-C (Fig. 4). When processed with a cobalt dopant, tris[2-(1H-pyrazol-1-yl)-4-tert-butylpyridine)-cobalt (Ⅲ) tris (bis (trifluoromethylsulfonyl) imide)] (FK209), these structures showed nearly identical performance (PCE=12.4%) to that of spiroOMeTAD based devices [50]. Electron rich 3, 4-ethylenedioxythiophene as core was investigated to couple with OMeTAD, yielding new HTL H101 (Fig. 4). Through optimizing with cobalt dopant, tris (2-(1H-pyrazol-1-yl) pyridine) cobalt (Ⅲ) (FK102), the device employing H101 HTL achieved PCE of 13.8%, which slightly surpassed the reference device with spiro-OMeTAD HTL [51]. Continued work by the same group then introduced low-cost and stable starting materials, thiophene and bithiopthene, to develop small molecules H111 and H112 (Fig. 4), which showed deeper HOMO levels, and better device stability than that of H101. Best performing devices revealed the efficiency of 15.4% and 15.2% for H111 and H112, respectively, whereas, the reference device with spiro-OMeTAD shows 14.4% PCE [52]. Triphenylamine (TPA) connected with 1, 3, 5-triazine based materials (Triazine-ThOMeTPA and triazine-Ph-OMeTPA, Fig. 4), revealing the competitive response to those of Spiro-OMeTAD and the former with thiophene showed excellent performance [53]. Cost-effective fluorene-dithiophene based new HTL FDT (Fig. 4) passivate the surface of lead of photoactive layer through sulfur atom, showed the state-of-the-art PCE of 20.2% [54]. Star shaped new HTL N2, N2, N2', N2', N7, N7, N7', N7'-octakis (4-methoxyphenyl)-10-phenyl-10Hspiro[acridine-9, 9'-fluorene]-2, 2', 7, 7'-tetraamine (SAF-OMe) (Fig. 4), showed better hole-mobility and resulted in improved stability which remarkably improved PCE of 16.73% and 12.39% with and without dopant [55].
|
Download:
|
| Figure 4. HTL materials containing OMeTAD moieties. | |
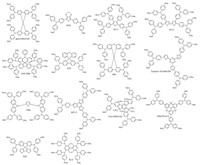
By introducing the HTL atop of transparent electrode, it is facile to build up planner p-i-n architecture. Recently, Huang et al. presented dopant-free HTL made with a C3h symmetrical Truxene core (Trux-OMeTAD) (Fig. 4) with hexyl side chains to ensure solubility and wettability, showed PCE of 18.6% with minimal hysteresis [35]. Note the mesogenic truxene allow forming an order arrangement of molecule stack in solid upon proper treatment. It facilitates high hole-mobility of HTL up to 10-3 cm2 V-1 s-1 upon thermal annealing of Trux-OMeTAD. Bi et al. developed X59 (Fig. 4) via two step synthesis and an optimized devices employing X59 as HTL exhibited significant decrease in hysteresis, excellent reproducibility and reasonable stability under dark and dry conditions approaching efficiency of 19.8% [56]. New star shaped HTL material based on benzotrithiophene (BTT-1, BTT-2 and BTT-3) were introduced, BTT-3 (Fig. 4) showed the comparable PCE of 18.2% to that of spiro-OMeTAD. Compound BTT-3 shows perfect band alignment with the HOMO level of MAPbI3 perovskite, can be used as alternative to spiro-OMeTAD to reduce the cost [57]. Low cost HTM, X60 (Fig. 4) showed performance (19.84%) under 100 mW cm-2 AM1.5G solar illumination [58]. Small-molecule HTL (methoxydiphenylamine-substituted fluorene) were synthesized from commercially available low cost material and introduced into perovskite solar cell, which displayed (V862) (Fig. 4) a power conversion efficiency of almost 20% [59]. Facile synthesis and solid state based device with tris{N, N-bis (4-methoxyphenyl)-N-phenyl}amine quinolizino arcidine (OMeTPA-FA) (Fig. 4) and tris{N, N-bis (4-methoxyphenyl)-N-biphenyl}amine (OMeTPA-TPA) as HTL materials revealed PCEs of 13.63% and 12.31% for and the device based on FTO/mp-TiO2/ MAPbI3/HTMs/Au and retained 11.64% performance for 500 h under outdoor conditions for OMeTPA-FA material [60]. Symmetrical, star shaped and planar and fused materials, tris {bis (4-methoxyphenylethenyl)-N-phenyl}amine (TPA-MeOPh) and highly stable tris{bis (4-methoxyphenylethenyl)-N-phenyl} amine quinolizino acridine (FA-MeOPh) (Fig. 5), showed a catchable PCE of 11.86% for (FA-MeOPh) [61]. Rakstys et al. developed triazatruxene based molecules, among those KR131 (Fig. 5), end-caped with electron rich methoxy substituents which potentially lowered the cost and remarkably furnished PCE of 17.7% for KR131 [62]. Nishimura et al. introduced triarylamine skeleton bridged with oxygen atoms and connected to azulene or biphenyl core units. Among four molecules, azulene 1 (Fig. 5) possess a PCE of 16.5% [63]. Meng et al. reported HTLs containing TPA with butadiene units and TPB derivatives were utilized in PVSCs with efficiencies of 11.63% and 13.10%, respectively [64, 65].

|
Download:
|
| Figure 5. Polymeric HTL materials for PVSCs. | |
Snaith et al. first reported (n-i-p) perovskite solar cells with planar structure composed of FTO/compact TiO2/perovskite/spiroOMeTAD/Au, and later same group showed the optimized device performance of 11.4% [14, 66]. Zhou et al. utilized spiro-OMeTAD as HTL and yetrium-dopped TiO2 (Y-TiO2) as ETL to construct planar PVSC. A high PCE of 19.3% is achieved through the careful control of device interface and the formation of perovskite film [19]. Sun et al. reported a hydrophobic small molecule containing rigid and flat benzodithiophene (BDT) as a donating, linker phenoxazine moiety which is end caped with a ethylrhodanine unit as an accepting part. The small molecule possesses unique properties with suitable energy levels and high hole mobility due to the strong π-π interactions, in turn effectively improving the lifetime of the charge-separated excited state. Small molecule M1 showed holemobility of 2.71 ×10-4 cm2 V-1 s-1, which is higher than SpiroOMeTAD (1.32 × 10-4 cm2 V-1 s-1). The doped M1 (Fig. 5) shows much higher conductivity (1.16 × 10-3 S cm-1) than that of (LiTFSI)-doped Spiro-OMeTAD (1.57 × 10-4 S cm-1). The overall PCE obtained was 13.2%, relatively higher than spiro-OMeTAD [67]. To demonstrate the relationship between the structure and photovoltaic properties, Yang et al. developed two molecules, the first of which contained alkylthienyl-substituted benzo[1, 2-b:4, 5-b']dithiophene (BDTT) and dithienosilole (DTS) as strong donating moieties and ethyl rhodanine as an accepting part (DERDTS-TBDT). In the second molecule, incorporation of a strong electron withdrawing unit 5, 6-difluoro-2, 1, 3-benzothiadiazole (DFBT) took place instead of a strong donating BDT component forming (DORDTS-DFBT). As HTL, a PCE of 16.2% was obtained for the device DERDTS-TBDT (Fig. 5) under AM 1.5G (100 mW cm-2) illumination, while the device based on DORDTS-DFBT showed an inferior efficiency of 6.2%. High hole-mobility and matchable energy levels of DERDTS-TBDT gives high photoresponse with external quantum efficiency (EQE) of 88%, while the DORDTS-DFBT with low hole-mobility and deeper HOMO level exhibits the decreased PCE [68]. Recently, Yang et al. demonstrated a dopant free small molecule (DOR3T-TBDT) (Fig. 5) constructed of donor-acceptor (D-A) strategy for HTL. The results indicated that this small molecule has the ability to extract efficiently holes from the perovskite to anode and optimized the device to exhibit an impressive PCE of 14.9%. Strong packing of the material gives relatively high hole-mobility and reduced the fabrication cost [69].
Reddy et al. synthesized new HTMs, CzPAFSBF and CzPAFSBFN (Fig. 5). The central N-atom is coupled with phenylcarbazole units and is end-caped with SBF and SBFN. The introduction of long alkyl groups improve solubility and hydrophobicity, resulting in the stability of the device. The device CzPAF-SBF-based delivered PCE 17.21% in comparison to spiro-OMeTAD (16.67%) under the same set of experimental conditions [70]. More recently, Hua et al. reported dopant free HTL by introducing silver metal (inorganicorganic) complex, the device based on HA1 (Fig. 5), showed efficiency of 11.98% which is slightly lower than expensive spiroOMeTAD [71]. PVSC device containing X21 as HTL showed impressive efficiency of 17.33% due to X21 possesses good solubility, ordered packing in film and excellent charge transport property [72]. Zhang et al. introduced dopants free HTL, chemical backbone (Z1011) (Fig. 5) looks like butterfly and presented PCE of 16.3%. This butterfly structured molecule possessed suitable energy levels to that of perovskite and also high hole-mobility [73].
Besides of the developed small molecule materials, the polymeric HTL, like PEDOT:PSS (Fig. 6) has also been frequently employed to build up planner PVSCs [74-77]. Inverted perovskite device with architecture ITO/PEDOT:PSS/MAPbI3/PC61BM/BCP/Al presented highest PCE of 15.4% with no hysteresis [78]. Continuous work on controlling grain size, techniques led PCE to 17.7% [79] and with usage of HI into perovskite produced pin-hole free film by giving PCE 18.1% [80]. Guo et al. worked on doping of P3HT as HTL and successfully increased the PCE from 9.2% to 12.4% [81]. Xue et al. tailored conjugated polymer back bone, yielding PTPAFSONa and PTPADCF3FSONa which are alcohol-soluble HTL materials (denoted as HSL1 and HSL2) (Fig. 6). These units contained polar functional groups which can be easily tuned to get desired energy levels with respect to perovskites. The engineering of polar sidechain potentially enhanced the surface energy of HSL materials which also exhibit better crystallinity. High PCE of 16.6% (VOC: 1.07 V) is achieved for HSL2 while HSL1 responded 15.4%, both with negligible hysteresis [82].
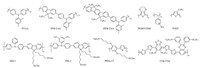
|
Download:
|
| Figure 6. Polymeric HTL materials for PVSCs. | |
Zhao et al. constructed an inverted device with PTAA (Fig. 6) as HTL and PCE of 13.85 was achieved [83]. Soek et al. developed device loaded with PTAA, PF8-TAA, PIF-TAA (Fig. 6) as HTL, they achieved very high VOC 1.4 eV for wide gap MAPbBr3 perovskite with PF8-TAA HTL. A PCE of 16.2% was received for PTAA using MAPbI3 as perovskite active material [42]. By employing nonwetting PTAA as HTL, Huang et al. is able to fabricate large grain perovskite with the reduce of charge trap density, which enhanced and stabilized the device PCE to 18.3% when comparing the device based on hydrophilic PEDOT:PSS HTL [36]. Seok et al. employed PTAA as HTL while utilized thinner layer of mesoporous TiO2 (80 nm) and an unprecedented PCE of 20.3% was measured via reverse bias scan, the PCE of around 17.3% obtained as an average [41]. Recently, Kim et al. documented donor-π-acceptor conducting material (TTB-TTQ) (Fig. 6) containing the strong electron withdrawing unit benzothiadiazole (BT). Utilizing additives lithium bis (trifluoromethanesulfonyl) imide salt and tert-butylpyridine to TTB-TTQ improved the PCE up to 14.1% showing priority to spiro-OMeTAD [84]. Jo et al. designed polymeric hole-transport material as HTL, which is composed of 1, 4-bis (4-sulfonatobutoxy) benzene and thiophene moieties (PhNa-1T) (Fig. 6) and is fabricated in high-performance inverted-type flexible PVSCs, (PhNa-1T) achieves high photovoltaic performances, potentially reduced energy loss, improved morphology of perovskite absorber showing better contact of HTL/perovskite. An impressive power conversion efficiency of 14.7% was obtained with high hole extraction and reduced charge recombination [85] (Table 1).
|
|
Table 1 HTL materials with their efficiencies. |
4. Organic π-functional materials as electron transport layer for efficient perovskite solar cells
Most PVSCs employed transition-metal oxides as electron transport layer (ETL). For instance, the mesoporous and compact type of TiO2 layers have been frequently employed in constructing efficient PVSCs. However, the development of new ETLs is strongly required because TiO2 usually require high temperature sintering and show performance degradation upon UV exposure. Regarding this, organic π-functional ETL exhibits advantages, likes mild solution-processing, mechanical flexibility and wide range of property tunabilities etc. Desired ETL made with organic material should exhibit suitable energetics and wide optical band gap, for instance, to have its LUMO align with the conduction band of perovskite, while maintain sufficiently deeper HOMO than the valence band of perovskite. In addition, ETL with wide optical band gap can ensure its transparence in the visible and near infrared range, to maximize the photon absorption by perovskite layer. Furthermore, efficient ETL needs to selectively extract electron while block hole at cathode side, to alleviate charge recombination at the device interface.
Xue et al. synthesized amino functionalized polymer material (PN4N) and introduced it as an ETL interlayer into perovskite devices (Fig. 7). A high PCE of 15% with FF of 72% was recorded, which is much improved to the reference device with PCBM as ETL (the PCE of 12% with FF of 62%). It revealed that PN4N potentially enhanced electron extraction and suppressed the charge recombination at cathode side, which resulted in enhancing device fill factor, and reduced hysteresis [88]. Recently amino-functionalized polymer was introduced as electron efficient layer, the backbone is composed of fluorene, naphthalene diimide, and thiophene used as spacers (PFN-2TNDI) (Fig. 7). Polymer bearing amine functionalities can passivate the traps on perovskite surface and by forming interfacial dipoles can also reduce the work function of the metal cathode [89]. As non-fullerene based ETL, Jen et al. further developed functional hexaazatrinaphthylene to construct planar p-i-n devices, which exhibited negligible hysteresis and superior power conversion efficiency of 17.6% for HATNASOC7-Cs ETL (Fig. 7).
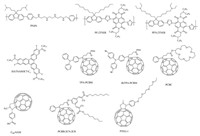
|
Download:
|
| Figure 7. Structures of ETL materials for PVSCs. | |
Peripheral functionalization with alkylsulfanyl chain onto HATNASOC7-CS ETL improved material solubility and significantly passivate the surface defeats of perovskite, in addition, lower the HOMO level of the molecule to block holes from interfacial recombination. And the device stability get significantly improved, which is mainly attributed to the hydrophobic nature of new material prevents moisture attacking perovskite layer [90]. Fullerene and its derivatives have been extensively used as ETLs due to their suitable electron affinity and good charge transport property [74, 75, 91-93]. Bolink et al. prepare perovskite layer through sublimation in vacuum chamber, which was sandwiched between two thin organic buffer layers (PTAA as HTL and PCBM as ETL). Such devices lead to PCE of 12% with high JSC of 16.12 mA cm-2 and VOC of 1.05 V [75]. Recently, Zhu et al. demonstrated that a folurolated chain containing fullerene ETL can facilitate the fabrication of stable and efficient PVSCs, which enables 80% of the initial PCE (15.5%) to be retained after being exposed in ambient condition with 20% relative humidity for 14 days [92]. Regarding to the chemical and physical defeats of TiO2 ETL, Grancini et al. demonstrated a fullerene self-assembled monolayer (C60-SAM) (Fig. 7) can effectively enhance electron extraction of TiO2 to achieve outstanding PCE with an averaged value of 14.8%. Anchoring fullerene SAM on metal oxide passivates the charge traps [94] and a fullerene unit toward perovskite reduces nonradiative recombination channels at the interface [95], which, significantly improve photovoltaic performance, with low hysteresis and potentially increased device stability [95-97].
Li et al. constructed planar p-i-n perovskite solar cells based on CH3NH3PbI3-xClx and double cathode buffer layers, in which fullerene derivative with a crown-ether (PCBC) (Fig. 7) was applied between PCBM and LiF. It suggests the dipole generated within cathode layer enhanced device FF and JSC which results in high PCE 15.53% [98]. Li et al. introduced a new fullerene ETL (PCBB-2CN-2C8) with multiple functionalities to optimize PVSC device, which presented a dramatic increase in VOC from 0.99 (PCBM) to 1.06 V (PCBB-2CN-2C8) and FF from 72.2% to 79.1%, eventually resulting in high PCE of 17.35% in the new fullerene ETL (PCBB-2CN-2C8) based device [99].
Li et al. demonstrated the modulation of organic-perovskite interfaces and their photovoltaic behaviors by tuning the properties functional fullerenes layered atop of perovskite. The fabricated devices showed that fullerene ETLs with intramolecular charge transfer characteristics not only improve molecular polarization but also significantly enhance carrier density, hence the transport capability upon light excitation, ultimately improve the device operation of PHJ PVSCs. Device with TPA-PCBM (Fig. 7) showed light-induced charge transfer from TPA to PCBM, which interestingly showed~13% enhancement in Jsc compared with those of PC61BM based devices [100]. Recently, hysteresis free and stable perovskite was also made with the vacuum-deposit C60 as ETL, which surprisingly delivered 19.1% PCE with MAPbI3 perovskite absorber and no HTL applied. It showed that the 35nm C60 layer enhance FF and VOC of planner PVSC device, which is attributed to the reduced leakage current and better electron extraction of C60 [101]. Loi et al. investigated the effect of fullerene ETL with different dielectric constant on device performance and lightsoaking phenomenon in PVSCs. High dielectric constant of PTEG-1 (Fig. 7) (dielectric constant 5.9) helps alleviate the electron trapping related recombination in the extraction layer. In addition, the electron donating side chains of PTEG-1 may passivate the perovskite defeats in comparison to PC60PBM (dielectric constant 3.9). High PCE of 15.7% was observed for PTEG-1 with device architecture ITO/PEDOT: PSS/CH3NH3PbI3xClx/PTEG-1/Al [102]. More recent research revealed that deposition of SnO2 allows yielding remarkable voltages of 1.21 V for the perovskite absorber with a band gap of 1.62 eV (approaching the thermodynamic limit of 1.32 V). Such PVSCs showed a final PCE of 20.7% after aging and dark storage [103]. Li et al. deposited TiO2 layers with native defects through electron beam evaporation in an oxygen-deficient environment, which provides photoconductive TiO2 electron transport layer leads to improved efficiency 19.0% of planar devices [104]. Lithium-doped TiO2 electrodes exhibited superior electronic properties with negligible hysteresis by reducing electronic trap states enabling faster electron transport and enabling the device to approach a PCE of 19% [105]. These results demonstrate that there have spaces for the development of new organic materials based ETL, comparing with those well-documented HTL materials and metal oxides (Table 2).
|
|
Table 2 ETL materials with their efficiencies. |
5. Conclusion and perspective
In summary, the exciting development has been made for exploring organic π-functional materials as HTL and ETL that enable the high-performance, mild-processing and stable PVSCs. A great number of newly designed small molecule and polymers exhibited a variety of functionalities, processbilities, which largely enriched the PVSC research. Both the planner (n-i-p and p-i-n) and mesoporous PVSCs have been rapidly improved to over 18% PCE, up to 22.1% PCE to date. For the perspectives of future HTL and ETL materials, the new structure-design of organics functional materials as buffer layers is required for achieving efficient, stable and practical PVSCs. Besides the considerations for charge mobility, energy level and work-function tunabilities of buffer materials, some other interesting features can be further explored, such as the capabilities to guide perovskite crystal growth, to protect perovskite from moisture degradation, and to eliminate the device hysteresis etc. Furthermore, little literature for the new ETL development has been documented, comparing to HTL materials. With the rapid development of this field, authors apologize for this short article cannot include all the nice works made from community. Nevertheless, the exciting progresses of organic π-functional materials based buffer layers are discussed. In order to transit the scientific curiosity into technology innovation for PVSC research, more determinations on the integrative exploration of buffer layers, perovskite and resilient device architectures are needed to eventually accomplish outdoor pre-requisites for long term stability, high-performance and low-cost perovskite solar cells.
AcknowledgmentsThe authors thank the financial support from the 973 program (No. 2014CB643503), and the National Natural Science Foundation of China (No. 21474088). C.-Z. Li thanks the financial support from NSFC (No. 21674093) and the National 1000 Young Talents Program hosted by China, and 100 Talents Program by Zhejiang University.
| [1] | C.W. Tang, Two-layer organic photovoltaic cell. Appl. Phys. Lett. 48 (1986) 183–185. DOI:10.1063/1.96937 |
| [2] | G. Yu, J. Gao, J.C. Hummelen, F. Wudl, A.J. Heeger, Polymer photovoltaic cells:enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 270 (1995) 1789–1791. DOI:10.1126/science.270.5243.1789 |
| [3] | B. O'Regan, M. Grätzel, A low-cost, high-efficiency solar cell based on dyesensitized colloidal TiO2 films. Nature 353 (1991) 737–740. DOI:10.1038/353737a0 |
| [4] | S. Mathew, A. Yella, P. Gao, Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 6 (2014) 242–247. DOI:10.1038/nchem.1861 |
| [5] | J.B. You, L.T. Dou, K. Yoshimura, A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 4 (2013) 1446. DOI:10.1038/ncomms2411 |
| [6] | J.B. Zhao, Y.K. Li, G.F. Yang, Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 1 (2016) 15027. DOI:10.1038/nenergy.2015.27 |
| [7] | W.C. Zhao, D.P. Qian, S.Q. Zhang, Fullerene-free polymer solar cells with over 11% efficiency and excellent thermal stability. Adv. Mater. 28 (2016) 4734–4739. DOI:10.1002/adma.v28.23 |
| [8] | A. Kojima, K. Teshima, Y. Shirai, T. Miyasaka, Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131 (2009) 6050–6051. DOI:10.1021/ja809598r |
| [9] | J.H. Im, C.R. Lee, J.W. Lee, S.W. Park, N.G. Park, 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 3 (2011) 4088–4093. DOI:10.1039/c1nr10867k |
| [10] | H.S. Kim, C.R. Lee, J.H. Im, Lead iodide perovskite sensitized all-solidstate submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2 (2012) 591. |
| [11] | J. Zhang, Y. Hua, B. Xu, The role of 3D molecular structural control in new hole transport materials outperforming spiro-OMeTAD in perovskite solar cells. Adv. Energy Mater. 6 (2016) 1601062. DOI:10.1002/aenm.201601062 |
| [12] | J.M. Frost, A. Walsh, What is moving in hybrid halide perovskite solar cells?. Accounts Chem. Res. 49 (2016) 528–535. DOI:10.1021/acs.accounts.5b00431 |
| [13] | M.M. Lee, J. Teuscher, T. Miyasaka, T.N. Murakami, H.J. Snaith, Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 338 (2012) 643–647. DOI:10.1126/science.1228604 |
| [14] | J.M. Ball, M.M. Lee, A. Hey, H.J. Snaith, Low-temperature processed mesosuperstructured to thin-film perovskite solar cells. Energy Environ. Sci. 6 (2013) 1739–1743. DOI:10.1039/c3ee40810h |
| [15] | J. Burschka, N. Pellet, S.J. Moon, Sequential deposition as a route to highperformance perovskite-sensitized solar cells. Nature 499 (2013) 316–319. DOI:10.1038/nature12340 |
| [16] | M.Z. Liu, M.B. Johnston, H.J. Snaith, Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 501 (2013) 395–398. DOI:10.1038/nature12509 |
| [17] | N.J. Jeon, H.G. Lee, Y.C. Kim, o-Methoxy substituents in spiro-OMeTAD for efficient inorganic-organic hybrid perovskite solar cells. J. Am. Chem. Soc. 136 (2014) 7837–7840. DOI:10.1021/ja502824c |
| [18] | N.G. Park, Perovskite solar cells:an emerging photovoltaic technology. Mater. Today 18 (2015) 65–72. DOI:10.1016/j.mattod.2014.07.007 |
| [19] | H.P. Zhou, Q. Chen, G. Li, Interface engineering of highly efficient perovskite solar cells. Science 345 (2014) 542–546. DOI:10.1126/science.1254050 |
| [20] | W.S. Yang, J.H. Noh, N.J. Jeon, High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 348 (2015) 1234–1237. DOI:10.1126/science.aaa9272 |
| [21] | H. Kim, K.G. Lim, T.W. Lee, Planar heterojunction organometal halide perovskite solar cells:roles of interfacial layers. Energy Environ. Sci. 9 (2016) 12–30. DOI:10.1039/C5EE02194D |
| [22] | C.Z. Li, H.L. Yip, A.K.Y. Jen, Functional fullerenes for organic photovoltaics. J. Mater. Chem. 22 (2012) 4161–4177. DOI:10.1039/c2jm15126j |
| [23] | H.L. Yip, A.K.Y. Jen, Recent advances in solution-processed interfacial materials for efficient and stable polymer solar cells. Energy Environ. Sci. 5 (2012) 5994–6011. DOI:10.1039/c2ee02806a |
| [24] | C.C. Chueh, C.Z. Li, A.K.Y. Jen, Recent progress and perspective in solutionprocessed interfacial materials for efficient and stable polymer and organometal perovskite solar cells. Energy Environ. Sci. 8 (2015) 1160–1189. DOI:10.1039/C4EE03824J |
| [25] | Q.F. Dong, Y.J. Fang, Y.C. Shao, Electron-hole diffusion lengths >175 μm in solution-grown CH3NH3PbI3 single crystals. Science 347 (2015) 967–970. DOI:10.1126/science.aaa5760 |
| [26] | J.H. Hou, H.Y. Chen, S.Q. Zhang, G. Li, Y. Yang, Synthesis characterization, and photovoltaic properties of a low band gap polymer based on silolecontaining polythiophenes and 2, 1, 3-benzothiadiazole. J. Am. Chem. Soc. 130 (2008) 16144–16145. DOI:10.1021/ja806687u |
| [27] | Z.T. Huang, G.B. Xue, J.K. Wu, Electron transport in solution-grown TIPSpentacene single crystals:effects of gate dielectrics and polar impurities. Chin. Chem. Lett. (2016) . DOI:10.1016/j.cclet.2016.05.016(inpress) |
| [28] | Z.T. Huang, C.C. Fan, G.B. Xue, Solution-grown aligned crystals of diketopyrrolopyrroles (DPP)-based small molecules:rough surfaces and relatively low charge mobility. Chin. Chem. Lett. 27 (2016) 523–526. DOI:10.1016/j.cclet.2016.01.054 |
| [29] | Q.Q. Lin, A. Armin, R.C.R. Nagiri, P.L. Burn, P. Meredith, Electro-optics of perovskite solar cells. Nat. Photonics 9 (2015) 106–112. |
| [30] | T.H. Liu, K. Chen, Q. Hu, R. Zhu, Q.H. Gong, Inverted perovskite solar cells:progresses and perspectives. Adv. Energy Mater. 6 (2016) 1600457. DOI:10.1002/aenm.v6.17 |
| [31] | P.W.M. Blom, V.D. Mihailetchi, L.J.A. Koster, D.E. Markov, Device physics of polymer:fullerene bulk heterojunction solar cells. Adv. Mater. 19 (2007) 1551–1566. DOI:10.1002/(ISSN)1521-4095 |
| [32] | S. Braun, W.R. Salaneck, M. Fahlman, Energy-level alignment at organic/metal and organic/organic interfaces. Adv. Mater. 21 (2009) 1450–1472. DOI:10.1002/adma.v21:14/15 |
| [33] | W.J. Potscavage Jr., A. Sharma, B. Kippelen, Critical interfaces in organic solar cells and their influence on the open-circuit voltage. Acc. Chem. Res. 42 (2009) 1758–1767. DOI:10.1021/ar900139v |
| [34] | E.L. Ratcliff, B. Zacher, N.R. Armstrong, Selective interlayers and contacts in organic photovoltaic cells. J. Phys. Chem. Lett. 2 (2011) 1337–1350. DOI:10.1021/jz2002259 |
| [35] | C.Y. Huang, W.F. Fu, C.Z. Li, Dopant-free hole-transporting material with a C3h symmetrical truxene core for highly efficient perovskite solar cells. J. Am. Chem. Soc. 138 (2016) 2528–2531. DOI:10.1021/jacs.6b00039 |
| [36] | C. Bi, Q. Wang, Y.C. Shao, Non-wetting surface-driven high-aspect-ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 6 (2015) 7747. DOI:10.1038/ncomms8747 |
| [37] | A. Werner, F. Li, K. Harada, n-Type doping of organic thin films using cationic dyes. Adv. Funct. Mater. 14 (2004) 255–260. DOI:10.1002/(ISSN)1616-3028 |
| [38] | Y.C. Shao, Y.J. Fang, T. Li, Grain boundary dominated ion migration in polycrystalline organic-inorganic halide perovskite films. Energy Environ. Sci. 9 (2016) 1752–1759. DOI:10.1039/C6EE00413J |
| [39] | D.W. de Quilettes, S.M. Vorpahl, S.D. Stranks, Impact of microstructure on local carrier lifetime in perovskite solar cells. Science 348 (2015) 683–686. DOI:10.1126/science.aaa5333 |
| [40] | B. Kan, M.M. Li, Q. Zhang, A series of simple oligomer-like small molecules based on oligothiophenes for solution-processed solar cells with high efficiency. J. Am. Chem. Soc. 137 (2015) 3886–3893. DOI:10.1021/jacs.5b00305 |
| [41] | N.J. Jeon, J.H. Noh, W.S. Yang, Compositional engineering of perovskite materials for high-performance solar cells. Nature 517 (2015) 476–480. DOI:10.1038/nature14133 |
| [42] | S. Ryu, J.H. Noh, N.J. Jeon, Voltage output of efficient perovskite solar cells with high open-circuit voltage and fill factor. Energy Environ. Sci. 7 (2014) 2614–2618. DOI:10.1039/C4EE00762J |
| [43] | X. Tong, F. Lin, J. Wu, Z.M. Wang, High performance perovskite solar cells. Adv. Sci. 3 (2016) 1500201. DOI:10.1002/advs.201500201 |
| [44] | B. Susrutha, L. Giribabu, S.P. Singh, Recent advances in flexible perovskite solar cells. Chem. Commun. 51 (2015) 14696–14707. DOI:10.1039/C5CC03666F |
| [45] | D.Y. Liu, T.L. Kelly, Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nat. Photonics 8 (2014) 133–138. |
| [46] | F.Z. Huang, Y. Dkhissi, W.C. Huang, Gas-assisted preparation of lead iodide perovskite films consisting of a monolayer of single crystalline grains for high efficiency planar solar cells. Nano Energy 10 (2014) 10–18. DOI:10.1016/j.nanoen.2014.08.015 |
| [47] | D. Bi, W. Tress, M.I. Dar, Efficient luminescent solar cells based on tailored mixed-cation perovskites. Sci. Adv. 2 (2016) e1501170. DOI:10.1126/sciadv.1501170 |
| [48] | T.H. Liu, K. Chen, Q. Hu, Fast-growing procedure for perovskite films in planar heterojunction perovskite solar cells. Chin. Chem. Lett. 26 (2015) 1518–1521. DOI:10.1016/j.cclet.2015.09.022 |
| [49] | M. Saliba, T. Matsui, J.Y. Seo, Cesium-containing triple cation perovskite solar cells:improved stability, reproducibility and high efficiency. Energy Environ. Sci. 9 (2016) 1989–1997. DOI:10.1039/C5EE03874J |
| [50] | J.W. Mun, I. Cho, D. Lee, Acetylene-bridged D-A-D type small molecule comprising pyrene and diketopyrrolopyrrole for high efficiency organic solar cells. Org. Electron. 14 (2013) 2341–2347. DOI:10.1016/j.orgel.2013.05.035 |
| [51] | H. Li, K. Fu, A. Hagfeldt, A simple 3, 4-ethylenedioxythiophene based hole-transporting material for perovskite solar cells. Angew. Chem. Int. Ed. 53 (2014) 4085–4088. DOI:10.1002/anie.201310877 |
| [52] | H.R. Li, K.W. Fu, P.P. Boix, Hole-transporting small molecules based on thiophene cores for high efficiency perovskite solar cells. ChemSusChem 7 (2014) 3420–3425. DOI:10.1002/cssc.v7.12 |
| [53] | K. Do, H. Choi, K. Lim, Star-shaped hole transporting materials with a triazine unit for efficient perovskite solar cells. Chem. Commun. 50 (2014) 10971–10974. DOI:10.1039/C4CC04550E |
| [54] | M. Saliba, S. Orlandi, T. Matsui, A molecularly engineered holetransporting material for efficient perovskite solar cells. Nat. Energy 1 (2016) 15017. DOI:10.1038/nenergy.2015.17 |
| [55] | Y.K. Wang, Z.C. Yuan, G.Z. Shi, Dopant-free spiro-triphenylamine/fluorene as hole-transporting material for perovskite solar cells with enhanced efficiency and stability. Adv. Funct. Mater. 26 (2016) 1375–1381. DOI:10.1002/adfm.v26.9 |
| [56] | D.Q. Bi, B. Xu, P. Gao, Facile synthesized organic hole transporting material for perovskite solar cell with efficiency of 19.8%. Nano Energy 23 (2016) 138–144. DOI:10.1016/j.nanoen.2016.03.020 |
| [57] | A. Molina-Ontoria, I. Zimmermann, I. Garcia-Benito, Benzotrithiophene-based hole-transporting materials for 18.2% perovskite solar cells. Angew. Chem. Int. Ed. 55 (2016) 6270–6274. DOI:10.1002/anie.201511877 |
| [58] | B. Xu, D.Q. Bi, Y. Hua, A low-cost spiro. Energy Environ. Sci. 9 (2016) 873–877. DOI:10.1039/C6EE00056H |
| [59] | T. Malinauskas, M. Saliba, T. Matsui, Branched methoxydiphenylaminesubstituted fluorene derivatives as hole transporting materials for highperformance perovskite solar cells. Energy Environ. Sci. 9 (2016) 1681–1686. DOI:10.1039/C5EE03911H |
| [60] | H. Choi, S. Paek, N. Lim, Efficient perovskite solar cells with 13.63% efficiency based on planar triphenylamine hole conductors. Chemistry 20 (2014) 10894–10899. DOI:10.1002/chem.201403807 |
| [61] | H. Choi, S. Park, S. Paek, Efficient star-shaped hole transporting materials with diphenylethenyl side arms for an efficient perovskite solar cell. J. Mater. Chem. A 2 (2014) 19136–19140. DOI:10.1039/C4TA04179H |
| [62] | K. Rakstys, A. Abate, M.I. Dar, Triazatruxene-based hole transporting materials for highly efficient perovskite solar cells. J. Am. Chem. Soc. 137 (2015) 16172–16178. DOI:10.1021/jacs.5b11076 |
| [63] | H. Nishimura, N. Ishida, A. Shimazaki, Hole-transporting materials with a two-dimensionallyexpanded π-system around an azulenecoreforefficient perovskite solar cells. J. Am. Chem. Soc. 137 (2015) 15656–15659. DOI:10.1021/jacs.5b11008 |
| [64] | S.T. Lv, L.Y. Han, J.Y. Xiao, Mesoscopic TiO2/CH3NH3PbI3 perovskite solar cells with new hole-transporting materials containing butadiene derivatives. Chem. Commun. 50 (2014) 6931–6934. DOI:10.1039/c4cc02211d |
| [65] | Y.K. Song, S.T. Lv, X.C. Liu, Energy level tuning of TPB-based holetransporting materials for highly efficient perovskite solar cells. Chem. Commun. 50 (2014) 15239–15242. DOI:10.1039/C4CC06493C |
| [66] | V.M. Eperon, P. Burlakov, A. Docampo, Morphological control for high performance, solution-processed planar heterojunction perovskite solar cells. Adv. Funct. Mater. 24 (2014) 151–157. DOI:10.1002/adfm.v24.1 |
| [67] | M. Cheng, B. Xu, C. Chen, Phenoxazine-based small molecule material for efficient perovskite solar cells and bulk heterojunction organic solar cells. Adv. Energy Mater. 5 (2015) 1401720. DOI:10.1002/aenm.201401720 |
| [68] | Y.S. Liu, Z.R. Hong, Q. Chen, et al, Perovskite solarcells employingdopant-free organic hole transport materials with tunable energy levels. Adv. Mater. 28 (2016) 440–446. DOI:10.1002/adma.v28.3 |
| [69] | Y.S. Liu, Q. Chen, H.S. Duan, A dopant-free organic hole transport material for efficient planar heterojunction perovskite solar cells. J. Mater. Chem. A 3 (2015) 11940–11947. DOI:10.1039/C5TA02502H |
| [70] | S.S. Reddy, K. Gunasekar, J.H. Heo, Highly efficient organic hole transporting materials for perovskite and organic solar cells with long-term stability. Adv. Mater. 28 (2016) 686–693. DOI:10.1002/adma.201503729 |
| [71] | Y. Hua, B. Xu, P. Liu, High conductivity Ag-based metal organic complexes as dopant-free hole-transport materials for perovskite solar cells with high fill factors. Chem. Sci. 7 (2016) 2633–2638. DOI:10.1039/C5SC03569D |
| [72] | J.B. Zhang, B. Xu, M.B. Johansson, Constructive effects of alkyl chains:a strategy to design simple and non-spiro hole transporting materials for highefficiency mixed-ion perovskite solar cells. Adv. Energy Mater. 6 (2016) 1502536. DOI:10.1002/aenm.201502536 |
| [73] | F. Zhang, C.Y. Yi, P. Wei, A novel dopant-free triphenylamine based molecular "butterfly" hole-transport material for highly efficient and stable perovskite solar cells. Adv. Energy Mater. 6 (2016) 1600401. DOI:10.1002/aenm.201600401 |
| [74] | P.W. Liang, C.Y. Liao, C.C. Chueh, Additive enhanced crystallization of solution-processed perovskite for highly efficient planar-heterojunction solar cells. Adv. Mater. 26 (2014) 3748–3754. DOI:10.1002/adma.v26.22 |
| [75] | O. Malinkiewicz, A. Yella, Y.H. Lee, Perovskite solar cells employing organic charge-transport layers. Nat. Photonics 8 (2014) 128–132. |
| [76] | S.Y. Sun, T. Salim, N. Mathews, The origin of high efficiency in lowtemperature solution-processable bilayer organometal halide hybrid solar cells. Energy Environ. Sci. 7 (2014) 399–407. DOI:10.1039/C3EE43161D |
| [77] | P. Docampo, J.M. Ball, M. Darwich, G.E. Eperon, H.J. Snaith, Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates. Nat. Commun. 4 (2013) 2761. |
| [78] | Z.G. Xiao, C. Bi, Y.C. Shao, Efficient, high yield perovskite photovoltaic devices grown by interdiffusion of solution-processed precursor stacking layers. Energy Environ. Sci. 7 (2014) 2619–2623. DOI:10.1039/C4EE01138D |
| [79] | W.Y. Nie, H. Tsai, R. Asadpour, High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 347 (2015) 522–525. DOI:10.1126/science.aaa0472 |
| [80] | J.H. Heo, H.J. Han, D. Kim, T.K. Ahn, S.H. Im, Hysteresis-less inverted CH3NH3PbI3 planar perovskite hybrid solarcells with 18.1% powerconversion efficiency. Energy Environ. Sci. 8 (2015) 1602–1608. DOI:10.1039/C5EE00120J |
| [81] | Y.L. Guo, C. Liu, K. Inoue, Enhancement in the efficiency of an organicinorganic hybrid solar cell with a doped P3HT hole-transporting layer on a void-free perovskite active layer. J. Mater. Chem. A 2 (2014) 13827–13830. DOI:10.1039/C4TA02976C |
| [82] | Q.F. Xue, G.T. Chen, M.Y. Liu, Improving filmformation and photovoltage of highly efficient inverted-type perovskite solar cells through the incorporation of new polymeric hole selective layers. Adv. Energy Mater. 6 (2016) 1502021. DOI:10.1002/aenm.201502021 |
| [83] | D.W. Zhao, M. Sexton, H.Y. Park, High-efficiency solution-processed planar perovskite solar cells with a polymer hole transport layer. Adv. Energy Mater. 5 (2015) 1401855. DOI:10.1002/aenm.201401855 |
| [84] | G.W. Kim, J. Kim, G.Y. Lee, A strategy to design a donor-π-acceptor polymeric hole conductor for an efficient perovskite solar cell. Adv. Energy Mater. 5 (2015) 1500471. DOI:10.1002/aenm.201500471 |
| [85] | J.W. Jo, M.S. Seo, M. Park, Improving performance and stability of flexible planar-heterojunction perovskite solar cells using polymeric holetransport material. Adv. Funct. Mater. 26 (2016) 4464–4471. DOI:10.1002/adfm.v26.25 |
| [86] | N.J. Jeon, J. Lee, J.H. Noh, Efficient inorganic-organic hybrid perovskite solar cells based on pyrene arylamine derivatives as hole-transporting materials. J. Am. Chem. Soc. 135 (2013) 19087–19090. DOI:10.1021/ja410659k |
| [87] | S.D. Sung, M.S. Kang, I.T. Choi, 14.8% perovskite solar cells employing carbazole derivatives as hole transporting materials. Chem. Commun. 50 (2014) 14161–14163. DOI:10.1039/C4CC06716A |
| [88] | Q.F. Xue, Z.C. Hu, J. Liu, Highly efficient fullerene/perovskite planar heterojunction solar cells via cathode modification with an aminofunctionalized polymer interlayer. J. Mater. Chem. A 2 (2014) 19598–19603. DOI:10.1039/C4TA05352D |
| [89] | C. Sun, Z.H. Wu, H.L. Yip, Amino-functionalized conjugated polymer as an efficient electron transport layer for high-performance planarheterojunction perovskite solar cells. Adv. Energy Mater. 6 (2016) 1501534. DOI:10.1002/aenm.201501534 |
| [90] | D.B. Zhao, Z.L. Zhu, M.Y. Kuo, C.C. Chueh, A.K.Y. Jen, Hexaazatrinaphthylene derivatives:efficient electron-transporting materials with tunable energy levels for inverted perovskite solar cells. Angew. Chem. Int. Ed. 55 (2016) 8999–9003. DOI:10.1002/anie.201604399 |
| [91] | K. Wojciechowski, T. Leijtens, S. Siprova, C60 as an efficient n-type compact layer in perovskite solar cells. J. Phys. Chem. Lett. 6 (2015) 2399–2405. DOI:10.1021/acs.jpclett.5b00902 |
| [92] | Z.L. Zhu, C.C. Chueh, F. Lin, A.K.Y. Jen, Enhanced ambient stability of efficient perovskite solar cells by employing a modified fullerene cathode interlayer. Adv. Sci. 3 (2016) . DOI:10.1002/advs.201600027 |
| [93] | P.W. Liang, C.C. Chueh, S.T. Williams, A.K.Y. Jen, Roles of fullerene-based interlayers in enhancing the performance of organometal perovskite thinfilm solar cells. Adv. Energy Mater. 5 (2015) . DOI:10.1002/aenm.201402321 |
| [94] | G. Grancini, R.S. Santosh Kumar, A. Abrusci, Boosting infrared light harvesting by molecular functionalization of metal oxide/polymer interfaces in efficient hybrid solar cells. Adv. Funct. Mater. 22 (2012) 2160–2166. DOI:10.1002/adfm.201102360 |
| [95] | K. Wojciechowski, S.D. Stranks, A. Abate, Heterojunction modification for highly efficient organic-inorganic perovskite solar cells. ACS Nano 8 (2014) 12701–12709. DOI:10.1021/nn505723h |
| [96] | C.Z. Li, J. Huang, H. Ju, Modulate organic-metal oxide heterojunction via[1, 6] azafulleroid for highly efficient organic solar cells. Adv. Mater. 28 (2016) 7269–7275. DOI:10.1002/adma.201601161 |
| [97] | A. Abrusci, S.D. Stranks, P. Docampo, High-performance perovskitepolymer hybrid solar cells via electronic coupling with fullerene monolayers. Nano Lett. 13 (2013) 3124–3128. DOI:10.1021/nl401044q |
| [98] | X.D. Liu, M. Lei, Y. Zhou, B. Song, Y.F. Li, High performance planar p-i-n perovskite solar cells with crown-ether functionalized fullerene and LiF as double cathode buffer layers. Appl. Phys. Lett. 107 (2015) 063901. DOI:10.1063/1.4928535 |
| [99] | Y.W. Li, Y. Zhao, Q. Chen, Multifunctional fullerene derivative for interface engineering in perovskite solar cells. J. Am. Chem. Soc. 137 (2015) 15540–15547. DOI:10.1021/jacs.5b10614 |
| [100] | C.Z. Li, P.W. Liang, D.B. Sulas, Modulation of hybrid organic-perovskite photovoltaic performance by controlling the excited dynamics of fullerenes. Mater. Horiz. 2 (2015) 414–419. DOI:10.1039/C5MH00026B |
| [101] | H. Yoon, S.M. Kang, J.K. Lee, M. Choi, Hysteresis-free low-temperatureprocessed planar perovskite solar cells with 19.1% efficiency. Energy Environ. Sci. 9 (2016) 2262–2266. DOI:10.1039/C6EE01037G |
| [102] | S.Y. Shao, M. Abdu-Aguye, L. Qiu, Elimination of the light soaking effect and performance enhancement in perovskite solar cells using a fullerene derivative. Energy Environ. Sci. 9 (2016) 2444–2452. DOI:10.1039/C6EE01337F |
| [103] | E.H. Anaraki, A. Kermanpur, L. Steier, Highly efficient and stable planar perovskite solar cells by solution-processed tin oxide. Energy Environ. Sci. 9 (2016) 3128–3134. DOI:10.1039/C6EE02390H |
| [104] | Y.B. Li, J.K. Cooper, W.J. Liu, Defective TiO2 with high photoconductive gain for efficient and stable planar heterojunction perovskite solar cells. Nat. Commun. 7 (2016) 12446. DOI:10.1038/ncomms12446 |
| [105] | F. Giordano, A. Abate, J.P.C. Baena, Enhanced electronic properties in mesoporous TiO2 via lithium doping for high-efficiency perovskite solarcells. Nat. Commun. 7 (2016) 10379. DOI:10.1038/ncomms10379 |
| [106] | P. Docampo, J.M. Ball, M. Darwich, G.E. Eperon, H.J. Snaith, Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates. Nat. Commun. 4 (2013) 2761. |
 2017, Vol. 28
2017, Vol. 28