b Tianjin Engineering Laboratory of Quality Control Technology of Traditional Chinese Medicine, Tianjin Institute of Pharmaceutical Research, Tianjin 300193, China;
c The Tianjin Engineering Center of Injection Package, China Otsuka Pharmaceutical Company Limited, Tianjin 300382, China
Respiratory system infection is the common factor that leads to significant morbidity among humans [1]. In addition, it is more dangerous to children and the elderly. When respiratory system infection occurs, fever and inflammation are the main symptoms [2]. Although mortality due to respiratory system infection is low, its complications, including otitis media, sinusitis, exacerbations of asthma and chronic obstructive pulmonary disease significantly contribute to its harmfulness [3]. The infection may also be exacerbated by air pollution, which is a worldwide public health problem [4]. Therefore, effective drug therapies are in urgent need.
About ninety percent of patients with a respiratory system infection are dosed with antimicrobials and antibiotic for treatment [5]. However, excessive and inappropriate use of antibiotics will cause the development of bacterial resistance, which may spread across borders. More specifically, unnecessary use of antibiotic leads to antibiotic resistance [6] and may result in reduced efficacy in other diseases [7]. Thus, it is essential to pay attention to this important issue, which will have a potentially serious impact on all countries.
Traditional Chinese medicine (TCM) has been widely used for a long time in China. Considerable experience on the application of TCM in various diseases has accumulated. The Chinese herbal medicine Cimicifugae Rhizoma (CR) originates from the genus Cimicifuga including Cimicifuga dahurica (Turcz.) Maxim., Cimicifuga foetida L. and Cimicifuga heracleifolia Kom. It is known as “Sheng-ma” in China, and listed in Chinese Pharmacopoeia officially [8]. CR has been historically used as an effective drug for fever and headache [9, 10], which is now attributed to its antiinflammation and antipyretic effects. As is known, triterpenoids and phenolic derivatives are the main components in extracts of CR. Many studies suggest that various phenol-type derivatives are the major active compounds with anti-inflammatory effects [9]. A recent investigation indicated that CR extracts inhibit the production of inflammatory cytokines, such as histamine, bradykinin, COX-2, IL-8, and TNF-α [11]. Additionally, some studies have suggested that CR has antipyretic effects due to the relaxation of blood vessels [12]. Due to these biological activities, CR might exert protective effects on respiratory system infections.
In this study, the protective effects of CR in Pseudomonas aeruginosa (P. aeruginosa, PAK) -induced pneumonia were evaluated in mice. Then, using an established ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry (UPLC/Q-TOF-MS) integrated nuclear factor κB (NF-κB) luciferase reporter gene assay system, series of anti-inflammatory ingredients were identified from CR.
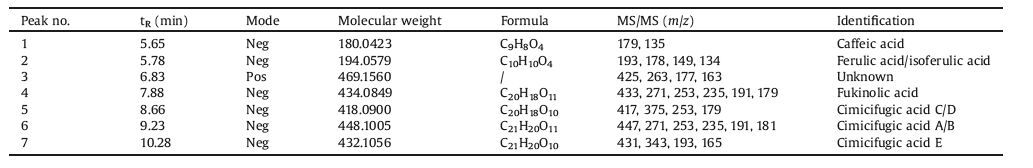
2. Results and discussionTo evaluate the anti-inflammatory effects of CR, P. aeruginosa- induced pneumonia was established in mice. Compared with the control group, the lung section in the model group displayed inflammatory cell infiltration, widened alveolar septum and capillary congestion around the vessels. This finding indicated that the pneumonia model was successfully established. The protective effects of different doses of CR extract (CRE) were evaluated. Cephalosporin was used as the positive control to reduce inflammation. Histological injury was markedly alleviated by a high dose of CRE (CR-H), with reduced inflammatory infiltration and neutrophil recruitment. Moreover, low (CR-L) and middle (CR-M) doses of CRE exerted protective effects on pneumonia to a lesser extent (results were shown in Fig. S1 in Supporting information). This result suggested that CRE exerted anti-inflammatory effects on the mouse pneumonia model in a dose-dependent manner. According to Fig. 1A and B, the inflammatory cytokines, including IL-8 and TNF-α, notably increased at 12 h after infection vs. the control group. CRE and cephalosporin could significantly reduce the expressions of the inflammatory cytokines in lung tissue and plasma. These results demonstrated that CRE had a protective effect on PKA-induced pneumonia, and this effect was at least partially due to its antiinflammatory activity.
|
Download:
|
| Figure 1. The effects of CRE on PKA-induced pneumonia in mice shown by the levels of IL-8 (A) and TNF-α (B) in mice lung tissue and blood. CR-L, CR-M and CR-H were set as the doses of 1.5 g/kg, 3 g/kg and 6 g/kg of CRE, respectively. The values are presented as the means ± SEM; n = 10 for each group. *** indicates P<0.001 vs. the control group; #, ## or ### indicates P<0.05, P<0.01 or P<0.001 vs. the model group, respectively. | |
The pneumonia model induced by PKA in mice used in this study resembles the disease in humans. This system has been established as a reliable model to evaluate the therapeutic effects of diverse compounds on pneumonia [13, 14]. DiMango et al. reported that inflammatory cytokines, including NF-κB and IL-8, were activated by adherent P. aeruginosa in normal and cystic fibrosis respiratory epithelial cells [15]. In addition, the reduction or disappearance of cytokines, such as IL-1, IL-6, and TNF-α, could reflect the amelioration of pneumonia [16]. NF-κB, the important transcription factor, is related to gene activation of many of the mediators involved in the inflammatory response, such as TNF-α, IL-8 and iNOS [17, 18]. For example, Chai et al. reported that the inhibitor of NF-κB, pyrrolidine dithiocarbamate (PDTC), relieved the lung damage produced by P. aeruginosa pneumonia [19]. Therefore, NF-κB inhibition effect was used to identify potential anti-inflammatory ingredients from CR.
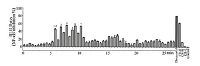
To clarify the effective ingredients, the UPLC/Q-TOF/MS method was applied to separate and identify components in CR. Then the column effluent was split at a ratio of 1:9 after substance separation. The majority of the substance was directed toward Q-TOF/MS analysis, and the other part was directed toward diode array detection (DAD) in Fig. 2A. The total ion current (TIC) chromatograms in positive and negative ESI modes are presented in Fig. 2B and C, respectively. NF-κB luciferase reporter assay was integrated to UPLC/Q-TOF/MS analysis to screen the anti-inflammatory components from these UPLC fractions. The percentage of NF-κB inhibition of each UPLC fraction was shown in Fig. 3. Also, dexacortal (10-5 mol/L), the positive drug with anti-inflammatory activity, and a high dose of CRE (CR-H, 100 mg/mL) significantly inhibited the activation of NF-κB induced by TNF-α in this analysis system. Finally, six fractions with potent NF-κB inhibitory effects of greater than 50% were considered to have anti-inflammatory constituents. As a result, seven ingredients including six cinnamic acid derivatives (caffeic acid, ferulic acid/isoferulic acid, fukinolic acid, and cimicifugic acid C/D, A/B and E) were identified as the potential effective compounds from CR. The structural formulas of these compounds were presented in Fig. 2A, and the detailed information was listed in Table 1. Some peaks that had the same protonate molecules in the MS spectra and similar fragment ions in the MS/MS spectra as those have been reported in literatures could be distinguished and identified due to their different retention behaviors [20-22]. The detailed identification information could be referred to the Supporting information. Among these compounds, caffeic acid, ferulic acid and cimicifugic acids have all been reported to be constituents of CR [23].

|
Download:
|
| Figure 2. UPLC/Q-TOF/MS analysis of CRE. (A) UPLC/UV chromatograms of CRE and the structural formulas of the biocative compounds. TIC chromatograms in positive ESI mode (B) and negative ESI mode (C). | |
|
Download:
|
| Figure 3. The bioactivity chromatograms of CRE obtained via the dual-luciferase reporter assay system for NF-κB inhibition. | |
|
|
Table 1 MS/MS data from ESI-MS and the identification of the bioactive compounds of CRE. |
As these cinnamic acid derivatives were all derived from caffeic acid groups, and possessed similar fragment ions such as 179 (caffeic acid [M-H]-; fukinolic acid [M-H-C11H10O7]-; cimicifugic acid C/D [M-H-C11H10O7]-) and 191 (fukinolic acid [M-H-C10H10O7]- and cimicifugic acid A/B [M-H-C11H12O7]-), then caffeic acid was chosen as a representative compound to confirm the anti-inflammatory effects. As shown in Fig. 4A, the levels of activated NF-κB were significantly increased in BEAS-2B cells treated with TNF-α. In contrast, caffeic acid significantly restrained the activation of NF-κB in a dose-dependent manner. In addition, as shown in Fig. 4B and C, IL-6 and IL-8 markedly increased after treated with TNF-α. Similarly, caffeic acid could significantly reduce the increase in BEAS-2B cells. Conclusively, caffeic acid, as a representative of these screened compounds, possessed antiinflammatory capacity; and this confirmed our screening results.

|
Download:
|
| Figure 4. The anti-inflammatory effects of caffeic acid on NF-κB (A), IL-6 (B) and IL-8 (C) levels in TNF-α-treated BEAS-2B cells. The values are presented as the means ± SEM; n = 6 for each group. *** indicates P<0.001 vs. the control group; #P<0.05, ##P<0.01 or ###P<0.001 vs. model group, respectively | |
The UPLC/Q-TOF-MS-integrated NF-κB luciferase reporter gene assay system established in our laboratory [24] has been successfully used to screen anti-inflammatory ingredients from TCM. For example, Sun et al. used the NF-κB luciferase reporter system to identify the anti-inflammatory ingredients from Menispermi Rhizoma [25]. Compounds including sinomenine, norsinoacutin, magnoflorine, laurifloline and dauricinoline were identified and further verified as effective components. Hou et al. also successfully identified several anti-inflammatory ingredients from Qingfei Xiaoyan Wan (a TCM formula, which has been used clinically in China for upper respiratory tract infections), such as arctigenin, cholic acid, chlorogenic acid and sinapic acid [26]. In this experiment, this approach was carried out again for screening anti-inflammatory ingredients from CR.
Previous research reported that ferulic acid and isoferulic acid are the anti-inflammatory compounds in CR [27, 28]. The antiinflammatory effects of caffeic acid and its derivatives are associated with its antioxidant activity, inhibition of 5-lipoxygenase and protein kinase C activity [29]. For example, Satoru et al. reported that fukinolic acid exerted its antiinflammatory effects through radical scavenging and inhibition of macrophage NO production as an antioxidant [30]. In our study, the anti-inflammatory activities of these compounds were also detected as having been reported. The other studies demonstrated that cinnamic acid derivatives, including caffeic acid, fukinolic acid as well as cimicifugic acids A, B, E and F isolated from the rhizomes of Cimicifuga racemose, possessed inhibition activity of neutrophil elastase [31]. As is known, neutrophil elastase contributes to the destruction of basement membranes during active inflammation with a typical feature of an elevated plasma level of neutrophil elastase [32, 33]. Thus, the inhibition of neutrophil elastase contributes to the protection from injury of inflammation. Moreover, the caffeic acid compounds also related to the neuroprotective activity against glutamate-induced neurotoxicity [34]; vasoactive effects of cimicifugic acids A-E, fukinolic acid and fukiic acid isolated from Cimicifuga plants were also verified by rat aortic strips test [35]. To the best of our knowledge, it was first found that cimicifugic acid A/B, C/D and E can regulate the activation of NF-κB.
3. ConclusionOur study revealed that CR ameliorated PKA-induced pneumonia in mice. Caffeic acid derivatives were characterized as NF-κB inhibitors that might be responsible for the anti-inflammatory effects of CR. This study suggested that CR and its antiinflammatory components have the possibility to be developed as complementary therapies in the treatment of respiratory system infection in clinics.
4. Experimental 4.1. Chemicals and materialsLC-MS grade acetonitrile was purchased from Fisher (Pittsburgh, PA, USA); formic acid for MS analysis was LC grade and from Acros Organics (Geel, Belgium). Ethanol was of analytical grade and purchased from Dingguo Biotechnology Co., Ltd. (Tianjin, China). The water used in the analysis was purified by a Milli-Q academic water purification system (Millipore, Milford, MA, USA). CR was purchased from a medicine market at Anguo China. The herbal medicine was identified as Cimicifugae Rhizoma by Professor Tiejun Zhang from the Tianjin Institute of Pharmaceutical Research. The voucher specimens are currently deposited in Composite Drugs and Systems Biology Laboratory in Nankai University. Human TNF-α was obtained from PeproTech (Rocky Hill, USA). Dexacortal (Dex) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). The reporter plasmid pGL 4.32 and Renilla luciferase reporter vector plasmid pRL-TK were purchased from Promega (WI, USA). All reagents for cell culture were purchased from Gibco BRL Life Technologies (Rockville, MD, USA). Lipofectamine 2000 transfection reagent was obtained from Invitrogen (Carlsbad, CA, USA). ELISA kits for mouse and human were purchased from West Tang Bio-Tech Co., Ltd. (Shanghai, China). All other reagents used in this study were of analytical grade.
4.2. Sample preparationHerbal medicine CR was crushed. The dried powder (2.5 g) was added to 25 mL of 75% aqueous ethanol in a conical flask. Then, ultra-sonication (40 kHz) was performed at room temperature for 30 min. Subsequently, the extraction was filtered through filter paper, and the filtrate was centrifuged at 12, 000 rpm for 10 min to obtain the supernatant. The supernatant was then dried in a vacuum drier prior to use in later studies and named CR extract (CRE). CRE was stored in the refrigerator at 4 ℃ and allowed to reach room temperature before analysis.
4.3. Cell culture, transfection and dual-luciferase assayHEK 293 (a human embryonic kidney 293 cell line) was chosen based on its high transfection efficiency; BEAS-2B (a cell line derived from human bronchial epithelial cells) was used in evaluation of cytokines for pulmonary inflammation. HEK 293 and BEAS-2B cells were obtained from the American Type Culture Collection (Rockville, MD). HEK 293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco BRL) containing 10% fetal bovine serum (Gibco BRL), whereas BEAS-2B cells were cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum. The cells were cultured in culture flasks at 37 ℃ and 5% CO2 in a humidified incubator.
The cells were transfected with plasmid pGL 4.32 and pRL-TK by Lipofectamine 2000 as previously reported [36]. After TNF-α-stimulated (10 ng/mL) cells were treated with the prepared samples, they were washed with sodium chloride physiological solution (SCPS) (Otsuka Pharmaceutical Co., Ltd., Tianjin, China), and lysed for luciferase activity detection using the dual-luciferase reporter assay system (Promega) according to the manufacturer’s instruction. Relative luciferase activity was obtained by normalizing the firefly luciferase activity against that of the internal control (Renilla luciferase).
4.4. AnimalsKunming mice weighing 20-25 g were obtained from the Experimental Animal Center, Academy of Military Medical Science (Beijing, China, lot number 0034445). The mice were raised in rat cages (48 cm × 29 cm × 18 cm) in a unidirectional airflow room under controlled temperature (20-24 ℃), relative humidity (40%- 60%) and a 12-h light/dark cycle. Filtered tap water and commercial rat chow were available ad libitum. The mice were acclimated to the environment of the cages for 1 week. Before the experiments, they were fasted for 12 h. All experimental protocols conformed to the Guide for the Care and Use of Laboratory Animal Care and Use in Research (Ministry of Health, Beijing, China) and were approved by the Animal Ethics Committee of Nankai University.
4.5. P. aeruginosa culture and infectionP. aeruginosa (PAK) used in our laboratory is a wild-type strain that was clinically isolated from the sputum of a patient with bronchiectasis [37]. Sixty Kunming mice were randomly divided into 6 groups, including the control group, model group, positive drug (cephalosporin) group, and high, middle, and low dose of CRE groups, with 10 mice in each group. These mice were treated as follows for one week before PAK infection. The mice in the control group and model group had free access to food and water without any drugs. The mice in the positive drug group were administered cephalosporin (6 g/kg) via gavage once daily. The mice in the high, middle and low doses groups were administered CRE at 6 g/kg, 3 g/ kg and 1.5 g/kg, respectively, via gavage once daily. Cephalosporin and different concentrations of CRE were all prepared in water. Then, the pneumonia model was established through nasal instillation of PAK suspension (2 × 109/mL, 50mL) to mice [25]. The doses of CRE in high, middle and low doses groups were chosen based on the results of pre-experiments (data not shown) and referred to the dose used in clinics.
4.6. Pathological analysis of the lung tissuesFresh lung tissue samples were fixed in 10% formalin and embedded in paraffin. Samples were crosscut into 40-50 slices with a thickness of 4-5 mm. Hematoxylin-eosin (HE) staining of sections from the lung tissues of mice was conducted using the regular method [38]. Then, the stained sections were observed under a light microscope (40 × and 200 × magnification).
4.7. ELISA assay of inflammatory cytokinesThe lung tissues were homogenized in 2 mL of SCPS and centrifuged at 3000 rpm for 10 min, and then, the supernatant was collected. Blood drawn from the vena angularis of mice was centrifuged at 3000 rpm for 10 min to obtain plasma. The homogenate and plasma were stored at -80 ℃ until used for ELISA assay of inflammatory cytokines, such as TNF-α and IL-8.
After TNF-α stimulated (10 ng/mL), BEAS-2B cells were treated with the prepared samples including dexacortal (1 × 10-5 mol/L) and caffeic acid (1 × 10-6, 1 × 10-5 and 1 × 10-4 mol/L) for 5 h, the culture media were collected for measurement of inflammatory cytokines including IL-6 and IL-8 by ELISA kits according to the manufacturer’s protocols.
4.8. UPLC/Q-TOF-MS analysisA Waters Acquity UPLC System (Waters Co., Milford, MA, USA) equipped with a photodiode array detector (PAD) was used. UV detection was achieved in the range of 190-400 nm. The system was controlled by MassLynx V 4.1 software (Waters Co.). The separation of the sample was performed on a Waters Acquity UPLC BEH C18-column (2.1 mm × 100 mm, 1.7 mm) with a column temperature maintained at 35 ℃. For the screening of active ingredients that have an antergic effect on NF-κB, the gradient elution of acetonitrile (A) and 0.1% formic acid (B) was performed as follows: 0-1 min, isocratic of 2% A; 1-3 min, isocratic of 10% A; 3-7 min, isocratic of 15% A; 7-15 min, isocratic of 30% A; 15- 20 min, isocratic of 50% A; 20-23 min, isocratic of 80% A; 23- 24 min, isocratic of 100% A; 24-25 min, 100-2% A. The flow rate was set at 0.4 mL/min. Five microliters of the test sample (20 mg/mL, raw material) was injected into the UPLC system. Subsequently, the UPLC fractions were collected in a 96-deep-well plate (2.2 mL) every 30 s followed by evaporation to dryness in a vacuum drying oven. The residues were dissolved in 100 mL of cell culture medium for the dual-luciferase assay.
4.9. MS and MS/MS conditionsAccurate mass measurements and MS/MS were obtained with a Waters Q/TOF Premier equipped with an electrospray ionization (ESI) system (Waters, Manchester, UK). The parameters were set as follows: capillary voltage, 3.0 kV (positive mode) and 2.5 kV (negative mode); sample cone voltage, 30 V; high-purity nitrogen was used as the nebulization and auxiliary gas; nebulization gas, 600 L/h at 350 ℃; cone gas, 50 L/h; and source temperature, 100 ℃. The Q/TOF Premier acquisition rate was 0.1 s with a 0.02 s interscan delay. The collision gas was argon at a pressure of 5.3 × 10-5 Torr. The most noteworthy result is that the Q/TOF Premier can achieve a mass accuracy of 1-5 ppm and mass resolution with m/Δm at 10, 000 for accurate analysis. The instrument was operated with the collision cell operating at 30 eV. Leucine enkephalinamide acetate was used as the lock mass ([M+H]+ 555.2931, [M-H]- 553.2775) and a flow rate of 20 μL/min.
4.10. Statistical analysisThe results were expressed as the standard error of the mean (SEM). Multiple comparisons were performed using one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test. For single comparisons, significant differences between means were determined using Student’s t-test. P<0.05 was considered statistically significant.
AcknowledgmentThis project was financially supported by the National Natural Science Foundation of China (Nos. 81303291, 81373506, 81473403).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.11.021
| [1] | E.C. Goodall, A.C. Granados, K. Luinstra, Vitamin D3 and gargling for the prevention of upper respiratory tract infections:a randomized controlled trial. BMC Infect. Dis. 14 (2014) 273–281. DOI:10.1186/1471-2334-14-273 |
| [2] | J.D. Hasday, N. Shah, P.A. Mackowiak, Fever, hyperthermia, and the lung:it's all about context and timing. Trans. Am. Clin. Climatol. Assoc. 122 (2011) 34–47. |
| [3] | D. Proud, Upper airway viral infections. Pulm. Pharmacol. Ther. 21 (2008) 468–473. DOI:10.1016/j.pupt.2007.06.004 |
| [4] | L.A. Darrow, M. Klein, W.D. Flanders, Air pollution and acute respiratory infections among children 0-4 years of age:an 18-year time-series study. Am. J. Epidemiol. 180 (2014) 968–977. DOI:10.1093/aje/kwu234 |
| [5] | C.C. Butler, K. Hood, T. Verheij, Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care:prospective study in 13 countries. BMJ 338 (2009) b2242–b2250. DOI:10.1136/bmj.b2242 |
| [6] | J. Davies, D. Davies, Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 74 (2010) 417–433. DOI:10.1128/MMBR.00016-10 |
| [7] | I. Fredericks, S. Hollingworth, A. Pudmenzky, Consumer knowledge and perceptions about antibiotics and upper respiratory tract infections in a community pharmacy. Int. J. Clin. Pharm. 37 (2015) 1213–1221. DOI:10.1007/s11096-015-0188-y |
| [8] | Chinese Pharmacopoeia Commission, The Chinese Pharmacopoeia, The Chemical Industry Publishing House, Beijing, 2005 p. 50. |
| [9] | J.X. Li, Z.Y. Yu, Cimicifugae rhizoma:from origins bioactive constituents to clinical outcomes. Curr. Med. Chem. 13 (2006) 2927–2951. DOI:10.2174/092986706778521869 |
| [10] | J.W. Yun, J.R. You, Y.S. Kim, Pre-clinical in vitro and in vivo safety evaluation of Cimicifuga heracleifolia. Regul. Toxicol. Pharmacol. 73 (2015) 303–310. DOI:10.1016/j.yrtph.2015.07.006 |
| [11] | S.J. Kim, M.S. Kim, Inhibitory effects of cimicifugae rhizoma extracts on histamine, bradykinin and COX-2 mediated inflammatory actions. Phytother. Res. 14 (2000) 596–600. DOI:10.1002/(ISSN)1099-1573 |
| [12] | L. Mun, M.S. Jun, Y.M. Kim, 7,8-Didehydrocimigenol from Cimicifugae rhizoma inhibits TNF-α-induced VCAM-1 but not ICAM-1expression through upregulation of PPAR-gamma in human endothelial cells. Food Chem. Toxicol. 49 (2011) 166–172. DOI:10.1016/j.fct.2010.10.012 |
| [13] | N. Aoki, K. Tateda, Y. Kikuchi, Efficacy of colistin combination therapy in a mouse model of pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. J. Antimicrob. Chemother. 63 (2009) 534–542. DOI:10.1093/jac/dkn530 |
| [14] | Q. Lu, C. Girardi, M. Zhang, Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Med. 36 (2010) 1147–1155. DOI:10.1007/s00134-010-1879-4 |
| [15] | E. DiMango, A.J. Ratner, R. Bryan, Activation of NF-kappaB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J. Clin. Invest. 101 (1998) 2598–2605. DOI:10.1172/JCI2865 |
| [16] | M.J. Schultz, A.W. Rijneveld, S. Florquin, Role of interleukin-1 in the pulmonary immune response during Pseudomonas aeruginosa pneumonia. Am. J. Physiol. Lung Cell Mol. Physiol. 282 (2002) L285–L290. DOI:10.1152/ajplung.00461.2000 |
| [17] | R.H. Mohamed, R.A. Karam, M.G. Amer, Epicatechin attenuates doxorubicininduced brain toxicity:critical role of TNF-α, iNOS and NF-кB. Brain Res. Bull. 86 (2011) 22–28. DOI:10.1016/j.brainresbull.2011.07.001 |
| [18] | A. Kaulmann, S. Legay, Y.J. Schneider, Inflammation related responses of intestinal cells to plum and cabbage digesta with differential carotenoid and polyphenol profiles following simulated gastrointestinal digestion. Mol. Nutr. Food Res. 60 (2016) 992–1005. DOI:10.1002/mnfr.v60.5 |
| [19] | W.S. Chai, J.X. Fan, Z.M. Qi, Anti-inflammation effect of pyrrolidine dithiocarbamate on Pseudomonas aeruginosa induced pneumonia in rats. Chin. Pharmacol. Bull. 24 (2008) 206–210. |
| [20] | M. Takahira, A. Kusano, M. Shibano, ChemInform Abstract:three new fukiic acid esters, cimicifugic acids A, B and C, from Cimicifuga simplex WORMSK. Cheminform 46 (1998) 362–365. |
| [21] | W. Li, Y. Sun, W. Liang, Identification of caffeic acid derivatives in Actea racemosa (Cimicifuga racemosa, black cohosh) by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 17 (2003) 978–982. DOI:10.1002/(ISSN)1097-0231 |
| [22] | S.O. Kruse, A. Lohning, G.F. Pauli, Fukiic and piscidic acid esters from the rhizome of Cimicifuga racemosa and the in vitro estrogenic activity of fukinolic acid. Planta Med. 65 (1999) 763–764. DOI:10.1055/s-2006-960862 |
| [23] | X.H. Zhao, D.H. Chen, J.Y. Si, Studies on the phenolic acid constituents from Chinese medicine "sheng-ma", rhizome of Cimicifuga foetida L. Acta Pharm. Sin. 37 (2002) 535–538. |
| [24] | M.G. Zhou, M. Jiang, X. Ying, Identification and comparison of antiinflammatory ingredients from different organs of Lotus nelumbo by UPLC/QTOF and PCA coupled with a NF-кB reporter gene assay. PLoS One 8 (2013) e81971. DOI:10.1371/journal.pone.0081971 |
| [25] | D. Sun, M.G. Zhou, X.H. Ying, Identification of nuclear factor-kB inhibitors in the folk herb Rhizoma Menispermi via bioactivity-based ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry analysis. BMC Complement Altern. Med. 14 (2014) 356–366. DOI:10.1186/1472-6882-14-356 |
| [26] | Y.Y. Hou, Y. Nie, B.F. Cheng, Qingfei Xiaoyan Wan, a traditional Chinese medicine formula, ameliorates Pseudomonas aeruginosa-induced acute lung inflammation by regulation of PI3K/AKTand Ras/MAPK pathways. Acta Pharm. Sin. B 6 (2016) 212–221. DOI:10.1016/j.apsb.2016.03.002 |
| [27] | S. Sakai, H. Kawamata, T. Kogure, Inhibitory effect of ferulic acid and isoferulic acid on the production of macrophage inflammatory protein-2 in response to respiratory syncytial virus infection in RAW264.7 cells. Mediat. Inflamm. 8 (1999) 173–175. DOI:10.1080/09629359990513 |
| [28] | J.K. Kim, Y.I. Kwon, H.D. Jang, Anti-inflammatory effects of catechol and ferulic acid derivatives through NF-кB activation in Raw264.7 cells. FASEB J. 28 (2014) s821–s830. |
| [29] | G.D. Gamaro, E. Suyenaga, M. Borsoi, et al., Effect of rosmarinic and caffeic acids on inflammatory and nociception process in rats, ISRN Pharmacol. 2011(2011), doi:http://dx.doi.org/10.5402/2011/451682. |
| [30] | W. Satoru, H. Ken, T. Hiroyuki, Radical scavenging activity and inhibition of macrophage NO productionby fukinolic acid, a mainphenolic constituent in Japanese Butterbur (Petasites japoni). Food Sci. Technol. Res. 13 (2007) 366–371. DOI:10.3136/fstr.13.366 |
| [31] | B. Loser, S.O. Kruse, M.F. Melzig, Inhibition of neutrophil elastase activity by cinnamic acid derivatives from Cimicifuga racemosa. Planta. Med. 66 (2000) 751–753. DOI:10.1055/s-2000-9563 |
| [32] | D.J. Pipoly, E.C. Crouch, Degradation of native type IV procollagen by human neutrophil elastase. Implications for leukocyte-mediated degradation of basement membranes. Biochemistry 26 (1987) 5748–5754. DOI:10.1021/bi00392a025 |
| [33] | W. Fischbach, W. Becker, J. Mossner, Leukocyte elastase in chronic inflammatory bowel diseases:a markerof inflammatoryactivity?. Digestion 37 (1987) 88–95. |
| [34] | J.P. Bai, X.L. Hu, X.W. Jiang, Caffeic acids from roots of Arctium lappa and their neuroprotective activity. Chin. Tradit. Herb. Drugs 46 (2015) 163–168. |
| [35] | M. Noguchi, M. Nagai, M. Koeda, Vasoactive effects of cimicifugic acids C and D, and fukinolic acid in cimicifuga rhizome. Biol. Pharm. Bull. 21 (1998) 1163–1168. DOI:10.1248/bpb.21.1163 |
| [36] | M. Jiang, Y.Q. Han, M.G. Zhou, The screening research of antiinflammatory bioactive markers from different flowering phases of Flos Lonicerae Japonicae. PLoS One 9 (2014) e96214. DOI:10.1371/journal.pone.0096214 |
| [37] | F. Bai, H.J. Xu, Q. Zhang, Functional characterization of pfm in protein secretion and lung infection of Pseudomonas aeruginosa. Can. J. Microbiol. 57 (2011) 829–837. DOI:10.1139/w11-075 |
| [38] | X.M. Fan, M. Shi, Y.M. Wang, Transcriptional profiling analysis of HMPtreated rats with experimentally induced myocardial infarction. J. Ethnopharmacol. 137 (2011) 199–204. DOI:10.1016/j.jep.2011.05.010 |
 2017, Vol. 28
2017, Vol. 28