b Center of R & D, China Tobacco Jiangxi Industrial Co., Ltd., Nanchang 330096, China
Fluorine is generally regarded as a remarkable element because incorporation of fluorine into an organic molecule can dramatically alter its physical, chemical and biological properties [1]. Carbohydrates play many key roles in biological systems and their fluorinated counterparts have found a variety of applications in life science. For example, fluorinated carbohydrates have been used as biological probes for the structure elucidation of biological targets [2]. They can also act as highly efficient enzymatic inhibitors, which have broad applications in the treatment of diseases such as viral infections and cancer [3]. In this regard, many fluorinated nucleosides are marker drugs including sofosbuvir [4]. Furthermore, they are also used as positron emission tomography (PET) agents, with [18F] fluorodeoxyglucose being the standard radiotracer for PET neuroimaging and as a cancer diagnostic tool [5, 6].
Typically, fluorinated carbohydrates [7] can be prepared via deoxyfluorination of hydroxyl groups using DAST, deoxy-fluor or via nucleophilic substitution of an activated hydroxyl group with fluoride [8, 9]. One major drawback associated with the aforementioned transformations is the unpredictable nature of stereochemical outcomes. Competing reactions involving anchimeric assistance effects, rearrangement and SN1 pathways is often difficult to control [10]. One important, yet under-developed alternative is the nucleophilic ring-opening reactions of sugarderived epoxides with fluoride. Examples of this class of reactions on sugar derived epoxides are limited to the use of KHF2 [11] and Et3N·3HF [12]. However, these reagents have their own limitations. For instance, KHF2 requires high temperature (up to 200 ℃) and prolonged reaction time, while Et3N·3HF is both corrosive and toxic. Recently, the ring-opening reactions of sugar-derived epoxides have been reported using tetrabutylammonium bifluoride (TBABF/KHF2) under milder condition [13]. However, TBABF is a viscous liquid and difficult to handle [13]. Furthermore, TBABF needs to be prepared from tetrabutylammonium fluoride (TBAF) and concentrated HF solution (highly toxic) coupled with an extensive drying process prior to use [13]. Thus, ring opening of sugar-derived epoxides under mild conditions with easy-tohandle reagents is desirable for the synthesis of fluorinated carbohydrates.
In the continued interest of developing novel fluorination methodology [14], this study aims to demonstrate the feasibility of substituting TBABF/KHF2 with the safe and commercially available reagent mixture TBAF/KHF2, based on the work of Percy [15] and Hu [14a]. The reactions described herein are the first application of TBAF/KHF2 for the ring-opening of sugar-derived epoxides. Different substrates including 1-thioglycoside can be ring-opened to afford the corresponding fluorinated carbohydrates in high yields and short reaction times.
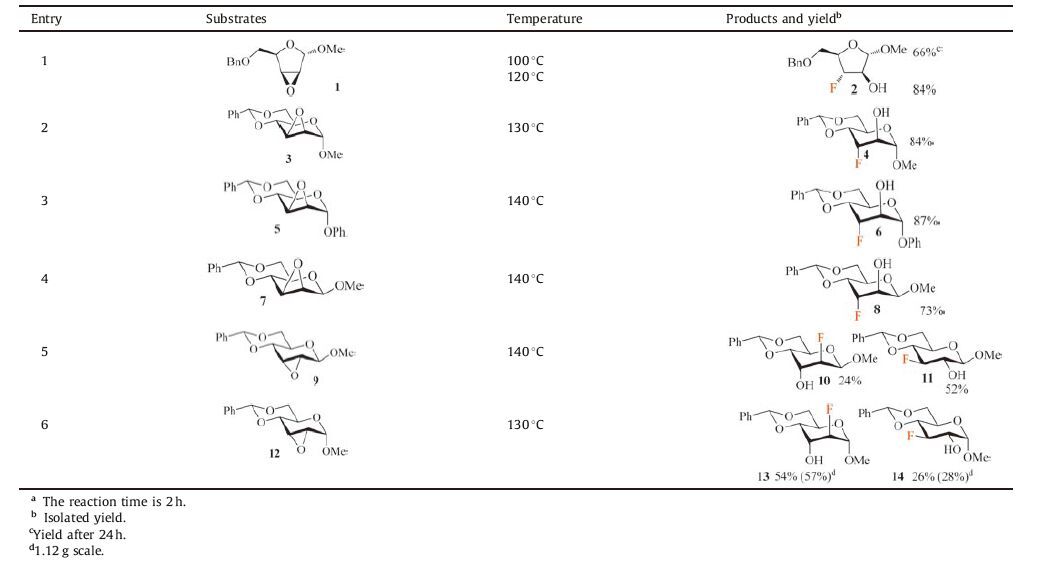
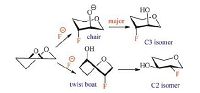
2. Results and discussionThe investigation was initiated with D-arabinose derived epoxide 1. Based on our previous work [14a], A neat solution of 1 in TBAF/KHF2 was heated at 100 ℃ for 24 h. To our delight, the desired product 2 could be obtained as a single regioisomer, although the yield was only 66% (Table 1, entry 1). Greater conversion was achieved when the reaction was increased to 120 ℃, affording fluorohydrin 2 in 84% yield (Table 1, entry 1). Using this testablished conditions, TBAF/KHF2 was reacted with other five epoxide substrates. For sugar-derived epoxides 3, 5, 7, the C3 regioisomer was obtained as the only product in 73%-87% yields (Table 1, entries 2-4). The regioselectivity is explained by the model in Fig. 1. Ring-opening of epoxide at C3 is energetically favorable over C2 attack which would incur an unfavourable twist boat transition state. Similar regioselectivity was observed by Aube [16] and Hu [14a] in ring-opening reactions of structurally related substrates. For epoxides 9 and 12, both C2 and C3 regioisomers were obtained in good combined yields (76%-80%, ratio ca. 2:1). Although the model described in Fig. 1 is able to predict the major product of 12, it is unable to do so for epoxide 9 [17]. Among the substrates tested, only a minimum amount of elimination product was observed for epoxide 9 (Supporting information), thus reflecting the mild basicity of the TBAF/KHF2 reagent. The practicality of this method was demonstrated by reaction of epoxide 12 on gram scale (entry 6, Table 1). The yields (57% and 28%) were slightly higher than those (54% and 26%) obtained from a milligram scale reaction.
|
|
Table 1 Ring-opening reactions of sugar-derived epoxides. a |
|
Download:
|
| Figure 1. Model of epoxide ring-opening (substituents are omitted for clarity). | |
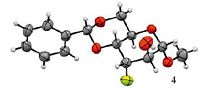
To compare the reactivity of the conventional fluorinating reagents, reactions of all substrates with Et3N·3HF [2b, 12] or TBABF/KHF2 [13] were conducted, resulting in both reagents displaying inferior reactivity under identical condition (Supporting information). A possible explanation for the lower yields obtained from the TBABF/KHF2 reactions may be due to the instability of the reagent mixture under high temperatures, which can not be avoided for the ring opening of sugar epoxides. The chemical structures of all the products obtained were determined by the NMR spectroscopy and the X-ray structure of product 4 is provided (Fig. 2) (Supporting information) [18].
|
Download:
|
| Figure 2. X-ray crystal structure of compound 4 | |
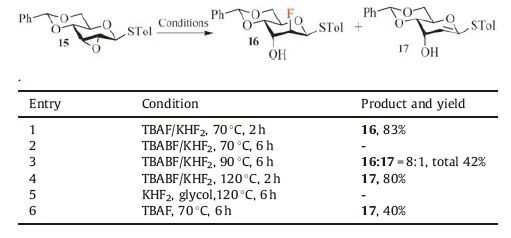
Having established the fluorination methodology, efforts were directed towards the synthesis of fluorinated 1-thiogylcoside derivatives, one kind of useful glycosylation donor [19]. Reaction of 15 with TBAF/KHF2 at 70 ℃ for 2 h furnished the C2 fluorinated product 16 in 83% yield (Table 2, entry 1). In contrast, reaction of 15 with TBABF/KHF2 gave poorer reactivity and selectivity (Table 2, entry 2-4) [20]. Furthermore, the use of TBAF/KHF2 is more attractive from an operational perspective due to its easy-tohandle nature. To verify the importance of this reagents combination, TBAF and KHF2 were then tested separately for this reaction. TBAF yielded completely the elimination product 17 along with recovery of starting material 15 at 70 ℃, whereas KHF2 alone brought about no reaction even at 120 ℃ (Table 2, entry 5-6).
|
|
Table 2 Synthesis of C-2 fluorinated 1-thioglycoside 16 and a comparison of TBAF/KHF2 and other fluorinating reagents. |
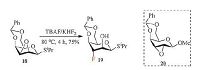
This method could also be applied for the synthesis C3 fluorinated 1-thioglycoside. As shown in Scheme 1, treatment of 18 with TBAF/KHF2 furnished fluorinated sugar 19 in 75% yield in 4 h. The regioselectivity in Table 2 and Scheme 1 is consistent with both the results obtained in Table 1 and epoxide ring-opening model in Fig. 1. It is worth noting that reaction of galactose-derived epoxide 20 and TBAF/KHF2 failed to give the corresponding fluorinated sugar (Scheme 1). The subtle structural differences between 18 and 20 represents a good example of the delicate balance of substituent electronic effects and the chemical reactivity of sugar substrates.
|
Download:
|
| Scheme 1. Synthesis of C-3 fluorinated 1-thioglycoside derivative 19. | |
3. Conclusion
TBAF/KHF2, a commercially available and stable reagent has been demonstrated to be an effective fluorinating agent for the ring-opening reactions of sugar-derived epoxides. Different sugarderived epoxides, including 1-thioglycoside substrates, can be ring-opened to afford fluorinated carbohydrates in high yields and in short reaction times. The availability, reactivity and easy-tohandle nature of this reagent mixture makes it an attractive alternative to TBABF/KHF2 and Et3N·3HF for the synthesis of fluorinated carbohydrates.
4. ExperimentalGeneral procedure for the ring-opening of epoxides: TBAF·3H2O (2 equiv.) and KHF2 (1 equiv.) was added to epoxide (1 equiv.) in a glove box at room temperature, the reaction vessel was removed from the glove box and stirred under argon for 2 h at the specified temperature. The mixture was diluted with EtOAc (20 mL) /H2O (5 mL), and saturated aqueous NaHCO3 was added to neutralize the resulting HF. The organic layer was washed with saturated aqueous NaCl (2 × 10 mL) and concentrated under reduced pressure. The crude product was purified by flash column chromatography.
Methyl-4, 6-O-benzylidene-3-deoxy-3-fluoro-α-D-altropyranoside (4) : White solid (110.8 mg, 84%); Reaction temperature: 130 ℃. Column chromatography (silica gel, petroleum ether/ EtOAc: 7:1). Mp: 151-152 ℃ (recrystallized with petroleum ether/ EtOAc 8:1 before measuring the Mp); [α]D +113.6 (c 1.0, CHCl3); IR (cm-1) : 3450, 1462, 1388, 1139, 1043; 1H NMR (400 MHz, CDCl3) : δ 7.51-7.35 (m, 5H), 5.60 (s, 1H, H7), 4.80 (ddd, 1H, J = 3.0, 3.0, 49.9 Hz, H3e), 4.61 (s, 1H, H1), 4.36-4.25 (m, 2H, H5 and H6), 4.06 (ddd, 1H, J = 3.0, 6.5, 9.6 Hz, H2), 3.96 (ddd, 1H, J = 2.5, 9.6, 30.3 Hz, H4a), 3.79 (td, 1H, J = 1.4, 10.4 Hz, H6), 3.42 (s, 3H), 2.38 (d, J = 5.6 Hz, OH); 13C NMR (100 MHz, CDCl3) : δ 137.1 (CAr), 129.3 (CAr), 128.4 (CAr), 126.3 (CAr), 102.2 (C7), 101.4 (C1), 87.0 (d, J = 185.3 Hz, C3), 74.8 (d, J = 16.9 Hz, C4), 69.2 (C6), 68.9 (d, J = 25.5 Hz, C2), 58.4 (d, J = 3.0 Hz, C5), 55.7 (OCH3); 19F {1H} NMR (376 MHz, CDCl3) : δ -205.1 (s, 1F); 19F NMR (376 MHz, CDCl3) : δ -205.1 (ddd, J = 6.5, 30.2, 49.9 Hz, 1F); HRMS (ESI) : calcd. for C14H17FNaO5+ [M + Na]+ 307.0952, found 307.0940.
Methyl-4, 6-O-benzylidene-3-deoxy-3-fluoro-β-D-altropyranoside (8) : White solid (82.4 mg, 0.290 mmol, 73%); Reaction temperature: 140 ℃; Column chromatography (silica gel, petroleum ether/EtOAc: 7:1); Mp: 166-168 ℃; [α]D -44.0 (c 1.1, CHCl3); IR (cm-1) : 3463, 2927, 1452, 1390, 1050; 1H NMR (400 MHz, CDCl3) : δ 7.52-7.34 (m, 5H), 5.58 (bs, 1H, H7), 5.00 (ddd, 1H, J = 1.9, 3.7, 49.9 Hz, H3), 4.75 (dd, 1H, J = 1.3, 2.8 Hz, H1), 4.39 (dd, 1H, J = 4.9, 10.5 Hz, H5), 4.09-3.97 (m, 3H, H2, H6 and H4), 3.84-3.79 (m, 1H, H6), 3.59 (s, 3H, OCH3), 2.48 (bs, OH); 13C NMR (100 MHz, CDCl3) : δ 137.0 (CAr), 129.2 (CAr), 128.3 (CAr), , 126.2 (CAr), 102.5 (C7), 99.0 (C1), 87.8 (d, J = 177.3 Hz, C3), 75.2 (d, J = 16.5 Hz, C4), 69.3 (d, J = 27.7 Hz, C5), 69.0 (C6), 63.1 (d, J = 3.3 Hz, C2), 57.2 (OCH3); 19F {1H} NMR (376 MHz, CDCl3) : δ -208.6 (s, 1F); 19F NMR (376 MHz, CDCl3) : δ -208.6 (m, 1F); HRMS (ESI) : calcd. For C14H17FNaO5+ [M + Na]+ 307.0952, found 307.0944.
Methyl-4, 6-O-benzylidene-2-deoxy-2-fluoro-α-D-altropyranoside (13) : Colorless oil (128 mg, 54%) and (684 mg, 57%); Reaction temperatue:130 ℃; Column chromatography (silica gel, petroleum ether/EtOAc: 5:1); [α]D +142.2 (c 1.0, CHCl3); IR (cm-1) : 3446, 1452, 1378, 1045; 1H NMR (400 MHz, CDCl3) : δ 7.50 -7.35 (m, 5H, HAr), 5.64 (s, 1H, H7), 4.82 (d, 1H, J = 10.6 Hz, H7), 4.73 -4.61 (m, 1H, H2), 4.35 (dd, 1H, J = 5.2, 10.3 Hz, H6), 4.30 -4.20 (m, 2H, H3 and H5), 3.90 (dt, 1H, J = 3.2, 9.8, Hz, H4), 3.82 (t, 1H, J = 10.3 Hz, H6), 3.46 (s, 3H, OMe), 2.77 (dd, 1H, J = 1.5, 5.5 Hz, OH); 13C NMR (100 MHz, CDCl3) : δ 137.1 (CAr), 129.3 (CAr), 128.3 (CAr), 126.2 (CAr), 102.3 (C7), 98.9 (d, J = 33.1 Hz, C1), 87.7 (d, J = 173.9 Hz, C2), 76.1 (d, J = 1.7 Hz, C4), 69.1 (C6), 66.5 (d, J = 27.9 Hz, C3), 58.1 (C5), 56.0 (OCH3); 19F {1H} NMR (376 MHz, CDCl3) : δ -194.0 (s, 1F); 19F NMR (376 MHz, CDCl3) : δ -194.0 (m, 1F); HRMS (ESI) : calcd. For C14H17FNaO5+ [M + Na]+ 307.0952, found 307.0956.
Methyl-4, 6-O-benzylidene-3-deoxy-3-fluoro-α-D-glucopyranoside (14) : White soild (61.7 mg, 26%) and (335 mg, 28%); Reaction temperatue:130 ℃; Column chromatography (silica gel, petroleum ether/EtOAc: 5:1); Mp: 164-166 ℃; [α]D +172.68 (c 1.0, CHCl3); IR (cm-1) : 3452, 1450, 1385, 1052; 1H NMR (400 MHz, CDCl3) : δ 7.51-7.49 (m, 2H), 7.39-7.35 (m, 3H), 5.55 (s, 1H, H7), 4.83 (dd, 1H, J = 2.7, 4.1 Hz, H1), 4.69 (ddd, 1H, J = 8.7, 8.7, 54.7 Hz, H3a), 4.34-4.30 (m, 1H, H6), 3.88-3.68 (m, 4H, H5, H6, H4 and H2), 3.45 (s, 3H), 2.43 (d, 1H, J = 9.7 Hz, OH); 13C NMR (100 MHz, CDCl3) : δ 136.8 (CAr), 129.3 (CAr), 128.3 (CAr), 126.2 (CAr), 101.7 (C7), 101.1 (d, J = 9.9 Hz, C1), 91.8 (d, J = 186.6 Hz, C3), 79.2 (d, J = 17.1 Hz, C4), 71.5 (d, J = 17.9 Hz, C2), 68.8 (C6), 61.9 (d, J = 7.5 Hz, C5), 55.7 (OCH3); 19F {1H} NMR (376 MHz, CDCl3) : d-200.0 (s, 1F); 19F NMR (376 MHz, CDCl3) : δ -200.0 (m, 1F); HRMS (ESI) : calcd. For C14H17FNaO5+ [M + Na]+ 307.0952, found 307.0960.
p-Tolyl-2-deoxy-2-fluoro-4, 6-O-benzylidene-1-thio-β-D-altropyranoside 16: White solid (109.1 mg, 0.290 mmol, 83%); Reaction temperatue: 70 ℃. Column chromatography (silica gel, petroleum ether/EtOAc: 10:1); Mp: 164-165 ℃; [α]D -23.2 (c 1.0, CHCl3); IR (cm-1) : 3419, 2913, 1493, 1456, 1387, 1115; 1H NMR (400 MHz, CDCl3) : δ 7.48-7.37 (m, 6H), 7.15 (d, 2H, J = 7.9 Hz), 5.64 (s, 1H), 5.18 (bd, 1H, J = 32.3 Hz, H1), 4.87 (dd, 1H, J = 3.6, 44.5 Hz, H2), 4.39-4.34 (m, 2H, H6 and H3), 4.02-3.92 (m, 2H, H4 and H5), 3.89- 3.84 (m, 1H, H6), 2.42 (d, 1H, J = 1.5 Hz, OH), 2.35 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) : δ 138.1 (CAr), 136.9 (CAr), 132.1 (CAr), 129.9 (CAr), 129.4 (CAr), 128.4 (CAr), 126.1 (CAr), 102.2 (C7), 90.9 (d, J = 176.5 Hz, C2), 84.2 (d, J = 18.4 Hz, C1), 76.2 (C4), 68.9 (C6), 67.2 (d, J = 29.5 Hz, C3), 66.4 (C5), 21.1 (CH3); 19F {1H} NMR (376 MHz, CDCl3) : δ -201.3 (s, 1F); 19F NMR (376 MHz, CDCl3) : δ -201.3 (m, 1F); HRMS (ESI) : calcd. For C20H21FNaO4S+ [M + Na]+ 399.1037, found 399.1042
Iso-propyl-4, 6-O-benzylidene-3-deoxy-3-fluoro-1-thio-β-DIdopyranose 19: White soild (30.2 mg, 0.092 mmol, 75%); Reaction temperatue: 80 ℃; Column chromatography (silica gel, petroleum ether/EtOAc: 5:1); Mp: 172-174 ℃; [α]D -33.8 (c 1.0, CHCl3); IR (cm-1) : 3372, 2920, 1493, 1464, 1380; 1H NMR (400 MHz, CDCl3) : δ 7.48-7.36 (m, 5H, HAr), 5.54 (s, 1H, H7), 4.97 (d, 1H, J = 4.0 Hz, H1), 4.83 (dt, 1H, J = 3.3, 43.6 Hz, H3), 4.40 (dd, 1H, J = 1.6, 12.7 Hz, H6), 4.21-4.18 (m, 1H, H2), 4.10 (dd, 1H, J = 1.8, 12.7 Hz, H6), 3.87 (ddd, 1H, J = 3.1, 6.5, 10.7 Hz, H4), 3.72 (t, 1H, J = 1.9 Hz, H5), 3.24 (quintet, 1H, J = 6.7 Hz, CH-), 3.17 (dd, 1H, J = 1.9, 12.5 Hz, OH), 1.37-1.34 (m, 6H); 13C NMR (100 MHz, CDCl3) : δ 136.9 (CAr), 129.4 (CAr), 128.4 (CAr), 126.0 (CAr), 101.6 (C7), 86.4 (d, J = 175.2 Hz, C3), 81.8 (d, J = 1.4 Hz, C1), 72.1 (d, J = 30.5 Hz, C2), 69.6 (C6), 68.4 (d, J = 26.0 Hz, C4), 68.2 (C5), 35.1 (CH-), 23.8 (CH3-), 23.7 (CH3-); 19F NMR (376 MHz, CDCl3) : δ -197.1 (m, 1F); 19F {1H} NMR (376 MHz, CDCl3) : δ -197.1 (s, 1F); HRMS (ESI) : calcd. for C16H21FNaO4S+[M + Na]+ 351.1037, found 351.1044.
AcknowledgmentWe thank the National Natural Science Foundation of China (No. 21502076) for the financial support. Dr. Mohan M. Bhadbhade and Dr. Thanh Le from the University of New South Wales (UNSW) are acknowledged for their help in solving problems related to the Xray crystal structure data of 4. Dr. Thanh Le is also acknowledged for the proof reading.
| [1] | (a) D. O'Hagan, Understanding organofluorine chemistry. An introduction to the C-F bond, Chem. Soc. Rev. 37(2008) 308-319; (b) S. Purser, P.R. Moore, S. Swallow, V. Gouverneur, Fluorine in medicinal chemistry, Chem. Soc. Rev. 37(2008) 320-330; (c) E.P. Gillis, K.J. Eastman, M.D. Hill, D.J. Donnelly, N.A. Meanwell, Applications of fluorine in medicinal chemistry, J. Med. Chem. 58(2015) 8315-8359. |
| [2] | (a) H.A. Chokhawala, H.Z. Cao, H. Yu, X. Chen, Enzymatic synthesis of fluorinated mechanistic probes for sialidases and sialyltransferases, J. Am. Chem. Soc. 129(2007) 10630-10631; (b) C.D. Brown, M.S. Rusek, L.L. Kiessling, Fluorosugar chain termination agents as probes of the sequence specificity of a carbohydrate polymerase, J. Am. Chem. Soc. 134(2012) 6552-6555. |
| [3] | (a) J.H. Kim, R. Resende, T. Wennekes, et al., Mechanism-based covalent neuraminidase inhibitors with broad-spectrum influenza antiviral activity, Science 340(2013) 71-75; (b) K.E. van Straaten, J.R.A. Kuttiyatveeti, C.M. Sevrain, et al., Structural basis of ligand Binding to UDP-galactopyranose mutase from mycobacterium tuberculosis using substrate and tetrafluorinated Substrate Analogues, J. Am. Chem. Soc. 137(2015) 1230-1244. |
| [4] | P.Y. Wang, B.K. Chun, S. Rachakonda, An efficient and diastereoselective synthesis of psi-6130:a clinically efficacious inhibitor of HCV NS5B polymerase. J. Org. Chem. 74 (2009) 6819–6824. DOI:10.1021/jo901345j |
| [5] | G.R. Morais, R.A. Falconer, I. Santos, Carbohydrate-based molecules for molecular imaging in nuclear medicine. Eur. J. Org. Chem. (2013) 1401–1414. |
| [6] | P. Som, H.L. Atkins, D. Bandoypadhyay, A fluorinated glucose analog, 2-fluoro-2-deoxy-D-glucose (F-18):nontoxic tracer for rapid tumor detection. J. Nuc.l Med. 21 (1980) 670–675. |
| [7] | (a) Y. Cheng, A.L. Guo, D.S. Guo, Recent progress in synthesis and applications of fluorinated carbohydrates, Curr. Org. Chem., 2010,14: 977-999; (b) K. Dax, M. Albert, J. Ortner, B.J. Paul, Synthesis of deoxyfluoro sugars from carbohydrate precursors. Carbohydr. Res. 327 (2000) 47–86. DOI:10.1016/S0008-6215(00)00022-7 |
| [8] | (a) P.A. Champagne, J. Desroches, J.D. Hamel, M. Vandamme, J.F. Paquin, Monofluorination of organic compounds:10 years of innovation, Chem. Rev., 2015,115: 9073-9174; (b) X.Y. Yang, T. Wu, R.J. Phipps, F.D. Toste, Advances in catalytic enantioselective fluorination, mono-, di-, and trifluoromethylation, and trifluoromethylthiolation reactions, Chem. Rev., 2015,115: 826-870; (c) N. Al-Maharik, D. O'Hagan, Organofluorine chemistry:deoxyfluorination reagents for C-F bond synthesis. Aldrichim Acta 44 (2011) 65–75. |
| [9] | J.P. Card, Synthesis of fluorinated carbohydrates. J. Carbohydr. Chem. 4 (1985) 451–487. DOI:10.1080/07328308508082671 |
| [10] | (a) K. Dax, M. Albert, J. Ortner, B.J. Paul, Synthesis of deoxyfluoro sugars from carbohydrate precursors, Carbohydr. Res., 2000,327: 47-86; (b) X.G. Hu, L. Hunter, Stereoselectively fluorinated N-heterocycles:a brief survey. Beilstein J. Org. Chem. 9 (2013) 2696–2708. DOI:10.3762/bjoc.9.306 |
| [11] | (a) Y. Nishida, J. Thiem, Convenient synthetic approach towards C-3 modified methyl beta-lactone, Carbohydr. Res., 1994,263: 295-301; (b) J.M. Chen, A.X. Huang, R.L. Mackman, et al., Nucleoside analogs for antiviral treatment, PCT Int. Appl., 2008100447(c) JA. Wright, N.F. Taylor, Flourocarbohydrates:Part XVIII. 9-(3-deoxy-3-flouro-β-D-xylofuranosyl)adenine and 9-(3-deoxy-3-flouro-α-D-arabinofuranosyl)adenine. Carbohydr. Res. 6 (1968) 347–354. DOI:10.1016/S0008-6215(00)81457-3 |
| [12] | (a) M. Bols, I. Lundt, Preparation of 2,3-epoxyaldonolactones and their conversion into 2-fluoro-2-deoxy-aldonolactones and 2-fluoro-2-deoxy-sugars. Acta Chem. Scand. 44 (1990) 252–256. DOI:10.3891/acta.chem.scand.44-0252 |
| [13] | (a) Y. Akiyama, T. Fukuhara, S. Hara, Regioselective synthesis of fluorohydrines via SN2-type ring-opening of epoxides with TBABF-KHF2, Synlett (2003) 1530-1532; (b) Y. Akiyama, C. Hiramatsu, T. Fukuhara, S. Hara, Selective introduction of a fluorine atom into carbohydrates and a nucleoside by ring-opening fluorination reaction of epoxides, J Fluorine. Chem., 2006,127: 920-923; (c) M. Mastihubova, P. Biely, Deoxy and deoxyfluoro analogues of acetylated methyl (-D-xylopyranoside-substrates for acetylxylan esterases. Carbohydr. Res. 339 (2004) 2101–2110. DOI:10.1016/j.carres.2004.06.001 |
| [14] | (a) N. Yan, Z. Fang, Q.Q. Liu, X.H. Guo, X.G. Hu, Conformation-induced regioselective and divergent opening of epoxides by fluoride:facile access to hydroxylated fluoro-piperidines, Org. Biomol. Chem., 2016,14: 3469-3475; (b) X.G. Hu, A. Lawer, M.B. Peterson, et al., Diastereoselective synthesis and conformational analysis of (2R)-and (2S)-fluorostatines:an approach based on organocatalytic fluorination of a chiral aldehyde, Org. Lett., 2016,18: 662-665; (c) X.G. Hu, D.S. Thomas, R. Griffith, L. Hunter, Stereoselective fluorination alters the geometry of a cyclic peptide:exploration of backbone-fluorinated analogues of unguisin A. Angew. Chem. Int. Edit. 53 (2014) 6176–6179. DOI:10.1002/anie.201403071 |
| [15] | J.M. Percy, R. Roig, K. Singh, Fluorinated analogues of amicetose and rhodinose-novel racemic and asymmetric routes. Eur. J. Org. Chem. (2009) 1058–1071. |
| [16] | T.C. Coombs, G.H. Lushington, J. Douglas, J. Aube, 1,3-allylic strain as a strategic diversification element for constructing libraries of substituted 2-arylpiperidines. Angew. Chem. Int. Edit 50 (2011) 2734–2737. DOI:10.1002/anie.201007133 |
| [17] | M. Miljkovic, Electrostatic and Stereoelectronic Effects in Carbohydrate Chemistry,. Springer (2014) . |
| [18] | The crystal structure of 4 has been deposited at the Cambridge Crystallographic Data Centre and allocated the deposition number:CCDC 1473829. |
| [19] | (a) M. Salvado, B. Amgarten, S. Castillon, G.J.L. Bernardes, O. Boutureira, Synthesis of fluorosugar reagents for the construction of well-defined fluoroglycoproteins, Org. Lett., 2015,17: 2836-2839; (b) L.M. Chauvigne-Hines, L.N. Anderson, H.M. Weaver, et al., Suite of ActivityBased Probes for Cellulose-Degrading Enzymes, J. Am. Chem. Soc., 2012,134: 20521-20532; (c) D. Crich, L.F. Li, 4,6-O-benzylidene-directed (-mannopyranosylation and (-glucopyranosylation:The 2-deoxy-2-fluoro and 3-deoxy-3-fluoro series of donors and the importance of the O2-C2-C3-O3 interaction. J. Org. Chem. 72 (2007) 1681–1690. DOI:10.1021/jo062294y |
| [20] | Y. Wang, Q. Li, S.H. Cheng, Base-promoted rearrangement of sugar epoxides to unsaturated sugars. Org. Lett. 7 (2005) 5577–5579. DOI:10.1021/ol052128x |
 2017, Vol. 28
2017, Vol. 28