b Key Laboratory of Xinjiang Endemic Phytomedicine Resources, Ministry of Education, School of Pharmacy, Shihezi University, Shihezi 832000, China
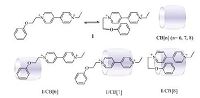
In recent years, the host-guest chemistry of the cucurbit[n]uril (CB[n], where n = 5-8, 10, and 14) family has been making big progress due to their tubular molecular structures [1-3]. In which the two polar “portals” together with a hydrophobic cavity and a modest or low water solubility provide an environmentally friendly water soluble confined medium for chemical reactions taken place. Among them, CB[6], CB[7] and CB[8] are more attractive due to their proper cavity size. Since CB[6] was pioneered as a reactor for 1, 3-dipolar cycloaddition reactions in 1983 [4], several other examples of photocycloadditions using CB[7] and CB [8] were reported [5]. Meanwhile, CB[7] was further employed as a barrel together with transition-metal ions as lids, to control phaseselective photolysis of bicyclic azoalkanes [6]. While CB[8] can mediate intermolecular photodimerization either in the solid state or in aqueous solution [7]. Furthermore, several other applications using CB[n] (n = 6-8) were reported in succession [8-11]. To gain further understanding on the CB[n] based molecular reactor, it would be interesting to employ the same reactant/reagent and perform the same reaction under the circumstance of different CB [n] cavity. To fulfill this, a fundamental question is whether the reactant can form a host-guest interaction with different CB[n], to provide a possibility to fine modulate the reaction through different binding mode aroused by the different CB[n] cavity. To touch this unexplored issue, N-phenyloxypropyl-N'-ethyl-4, 4'- bipyridium (1, Fig. 1) was designed based on our previous work [12]. In this molecule, the donor segment, phenoxy moiety, is covalently linked to the ethyl viologen dication (EV2+) fragment to facilitate the ICT interaction (as seen from its folded conformation in Fig. 1), permitting the phenol moiety inserted together with the viologen moiety into the cavity of CB[8]. Meanwhile, the viologen moiety can be included in CB[7], and the phenoxy moiety can be included in CB[6] due to the relative small cavity size (Fig. 1). All these provide a possibility to perform the same reaction in the presence of different CB[n] cavity.
|
Download:
|
| Figure 1. Structure of 1 and schematic representation of the assembly of 1/CB[n] (n = 6–8). | |
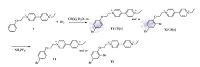
Considering that electrophilic aromatic substitution reactions such as bromination, nitration, sulfonation etc. are important ways to introduce functional groups onto benzene rings, especially, these reactions are quite fundamental either in basic organic chemistry books or for typical organic synthesis, herein, bromination of 1 and its corresponding inclusion complexes of CB[n] (n = 6- 8) was investigated. The results demonstrated that CB[8] performed pretty well as a molecular reactor to control bromination results of 1, more importantly, CB[8] can provide a safe bromination environment for 1, which is quite helpful to acquire 1- bormination product completely and enhance the stability of the corresponding reaction products. While the host-guest assembly of 1/CB[n] (n = 6, 7) would definitely decrease the ICT interaction of 1, leading to the molecule to be broken down easily during bromination (Scheme 1). Such an assay would be helpful for investigating functional systems centered on the CB[n] molecular reactor.
|
Download:
|
| Scheme 1. Schematic synthetic procedures of the bromination of 1 in the presence of CB[n] (n = 6–8). | |
2. Results and discussion
The inclusion of the viologen derivatives in CB[n] (n = 6-8) has been studied extensively in the literature [13]. Accordingly, formation of the including complex CB[n] (n = 6-8) and 1/CB[n] (n = 6-8) can be clearly observed on 1H NMR (Figs. S1-S3 in Supporting information). 1H NMR titration experiment supported the formation of a 1:1 host-guest complex 1/CB[n] [14-17]. Electrospray ionization mass spectrometry (ESI-MS, Fig. S4 in Supporting information) gave a positively charged peak at m/z 651.20 (calcd. for [1 + CB[6]-2Cl-]2+, 651.23) in the case of CB[6], m/z 734.17 (calcd. for [1 + CB[7]-2Cl-]2+, 734.25) for CB[7], and m/z 817.25 (calcd. for [1 + CB[8]-2Cl-]2+, 817.28) for CB[8], respectively, providing direct evidence for the formation of the 1:1 including complex. The formation of 1:1 including complex was further confirmed by UV/vis absorption titration experiments (Fig. S5 in Supporting information). It is noted that 1 revealed an absorption band at around 260 nm, whose absorption intensity decreased gradually with the addition of CB[n] (n = 6-8). In the case of 1/CB[8], a broad new absorption band appeared at about 310 nm, which can be attributed to the ICT resulting from the host-guest complex formation [12], providing an additional evidence for the host-guest complexes formation.
To make a reference for high performance liquid chromatograph (HPLC), standard substances T1 (1-bromination) and T2 (2- bromination) were synthesized (synthetic procedures were shown in Supporting information). The bromination experimental results could be analyzed through HPLC and chromatography mass spectrometry (HPLC-MS). The retention time for 1, T1, T2 was 4.98 min, 9.32 min, 13.10 min, respectively (Fig. S6 in Supporting information). As shown in Table 1, the reaction products T1 and T2 can be well modulated by the confined environment of CB[8] (Figs. S7-S8 in Supporting information). When the reaction lasted for 10 min, for 1 alone, there would be 84.0% T1 and 10.9% T2 products in aqueous solution. In the case of 1/CB[8], the folded ICT including mode is quite helpful to acquire T1 completely in confined hydrophobic environment of CB[8] (Figs. S7-S8 in Supporting information).
|
|
Table 1 Bromination result (HPLC isolated yield (%)) of 1 and 1/CB[n] (n = 6–8). |
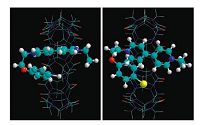
A molecular modeling research (HyperChem with Molecular Mechanics) also showed that the molecule 1 inside the cavity of CB [8] adopted a folded conformation to place the phenoxy moiety in a close interaction distance with EV2+ moiety (Fig. 2), which was in good accordance with the argument in the literature [12].
|
Download:
|
| Figure 2. Optimized molecular modeling of 1/CB[8] (left) and T1/CB[8] (right) with Br-, N-, C-, O- and H-atom in yellow, blue, cyan, red and white color, respectively. To make clarity, CB[8] is presented in sticks, and 1,T1 are in balls and cylinders for a clear view of the inclusion. | |
According to the results of HPLC-MS (Fig. S11 in Supporting information), bromination products of 1 could be decomposed into other by-products easily, such as 4, 4'-bipyridinum radical cation (V+·) (MS (ESI, m/z) : (V+·) C10H11N2+·, calculated for 159.1, found, 159.1) and 4-ethyl-4'-hydroxyethyl-bipyridinum radical cation (MS (ESI, m/z) : C14H17N2O+·, calculated for 230.1, found, 230.1) (Fig. S11a in Supporting information). Along with the extension of reaction time, other by-products could be ignored due to the little yield. For 1 alone, most of the bromination products were decomposed after the reaction lasted for 5 h. While, the folded conformation of 1 in CB[8] provides a close interaction distance between the phenoxy moiety and the EV2+ moiety, which quite facilitate the ICT [8]. So the bromination products of 1/CB[8] were quite stable under the strongly acidic conditions, even for 5 h.
Control experiments were also carried out for the bromination reaction of 1/CB[n] (n = 6, 7). The bromination result of 1/CB[n] (n = 6, 7) were presented in Table 1. Compared with the bromination of 1 alone, the bromination of 1/CB[6] would produce nearly 5% more T2 after the reaction lasted for 10 min. However, along with the extension of reaction time, the bromination products of 1/CB[6] are more likely to be decomposed (Fig. S9 in Supporting information). And after the reaction lasted for 5 h, there would be 83.0% by-products. This can be explained as follows. Including of CB[6] keeps the phenoxy moiety a little far away from the viologen cation moiety (Fig. S12 in Supporting information), which somehow increases the electron density of phenoxy moiety. Unfortunately, the host-guest assembly of 1/CB[6] would definitely decrease the ICT interaction of 1, leading to the molecule to be broken down easily.
In the case of 1/CB[7], the phenoxy moiety locates just outside the cavity of CB[7], and the viologen cation moiety is completely seated inside the cavity (Fig. S13 in Supporting information). The ICT interaction between the viologen cation moiety and the phenoxy moiety was decreased, when 1 was encapsulated in CB[7]. Although the yield of T2 can be improved, the decomposed products also increase from 11.0% to 49.2%, when the reaction time was prolonged from 10 min to 2 h. No further reaction was done considering of the significant decomposition of 1/CB[7].
3. ConclusionIn conclusion, 1 can form a host-guest inclusion compound with different CB[n] (n = 6-8) cavity through different binding modes, and CB[8] works pretty well as a molecular reactor to adjust the bromination results. Especially, CB[8] can provide a quite safe bromination environment for 1. All these shed some new light on CB[n] host-guest chemistry.
4. Experimental 4.1. Materials and apparatusDoubly purified water used in all experiments was from Milli-Q systems. Other solvents and reagents were of analytical grade and used without further purification. 1H NMR spectra were recorded on a VARIAN INOVA-400 spectrometer with chemical shifts reported as ppm. Mass spectrometric data were obtained on a Q-Tof MS spectrometer (Micromass, Manchester, England). Absorption spectra were measured on a PerkinElmer Lambda 35 UV-vis spectrophotometer.
4.2. Synthetic proceduresCB[n] (n = 6-8) was synthesized according to the literature [16]. The yield of CB[6], CB[7] and CB[8] was ca. 38%, 30% and 10%, respectively. 1H NMR (400 MHz, D2O), CB[6]: δ 5.82 (d, 12H, J = 15.6 Hz), 5.55 (s, 12H), 4.23 (d, 12H, J = 15.6 Hz); CB[7]: δ 5.78 (d, 14H, J = 15.4 Hz), 5.54 (s, 14H), 4.24 (d, 14H, J = 15.4 Hz); CB[8]: δ 5.72 (d, 16H, J = 15.4 Hz), 5.58 (s, 16H), 4.31 (d, 16H, J = 15.4 Hz).
N-Phenyloxyethyl-N'-ethyl-4, 4'-bipyridium (1) : This compound was synthesised according to the previous work [12]. The total yield of 1 was 30%. 1H NMR (400 MHz, D2O), 1: δ 9.20- 9.18 (d, 2H, J = 8.0 Hz), 9.10-9.08 (d, 2H, J = 8.0 Hz), 8.52-8.48 (m, 4H), 7.32-7.28 (t, 2H, J = 8.0 Hz), 7.02-6.98 (t, 1H, J = 4.0 Hz), 6.96- 6.94 (d, 2H, J = 4.0 Hz), 5.13-5.10 (t, 2H, J = 4.0 Hz), 4.75-4.70 (q, 2H, J = 4.0 Hz), 4.62-4.60 (t, 2H, J = 4.0 Hz), 1.67-1.64 (t, 3H, J = 4.0 Hz). HRMS: (m/z (%) ): 153.0861 (100) [M - 2Cl-]2+ (Calculated mass: 153.0868) (Synthetic procedures were shown in Supporting information).
Bromination of 1 and 1/CB[n] (n = 6-8) : Compound 1 (20 mg) was dissolved in 250 mL water. In the case of the including complexes, 20% excess of CB[n] was added to allow 1 to be completely included. To ensure the bromination reaction completely, 6 equiv. of bromine was added into the system. The reaction was last for 10 min, 30 min, 2 h, 5 h, respectively. Then saturated NaHCO3 solution was added to adjust the solution to neutral. The solution was concentrated to ca.10 mL and drop wised into a saturated NH4PF6 aqueous solution. The precipitate was collected and dried to give some crude product, which was redissolved in anhydrous acetonitrile, an aliquot of the supernate was injected into the HPLC systems to get final separation.
On HPLC system, a satisfactory separation was obtained using a 4.6 mm × 250 mm ultimate XB-C18 column with a diameter of 5 mm. Detection at 260 nm with a linear gradient containing methanol/H2O (added 0.3% triethylamine and 0.3% acetic acid) were found to be the most efficient eluents for this separation. In the first 20 min, the water ratio was changed from 70% to 30%, and then decreased from 30% to 0 in the later 10 min. The collection time was 40 min. Under the circumstance, the retention time for 1, T1, T2 was 4.98 min, 9.32 min, 13.10 min, respectively (Fig. S6).
AcknowledgmentsThis work was financially supported by State Key Laboratory of Fine Chemicals, Dalian University of Technology, National Natural Science Foundation of China (Nos. 21272030, 21472016, 21306019, 21576042).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.10.004.
| [1] | J.W. Lee, S. Samal, K. Kim, Cucurbituril homologues and derivatives:new opportunities in supramolecular chemistry. Acc. Chem. Res. 36 (2003) 621–630. DOI:10.1021/ar020254k |
| [2] | K. Kim, N. Selvapalam, D.J. Kim, Functionalized cucurbiturils and their applications. Chem. Soc. Rev. 36 (2007) 267–279. DOI:10.1039/B603088M |
| [3] | (a) J. Lagona, P. Mukhopadhyay, L. Isaacs, et al., The cucurbit[n]uril family, Angew. Chem. Int. Ed. 44(2005) 4844-4870; (b) J.X. Cheng, L.L. Liang, Z. Tao, et al., Twisted cucurbit[14] uril, Angew. Chem. 125(2013) 7393-7396. |
| [4] | (a) B. Zheng, F. Wang, F.H. Huang, et al., Supramolecular polymers constructed by crown ether-based molecular recognition, Chem. Soc. Rev. 41(2012) 1621-1636; (b) S.Y. Dong, B. Zheng, F.H. Huang, et al., Supramolecular polymers constructed from macrocycle-based host-guest molecular recognition motifs, Acc. Chem. Res. 47(2014) 1982-1994; (c) G. Yu, K. Jie, F.H. Huang, Supramolecular amphiphiles based on host-guest molecular recognition motifs, Chem. Rev. 115(2015) 7240-7303; (d)M.M.Zhang,X.Z.Yan,W.Harry,etal.,Stimuli-responsivehost-guestsystems basedontherecognitionofcryptandsbyorganicguests,Acc.Chem.Res.47(2014) 1995-2005. |
| [5] | W.L. Mock, T.A. Irra, T.L. Manimaran, Cycloaddition induced by cucurbituril:a case of Pauling principle catalysis. J. Org. Chem. 48 (1983) 3619–3620. DOI:10.1021/jo00168a070 |
| [6] | (a) J.W. Lee, K.P. Kim, K. Kim, et al., Unprecedented host-induced intramolecular charge-transfer complex formation, Chem. Commun. (2002) 2692-2693; (b) M. Pattabiraman, A. Natarajan, V. Ramamurthy, et al., Template directed photodimerization of trans-1,2-bis(n-pyridyl)ethylenes and stilbazoles in water, Chem. Commun. (2005) 4542-4544; (c) M. Pattabiraman, L.S. Kaanumalle, V. Ramamurthy, et al., Regioselective photodimerization of cinnamic acids in water:templation with cucurbiturils, Langmuir 22(2006) 7605-7609; (d) N. Barooah, B.C. Pemberton, J. Sivaguru, et al., Photodimerization and complexation dynamics of coumarins in the presence of cucurbit[8] urils, Photochem. Photobiol. Sci. 7(2008) 1473-1479; (e) L. Lei, L. Luo, C.H. Tung, et al., Cucurbit[8] uril-mediated photodimerization of alkyl 2-naphthoate in aqueous solution, Tetrahedron Lett. 49(2008) 1502-1505; (f) X.L. Wu, L. Luo, C.H. Tang, et al., Highly efficient cucurbit[8] uril-templated intramolecular photocycloaddition of 2-naphthalene-labeled poly (ethylene glycol) in aqueous solution, J. Org. Chem. 73(2008) 491-494; (g) B. Chen, S.F. Cheng, L.Z. Wu, et al., Highly efficient cucurbit[8] uriltemplated intramolecular photocycloaddition of 2-naphthalene-labeled poly (ethylene glycol) in aqueous solution, Photochem. Photobiol. Sci. 10(2011) 1441-1444. |
| [7] | W. Lali, P. Petrovi c, J.P. Djukic, The inhibition of iridium-promoted water oxidation catalysis (WOC) by cucurbit[n]urils. Dalton Trans. 41 (2012) 12233–12243. DOI:10.1039/c2dt31363d |
| [8] | (a) S.Y. Jon, Y.H. Ko, K. Kim, et al., A facile, stereoselective[2+2] photoreaction mediated by cucurbit[8] uril, Chem. Commun. (2001) 1938-1939; (b) M. Pattabiraman, A. Pattabiraman, V. Ramamurthy, et al., Templating photodimerization of trans-cinnamic acids with cucurbit[8] uril and γ-cyclodextrin, Org. Lett. 7(2005) 529-532; (c) R.B. Wang, L. Yuan, D.H. Macartney, et al., Cucurbit[7] uril mediates the stereoselective[4+4] photodimerization of 2-aminopyridine hydrochloride in aqueous solution, J. Org. Chem. 71(2006) 1237-1239; (d) B.C. Pemberton, N. Barooah, J. Sivaguru, et al., Supramolecular photocatalysis by confinement-photodimerization of coumarins within cucurbit[8] urils, Chem. Commun. 46(2010) 225-227. |
| [9] | A.L. Koner, C. Márquez, W.M. Nau, Chemoselektive photoreaktionen mithilfe von übergangsmetallen in cucurbiturilen. Angew. Chem. Int. Ed. 50 (2011) 545–548. DOI:10.1002/anie.201005317 |
| [10] | T. Fuenzalida, D. Fuentealba, A study of the Fenton-mediated oxidation of methylene blue-cucurbit[n]uril complexes. Photochem. Photobiol. Sci. 14 (2015) 686–692. DOI:10.1039/C4PP00362D |
| [11] | (a) Z.S. Hou, Y.B. Tan, K. Kim, et al., Preparation of novel side-chain pseudopolyrotaxanes consisting of cucurbituril[6] and polyamine salts, Chin. Chem. Lett. 16(2005) 1031-1034; (b)H.Y.Lu,J.R.Li,D.X.Shi,etal.,Anovelsynthesisofnitroformbythenitrolysisof cucurbituril, Chin. Chem. Lett. 26(2015) 365-368; (c) J.M. Yi, X. Xiao, Z. Tao, et al., Supramolecular self-assembly of cucurbit[8] uril with 2, 2'-(heptane-1,7-dily) dibenzimidazolium chloride, Acta Chim. Sin. 72(2014) 949-955; (d) M. Zhang, J. Gao, Z.Y. Tian, et al., Enhanced dSTORM imaging using fluorophores interacting with cucurbituri, Sci. China Chem. 59(2016) 848-852. |
| [12] | T.Y. Zhang, S.G. Sun, X.J. Peng, Redox-induced partner radical formation and its dynamic balance with radical dimer in cucurbit[8] uril. Phys. Chem. Chem. Phys. 11 (2009) 11134–11139. DOI:10.1039/b916591f |
| [13] | (a) L. Cera, C.A. Schalley, Stimuli-induced folding cascade of a linear oligomeric guest chain programmed through cucurbit[n]uril self-sorting (n=6, 7, 8), Chem. Sci. 5(2014) 2560-2567; (b) C. Hu, Y. Zheng, O.A. Scherman, et al., Cucurbit[8] uril directed stimuliresponsive supramolecular polymer brushes for dynamic surface engineering, Chem. Commun. 51(2015) 4858-4860; (c) E. Apple, M.J. Rowland, O.A. Scherman, et al., Enhanced stabilityand activity of temozolomide in primary glioblastoma multiforme cells with cucurbit[n] uril, Chem. Commun. 48(2012) 9843-9845; (d) Y.Y. Mao, K. Liu, T. Yi, et al., CB[8] gated photochromism of a diarylethene derivative containing thiazole orange groups, Chem. Commun. 51(2015) 6667-6669; (e) E.A. Apple, J.D. Barrio, O.A. Scherman, et al., Metastable single-chain polymer nanoparticles prepared by dynamic cross-linking with nor-secocucurbit[10] uril, Chem. Sci. 3(2012) 2278-2280. |
| [14] | (a) S. Choi, S.H. Park, K. Kim, et al., A stable cis-stilbene derivative encapsulated in cucurbit[7] uril, Chem. Commun. (2003) 2176-2177; (b) Y.H. Ko, K. Kim, K.J. Kim, et al., Designed self-assembly of molecular necklaces using host-stabilized charge-transfer interactions, J. Am. Chem. Soc. 126(2004) 1932-1937; (c) Y. Ling, W. Wang, A.E. Kaifer, et al., A new cucurbit[8] uril-based fluorescent receptor for indole derivatives, Chem. Commun. (2007) 610-612; (d) W. Ong, A.E. Kaifer, Salt effects on the apparent stability of the cucurbit[7] uril-methyl viologen inclusion complex, J. Org. Chem. 69(2004) 1383-1385; (e) J. Kim, I.S. Jung, K.J. Kim, et al., New cucurbituril homologues:syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n=5, 7, and 8), J. Am. Chem. Soc. 122(2000) 540-541. |
| [15] | (a) U. Rauwald, O.A. Scherman, Supramolecular block copolymers with cucurbit[8] uril in water, Angew. Chem. Int. Ed. 47(2008) 3950-3953; (b) W. Wang, A.E. Kaifer, Electrochemical switching and size selection in cucurbit[8] uril-mediated dendrimer self-assembly, Angew. Chem. Int. Ed. 45(2006) 7042-7046. |
| [16] | J.W. Lee, I. Hwang, K. Kim, Synthetic molecular machine based on reversible end-to-interior and end-to-end loop formation triggered by electrochemical stimuli. Chem. Asian J. 3 (2008) 1277–1283. DOI:10.1002/asia.v3:8/9 |
| [17] | A. Day, A.P. Arnold, B.J. Snushall, Controlling factors in the synthesis of cucurbituril and its homologues. J. Org. Chem. 66 (2001) 8094–8100. DOI:10.1021/jo015897c |
 2017, Vol. 28
2017, Vol. 28 



