Alkannin and shikonin (A/S, Fig. 1), an enantiomeric pair of naturally occurring isohexenylnaphthazarins, were isolated and identified from the roots of Alkanna tinctoria in Europe and Lithospermum erythrorhizonin in the Orient, respectively[1]. A/S and their derivatives exhibited a broad spectrum of biological activities [1-4] including wound healing, anti-inflammatory and immunoregulatory effects, while their potent antitumor activity mediated by multiple mechanisms [5-15] has aroused growing interest recently. The naphthazarin skeleton was found to be the crucial pharmacophore of the A/S, capable of generating ROS and covalently binding to cellular nucleophiles [14, 15], resulting in the apoptotic death of normal cells along with cancer cells.

|
Download:
|
| Figure 1. The chemical structures of A/S and biotinylated DMAKO derivatives. | |
Intriguingly, our previous investigations demonstrated that dimethylated A/S oxime (DMA/SKO) derivatives displayed more potent inhibitory effects than the parent compounds against cancer cell lines along with no apparent toxicity toward normal cells [16, 17]. Our continuous research found that DMAKO-05, displayed more potent antitumor activity and less toxicity in comparison to 5-FU both in vitro and in vivo, which was not a naphthoquinone prodrug due to its metabolism in rat liver microsome through hydrolysis of its side chain and oxidation of its naphthoquinone scaffold, rather than oxidation of hydroxyimine to ketone [18]. More recently, we have further reported that DMAKO-05, a potential candidate compound for melanoma, displayed potent cytotoxicity toward B16F0 cells by inhibiting Akt activation, inducing G1 arrest and further promoting B16F0 cell apoptosis [19]. Moreover, another hallmark molecular DMAKO-20 showed more excellent antitumor activities in a mouse xenograft model (unpublished data). A noteworthy finding was that their potent anti-proliferation effects were not closely associated with ROS and bioreductive alkylation [16, 17]. Meanwhile, the mode of action and mechanism of their antitumor activities remained unclear, which significantly hamper the further drug development of DMAKO derivatives.
Molecular probes, selectively bound biomolecular target (s), were widely utilized in molecular biology. In this article, more attention has been paid to the preparation of biotin-coupled activity based-probes (ABPs) of alkannin oxime derivatives incorporating a biotin moiety for avidin affinity "pull down" of covalently tagged target protein (s) [20, 21]. For effective labeling of target proteins, the anticancer activity of biotin probe should be retained. Meanwhile, the appropriate linker connecting the alkannin oxime and the affinity label should be taken into consideration in order to provide enough space for biotin to interact with avidin. Our preliminarily research concerning the structure-activity relationship of A/S oxime derivatives [17] revealed that the hydroxyl of oxime was a conserved functional group and thus was not suitable for incorporation of biotinylated linker. Nevertheless, modification of the 1'-OH on the side chain of A/S was conducive to enhancing anticancer activity [22-24]. Accordingly, the 1'-OH was a feasible position for introduction of biotin and linker. Based on these results, we designed and synthesized biotinylated DMAKO derivatives containing ether bond and ester group at 1'-position on the side chain (Fig. 1).
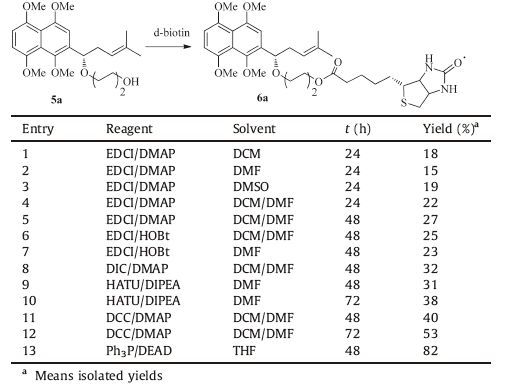
2. Results and discussion 2.1. ChemistryThe general synthesis strategy for chemical probes 8a-8c is illustrated in Scheme 1. 4-Methyl-1- (1, 4, 5, 8-tetramethoxynaphthalen-2-yl) pent-3-en-1-ol (1) was prepared with 1, 4, 5, 8- tetramethoxy-2-naphthaldehyde as the starting material according to the procedures previously reported by our research group [22]. The ketone 2 was obtained by the oxidation of alcohol 1 with Dess-Martin periodinane (DMP) and was then reduced by (-) -DIPCl to give (S) -4-methyl-1- (1, 4, 5, 8-tetramethoxynaphthalen-2- yl) pent-3-en-1-ol (3) in relatively high yield [17]. Etherification of 3 with (m-bromoalkyl) tetrahydro-2H-pyran (Br (CH2) mOTHP) through nucleophilic substitution provided 4a-4c under strong basic conditions. Subsequently, removal of THP protection quantitatively afforded alcohol 5a-5c in the presence of hydrochloric acid. However, succesive condensation of compound 5a-5c and d-biotin by treatment with 1-ethyl- (3- dimethylaminopropyl) carbodiimide hydrochloride (EDCI) provided ester 6a-6c in relatively low yield. Therefore, considerable efforts were taken to augument the yield of the key intermediate 6a-6c.

|
Download:
|
| Scheme 1. Synthesis of chemical probes 8a-8c. Reagents and conditions: (a) DMP, -10 ℃, 25 min; (b) (-)-DIP-Cl, THF, -20 ℃, 1 h; (c) 60% NaH, DMF, 0 ℃, 0.5 h, then Br(CH2CH2)mOTHP, KI, 60 ℃, 10 h; (d) 12 mol/L HCl, MeOH, 15 min; (e) Ph3P, DEAD, biotin 11, THF, r. t., 48 h; (f) CAN, CH3CN-EtOAc, 0 ℃, 8 min; (g) NH2OH.HCl, pyridine, reflux, 16 h. | |
Compound 5a was used as model substrate to probe the reaction conditions. Initially, treatment of 5a with EDCI/DMAP in different solvents (DCM, DMF, DMSO and DCM/DMF) afforded 6a in 15%-27% isolated yields (Table 1, entries 1-5). Subsequent replacement of DMAP with 1-hydroxybenzotriazole (HOBt) resulted in no apparent change in isolated yield (entries 6 and 7). Compared with EDCI, alcohol 5a coupled with N, N-diisopropylcarbodiimide (DIC) led to slightly higher yield (entry 8). Changing the coupling reagents from DIC to 2- (7-aza-1Hbenzotriazole-1-yl) -1, 1, 3, 3-tetramethylururonium hexafluorophosphate (HATU) and using N, N-diisopropy-lethylamine (DIPEA) as a solvent, we found that the yield of 6a was also no significantly improved (entry 9 and 10). Whereas the coupling reagent HATU was replaced by DCC and the reaction time was prolonged from 48 h to 72 h, it was surprising that this condensation reaction provided 6a in 53% isolated yield (entries 10 and 11). More importantly, a gratifying observation was that 6a was also obtained by a modified Mitsunobu reaction in 82% isolated yield (entry 12).
|
|
Table 1 Optimization of the coupling reaction between compound 5a and d-biotin. |
When compounds 6a-6c were successfully obtained through Mitsunobu reaction, subsequent oxidation of them with cerium (IV) ammonium nitrate (CAN) gave the 6-substituted 5, 8-O-dimethylalkannin derivatives 7a-7c in CH3CN/EtOAc (v/v = 1:3). Target compounds 8a-8c were prepared by the condensation reaction between 6-substituted 1, 4-naphthoquinone 7a-7c and hydroxylamine hydrochloride at 95 ℃ in the presence of pyridine. Additionally, it should be pointed out that much higher or lower temperature did not favor the generation of dioxime derivatives.
Our straightforward synthesis of chemical probe 16 is outlined in Scheme 2. The intermediate 12, readily obtained in good yield (80%) by the classical substitution reaction between d-biotin 11 and commercially available tert-butyl bromoacetate [25], could be converted into acid 13 by using trifluoroacetic acid in CH2Cl2 at room temperature. Compound 13 was then coupled with alcohol 3 in the presence of DCC and DMAP, followed by oxidation of ester 14 with CAN to afford 6-substituted 1, 4-naphthoquinone 15. Likewise, chemical probe 16 was synthesized using the method as described for compounds 8a-8c. The desired compounds were separated by HPLC in reasonable yield (37%-42%) since the polarity of biotinylated alkannin derivatives became higher than that of non-biotinylated alkannin derivatives and the polarity of byproducts was close to the corresponding desired compounds, which made it more difficult for chromatographic purification.
|
Download:
|
| Scheme 2. Synthesis of chemical probe 16. Reagents and conditions: (h) K2CO3, tert-butyl bromoacetate, DMSO, r. t.,8 h; (i) CF3COOH, CH2Cl2, r.t., 16 h; (e) DCC, DMAP, alcohol 3, DMF-CH2Cl2, r.t., 72 h; (f) CAN, CH3CN–EtOAc, 0 ℃, 8 min; (g) NH2OH·HCl, pyridine, 95 ℃, 16 h. | |
2.2. Cytotoxicities of biotinylated alkannin derivatives in vitro
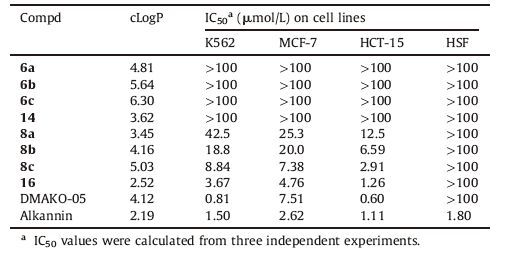
The cytotoxicities of biotinylated alkannin derivatives 6a-6c, 14, 8a-8c and 16 were evaluated against K562 (leukemia), HCT-15 (colorectal carcinoma), MCF-7 (breast cancer) and HSF (human skin fibroblast) cell using standard MTT assay [19]. Potency of alkannin, DMAKO-05 and biotinylated 1, 4, 5, 8-tetramethoxynathalenes 6a-6c and 14 on these cell lines were also examined for comparison purposes. As Table 2 shows, biotinylated 1, 4, 5, 8- tetramethoxynathalenes 6a-6c and 14 exhibited no cytotoxicity even at 100 mmol/L toward these cancer cell lines, whereas chemical probes 8a-8c and 16 displayed relatively potent cytotoxicity toward HCT-15 cells together with moderate cytotoxicity toward MCF-7 cells. Biotinylated DMAKO derivative 8c caused significant cytotoxicity at lower concentration (IC50 = 2.91, 7.38 and 8.84 mmol/L), which was superior to the cytotoxicity induced by 8a (IC50 = 12.5, 25.3 and 42.5 μmol/L) in HCT-15 cells, MCF-7 cells and K562 cells, respectively. This result indicated that extension of the linear C4 chain with another alkyl chain (C6 and C8) was responsible for the enhancement of biological activity. Compound 16 bearing ester moiety at 1'-OH on the side chain expressed stronger cytotoxic activity against MCF-7 cells with the IC50 value of 4.76 mmol/L than DMAKO-05 and was comparative effective to positive controls (DMAKO-05 and alkannin) toward HCT-15 cells. Table 2 also summarized their calculated LogP (cLogP) values obtained from ALOGPS 2.1 software [26]. Compounds (8a-8c, 16) containing oxime moiety showed the smaller value of cLogP at 3.45, 4.16, 5.03, 2.52, respectively than the corresponding biotinylated 1, 4, 5, 8-tetramethoxynathalenes (6a-6c, 14), suggesting the introduction of hydrophilic functional group on the naphthoquinone scaffold favored the enhancement of cytotoxicity. However, compared with 8a and 8b, 8c bearing eight carbon chain lengths had the higher value of cLogP, but displayed more potent cytotoxicty. Hence, roles of the cLogP values in cytotoxicities of DMAKO derivatives are needed to be further investigated.
|
|
Table 2 Cytotoxicity of biotinylated DMAKO derivatives against K562, MCF-7, HCT-15 and HSF cell lines. |
3. Conclusion
In summary, we synthesized four biotinylated DMAKO derivatives in this work. The biotin moiety was successfully introduced in the molecule through a modified Mitsunobu reaction. Besides, the cell-based investigation demonstrated that replacement of the linker C4 chain with another alkyl chain (C6 or C8) led to the increased cytotoxicity. Among these biotinyl derivatives, both compound 16 and 8c exhibited more potent anticancer activity than DMAKO-05 against MCF-7 cells and were comparatively effective to alkannin toward HCT-15 cells. Our present work could provide an available approach for the identification of the molecular target (s) of DMAKO derivatives. The purification and identification of compound (16 and 8c) targeted protein (s) by "protein pull down" are currently underway in our laboratory.
4. Experimental 4.1. ChemistryAll the commercial chemicals were of reagent grade and were used without further purification. Solvents were dried with standard procedures. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Varian MERCURY plus- 400 spectrometer with TMS as an internal standard. HRMS and MS were performed at the Analysis Center of Shanghai Jiao Tong University. Purity was evaluated by reversed phase high-pressure liquid chromatography (RP-HPLC) on an Agilent 1260 system using a Discovery C18 column (150 mm × 4.6 mm, 5 mm) at room temperature with a gradient elution using the mobile phase (A) water and (B) methanol (50% of B at 0-20 min, 50%-95% of B at 20- 30 min), with a flow rate of 1.0 mL min-1. The injection volume was 10 mL and the detection wavelength was set at 220 nm. All biologically evaluated compounds' purity were >95%.
Synthesis of compounds 2-7c and 12-15 were given in the Supplementary data.
General procedure for chemical probes (8a-8c and 16) : Hydroxylamine hydrochloride (3.5 mmol) was added to a solution of 6-substituted 1, 4-naphthoquione (0.5 mmol) in anhydrous pyridine (10.0 mL). The reaction mixture was refluxed for 16 h under nitrogen atmosphere. After cooling, the reaction mixture was concentrated and then poured to ice-water, followed by filteration to give the residual brown solid. The residue was purified by RP-HPLC [Agilent ZORBAX SB-C18 column (250 mm × 9.4 mm, 5 mm); a gradient elution using the mobile phase (A) H2O and (B) MeOH (0-50% of B at 0-20 min, 50%-95% of B at 20-30 min); 4.0 mL min-1; UV detection at 220 nm] to afford oxime (8a-8c and 16) as yellow solid.
4-(((S)-1-((5E, 8E) -5, 8-Bis (hydroxyimino) -1, 4-dimethoxy-5, 8- dihydronaphthalen-2-yl) -4-methylpent-3-en-1-yl) oxy) butyl 5- ( ((4R) -2-oxohexahydro-1H-thieno[3, 4-d]imidazol-4-yl) pentanoate (8a) : Yield: 32%. 1H NMR (400 MHz, DMSO-d6) : δ 12.06 (s, 2H), 7.39 (s, 2H), 7.08 (s, 1H), 6.43 (s, 1H), 6.38 (s, 1H), 5.21 (t, 1H, J = 8.0 Hz), 4.67 (t, 1H, J = 6.8 Hz), 4.30 (t, 1H, J = 6.8 Hz), 4.15-4.11 (m, 1H), 4.02 (t, 1H, J = 6.8 Hz), 3.79 (s, 3H), 3.60 (s, 3H), 3.22-3.29 (m, 2H), 3.10-3.04 (m, 1H), 2.81 (dd, 2H, J1 = 11.8 Hz, J2 = 3.8 Hz), 2.57 (d, 1H, J = 12.0 Hz), 2.39-2.32 (m, 2H), 2.26 (t, 2H, J = 6.4 Hz), 1.64 (s, 3H), 1.61-1.54 (m, 6H), 1.51 (s, 3H), 1.48-1.42 (m, 2H), 1.34-1.27 (m, 2H); 13C NMR (100 MHz, DMSO-d6) : δ 173.3, 163.2, 153.8, 149.0, 147.7, 138.0, 133.1, 124.4, 121.0, 120.2, 119.6, 111.0, 75.8, 68.4, 64.0, 61.5, 61.2, 59.6, 56.6, 55.8, 36.0, 33.7, 28.4, 26.3, 26.0, 25.6, 24.9, 18.1; HRMS (ESI) m/z calcd. for C32H45N4O8S [M+H]+, 645.2958; found: 645.2969; purity 97% (as determined by RP-HPLC, tR = 16.651 min).
6- (( (S) -1- ((5E, 8E) -5, 8-Bis (hydroxyimino) -1, 4-dimethoxy-5, 8- dihydronaphthalen-2-yl) -4-methylpent-3-en-1-yl) oxy) hexyl 5- ( (4R) - (2-oxohexahydro-1H-thieno[3, 4-d]imidazol-4-yl) pentanoate (8b) : Yield: 35%. 1H NMR (400 MHz, DMSO-d6) : δ 12.05 (s, 2H), 7.38 (s, 2H), 7.08 (s, 1H), 6.42 (s, 1H), 6.36 (s, 1H), 5.22 (t, 1H, J = 8.0 Hz), 4.66 (t, 1H, J = 6.8 Hz), 4.30 (t, 1H, J = 6.8 Hz), 4.14-4.10 (m, 1H), 3.98 (t, 2H, J = 6.8 Hz), 3.78 (s, 3H), 3.59 (s, 3H), 3.30-3.26 (m, 2H), 3.10-3.05 (m, 1H), 2.81 (dd, 2H, J1 = 11.8 Hz, J2 = 3.8 Hz), 2.57 (d, 1H, J = 12.0 Hz), 2.36-2.32 (m, 2H), 2.26 (t, 2H, J = 6.4 Hz), 1.64 (s, 3H), 1.58-1.50 (m, 10H), 1.35-1.27 (m, 7H); 13C NMR (100 MHz, DMSO-d6) : δ 173.3, 163.1, 153.9, 149.0, 147.8, 138.2, 133.0, 124.4, 121.1, 119.6, 119.4, 111.0, 75.8, 68.7, 64.1, 61.5, 61.2, 59.6, 56.6, 55.8, 36.1, 33.8, 29.7, 28.6, 28.4, 26.0, 25.9, 25.6, 25.0, 18.1; HRMS (ESI) m/z calcd. for C34H49N4O8S [M+H]+, 673.3271; found: 673.3264; purity 96% (as determined by RP-HPLC, tR = 18.926 min).
8- (( (S) -1- ((5E, 8E) -5, 8-Bis (hydroxyimino) -1, 4-dimethoxy-5, 8- dihydronaphthalen-2-yl) -4-methylpent-3-en-1-yl) oxy) octyl 5- ( (4R) - (2-oxohexahydro-1H-thieno[3, 4-d]imidazol-4-yl) pentanoate (8c) : Yield: 31%. 1H NMR (400 MHz, DMSO-d6) : δ 12.07 (s, 2H), 7.39 (s, 2H), 7.09 (s, 1H), 6.44 (s, 1H), 6.38 (s, 1H), 5.22 (t, 1H, J = 4.0 Hz), 4.68-4.63 (m, 1H), 4.30 (t, 1H, J = 6.8 Hz), 4.15-4.10 (m, 1H), 3.98 (t, 2H, J = 5.8 Hz), 3.79 (s, 3H), 3.59 (s, 3H), 3.30-3.25 (m, 2H), 3.11-3.05 (m, 1H), 2.81 (dd 2H, J1 = 11.8 Hz, J2 = 3.8 Hz, ), 2.58 (d, 1H, J = 12.0 Hz), 2.38-2.32 (m, 2H), 2.27 (t, 2H, J = 6.4 Hz), 1.65 (s, 3H), 1.55-1.49 (m, 10H), 1.34-1.23 (m, 11H); 13C NMR (101 MHz, DMSO-d6) : δ 173.3, 163.1, 153.8, 149.0, 147.7, 138.2, 133.0, 124.4, 121.2, 120.1, 119.5, 111.1, 75.8, 68.8, 64.1, 61.5, 61.2, 59.6, 56.6, 55.8, 36.1, 33.8, 32.9, 29.7, 29.1, 28.5, 28.4, 26.1, 26.0, 25.8, 25.0, 18.1; HRMS (ESI) m/z calcd. for C36H53N4O8S [M+H]+, 701.3584; found: 701.3584; purity 99% (as determined by RPHPLC, tR = 20.591 min).
2- (( (S) -1- ((5E, 8E) -5, 8-Bis (hydroxyimino) -1, 4-dimethoxy-5, 8-dihydronaphthalen-2-yl) -4-methylpent-3-en-1-yl) oxy) -2- oxoethyl 5- ((4R) -2-oxohexahydro-1H-thieno[3, 4-d]imidazol-4- yl) pentanoate (16) : Yield: 39%. 1H NMR (400 MHz, DMSO-d6) : δ 12.15 (s, 2H), 7.39 (s, 2H), 7.02 (s, 1H), 6.43 (s, 1H), 6.39 (s, 1H), 6.04 (t, 1H, J = 6.2 Hz), 5.13 (t, 1H, J = 7.2 Hz), 4.77 (s, 2H), 4.29 (t, 1H, J = 4.0 Hz), 4.12 (t, 1H, J = 4.0 Hz), 3.83 (s, 3H), 3.65 (s, 3H), 3.09-3.04 (m, 2H), 2.80 (dd, 2H, J1 = 11.8 Hz, J2 = 3.8 Hz), 2.60- 2.53 (m, 3H), 2.40 (t, 2H, J = 7.2 Hz), 1.66 (s, 3H), 1.61-1.56 (m, 2H), 1.54 (s, 3H), 1.50-1.40 (m, 2H), 1.39-1.33 (m, 2H), 1.27-1.20 (m, 2H); 13C NMR (100 MHz, DMSO-d6) : δ 173.0, 167.8, 163.1, 153.7, 148.0, 147.5, 135.4, 134.9, 124.5, 120.2, 120.0, 119.5, 119.1, 110.9, 71.9, 61.4, 61.1, 60.9, 59.6, 56.7, 55.8, 34.2, 33.3, 28.4, 28.3, 26.0, 24.8, 18.1; HRMS (ESI) m/z calcd. for C30H39N4O8S [M+H]+, 631.2438; found: 631.2435; purity 98% (as determined by RPHPLC, tR = 14.587 min).
4.2. Cytotoxicities assayCells at 70-80% confluency were trypsinized and plated into 96- well plates at a density of 1 × 104 cells/mL. After being cultured for 24 h at 37 ℃, 5% CO2 atmosphere, cells were treated with the tested compound of various concentrations for 72 h and control groups were treated with complete medium alone. The supernatants were then removed and replaced by 200 mL Roswell Park Memorial Institute 1640 (RMPI-1640) medium without serum. Subsequently, 20 μL 3- (4, 5-dimethylthiazol-2-yl) -2, 5-diphenyltetrazolium bromide (MTT) solution (5 mg/mL) was added to each well. Following incubation for another 4 h, the medium was aspirated, and the formazan crystals were dissolved in 100 mL DMSO for each well. The absorbance was recorded at a test wavelength of 570 nm using a Multiskan MK3 microplate reader (Thermo Scientific, USA). The percentage of survival was calculated as the absorbance ratio of treated cells to that of control cells. Half maximal inhibitory concentration (IC50) values were obtained by linear regression analysis using IBM SPSS statistics software (version 21.0).
AcknowledgmentsThe research was supported by National Natural Science Foundation of China (No. 81373274), Ph.D. Programs Foundation of Ministry of Education China (No. 20120073110068) and Shanghai Biomedical Supporting Funding (No. 15431900600). We are grateful to the Instrumental Analysis Center of Shanghai Jiao Tong University for recording the 1H NMR and 13C NMR spectra.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.09.015.
| [1] | V.P. Papageorgiou, A.N. Assimopoulou, E.A. Couladouros, D. Hepworth, K.C. Nicolaou, The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew. Chem. Int. Ed. 38 (1999) 270–300. DOI:10.1002/(ISSN)1521-3773 |
| [2] | I. Andujar, M.C. Recio, R.M. Giner, J.L. Ríos, Traditional Chinese medicine remedy to jury:the pharmacological basis for the use of shikonin as an anticancer therapy. Curr. Med. Chem. 20 (2013) 2892–2898. DOI:10.2174/09298673113209990008 |
| [3] | R.B. Wang, R.T. Yin, W. Zhou, D.F. Xu, S.S. Li, Shikonin and its derivatives:a patent review. Expert Opin. Ther. Pat. 22 (2012) 977–997. DOI:10.1517/13543776.2012.709237 |
| [4] | V.P. Papageorgiou, A.N. Assimopoulou, V.F. Samanidou, I.N. Papadoyannis, Recent advances in chemistry, biology and biotechnology of alkannins and shikonins. Curr. Org. Chem. 10 (2006) 2123–2142. DOI:10.2174/138527206778742704 |
| [5] | B.Z. Ahn, K.U. Baik, G.R. Kweon, K. Lim, B.D. Hwang, Acylshikonin analogues:synthesis and inhibition of DNA topoisomerase I. J. Med. Chem. 38 (1995) 1044–1047. DOI:10.1021/jm00006a025 |
| [6] | F. Yang, Y. Chen, W.H. Duan, SH-7, a new synthesized shikonin derivative, exerting its potent antitumor activities as a topoisomerase inhibitor. Int. J. Cancer 119 (2006) 1184–1193. DOI:10.1002/(ISSN)1097-0215 |
| [7] | H. He, L.P. Bai, Z.H. Jiang, Synthesis and human telomeric G-quadruplex DNAbinding activity of glucosaminosides of shikonin/alkannin. Bioorg. Med. Chem. Lett. 22 (2012) 1582–1586. DOI:10.1016/j.bmcl.2011.12.143 |
| [8] | F. Singh, D.Y. Gao, M.G. Lebwohl, H.C. Wei, Shikonin modulates cell proliferation by inhibiting epidermal growth factor receptor signaling in human epidermoid carcinoma cells. Cancer Lett. 200 (2003) 115–121. DOI:10.1016/S0304-3835(03)00239-8 |
| [9] | J. Guo, X.F. Chen, J. Liu, Novel shikonin derivatives targeting tubulin as anticancer agents. Chem. Biol. Drug Des. 84 (2014) 603–615. DOI:10.1111/cbdd.2014.84.issue-5 |
| [10] | W. Li, J. Liu, Y. Zhao, PKM2 inhibitor shikonin suppresses TPA-induced mitochondrial malfunction and proliferation of skin epidermal JB6 cells. Mol Carcinog. 53 (2014) 403–412. DOI:10.1002/mc.21988 |
| [11] | J. Liu, W. Zhou, S.S. Li, Modulation of orphan nuclear receptor Nur77-mediated apoptotic pathway by acetylshikonin and analogues. Cancer Res. 21 (2008) 8871–8880. |
| [12] | S.H. Kim, I.C. Kang, T.J. Yoon, Antitumor activities of a newly synthesized shikonin derivative, 2-hyim-DMNQ-S-33. Cancer Lett. 172 (2001) 171–175. DOI:10.1016/S0304-3835(01)00665-6 |
| [13] | J. Chen, J. Xie, Z. Jiang, Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene 30 (2011) 4297–4306. DOI:10.1038/onc.2011.137 |
| [14] | D.Z. Duan, B.X. Zhang, J. Yao, Y.P. Liu, J.G. Fang, Shikonin targets cytosolic thioredoxin reductase to induce ROS-mediated apoptosis in human promyelocytic leukemia HL-60 cells. Free Radical Biol. Med. 70 (2014) 182–193. DOI:10.1016/j.freeradbiomed.2014.02.016 |
| [15] | J. Ahn, M. Won, J.H. Hae Choi, Reactive oxygen species-mediated activation of the Akt/ASK1/p38 signaling cascade and p21Cip1 downregulation are required for shikonin-induced apoptosis. Apoptosis 18 (2013) 870–881. DOI:10.1007/s10495-013-0835-5 |
| [16] | R.B. Wang, X. Zhang, H.L. Song, S.S. Zhou, S.S. Li, Synthesis and evaluation of novel alkannin and shikonin oxime derivatives as potent antitumor agents. Bioorg. Med. Chem. Lett. 24 (2014) 4304–4307. DOI:10.1016/j.bmcl.2014.07.012 |
| [17] | X. Zhang, J.H. Cui, W. Zhou, S.S. Li, Design, synthesis and anticancer activity of ahikonin and alkannin derivatives with different substituents on the naphthazarin scaffold. Res. Chin. Univ. 3 (2015) 394–400. |
| [18] | X. Zhang, R.B. Wang, W. Zhou, Antitumor activity of DMAKO-05, a novel shikonin derivative, and its metabolism in rat liver microsome. AAPS PharmSciTech. 16 (2015) 259–266. DOI:10.1208/s12249-014-0217-5 |
| [19] | Y.Y. Yang, H.Q. He, J.H. Cui, Shikonin derivative DMAKO-05 inhibits Akt signal activation and melanoma proliferation. Chem. Biol. Drug Des. (2016) 1–10. |
| [20] | W.P. Heal, T.H.T. Dang, E.W. Tate, Activity-based probes:discovering new biology and new drug targets. Chem. Soc. Rev. 40 (2011) 246–257. DOI:10.1039/C0CS00004C |
| [21] | L.A.R. Carvalho, E.F.P. Ruivo, S.D. Lucas, R. Moreira, Activity-based probes as molecular tools for biomarker discovery. Med. Chem. Commun. 6 (2015) 536–546. DOI:10.1039/C4MD00417E |
| [22] | L.M. Zhao, T.P. Xie, Y.Q. He, D.F. Xu, S.S. Li, Synthesis and antitumor activity of 6-and 2-(1-substituted-thio-4-methylpent-3-enyl)-5,8-dimethoxynaphthalene-1,4-diones. Eur. J. Med. Chem. 44 (2009) 1410–1414. DOI:10.1016/j.ejmech.2008.09.039 |
| [23] | W. Zhou, X. Zhang, L. Xiao, Semi-synthesis and antitumor activity of 6-isomers of 5,8-O-dimethyl acylshikonin derivatives. Eur. J. Med. Chem. 46 (2011) 3420–3427. DOI:10.1016/j.ejmech.2011.05.006 |
| [24] | R.B. Wang, W. Zhou, Q.Q. Meng, Design, synthesis, and biological evaluation of shikonin and alkannin derivatives as potential anticancer agents via a prodrug approach. Chem. Med. Chem. 9 (2014) 2798–2808. DOI:10.1002/cmdc.v9.12 |
| [25] | K. Kim, H. Yang, S.Y. Jon, E. Kim, J. Kwak, Protein patterning based on electrochemical activation of bioinactive surfaces with hydroquinone-caged biotin. J. Am. Chem. Soc. 126 (2004) 15368–15369. DOI:10.1021/ja0459330 |
| [26] | I.V. Tetko, V.Y. Tanchuk, Application of associative neural networks for prediction of lipophilicity in ALOGPS 2.1 program. Chem. Inf. Comput. Sci. 42 (2002) 1136–1145. DOI:10.1021/ci025515j |
 2017, Vol. 28
2017, Vol. 28