b Department of Chemistry, Yazd Branch, Islamic Azad University, Yazd, Iran
Polyfunctionalized heterocyclic compounds play significant roles in the drug discovery process, and analysis of drugs [1, 2], therefore, the development of the design and synthesis of new diverse polycyclic heterocycles with potential medicinal and biological activity has received significant attention for research in organic, combinatorial, and medicinal chemistry [3, 4]. Multicomponent reactions involving a domino process (MDRs) [5, 6] with at least three or more reactants particularly performed under solvent-free conditions [7, 8] have become as a popular tool for the synthesis of chemically and biologically important organic frameworks. These processes avoid the isolation and purification of intermediates, higher productivity, minimize solvent waste, and enhance the greenness of the transformations. Also, microwaveassisted organic synthesis (MAOS) has become a very valuable tool, improving the outcome of multi-component reactions [9] because microwave heating is able to minimize side reactions, increase yields, reduce reaction times, improve reproducibility, and even enable unaccessible reactions by conventional heating and is particularly useful for the preparation of various biologically active heterocyclic compounds [10].
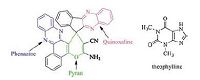
Heterocycles having phenazines, quinoxalines and pyrans moieties are important targets in the synthetic organic chemistry. While phenazines, quinoxalines and pyrans have attracted great attention in drug discovery, the preparation of compounds incorporating all of these motifs (Fig. 1) has not been reported.
|
Download:
|
| Figure 1. The structure of designed compound and theophylline. | |
Phenazine based compounds are nitrogen-containing heterocycles that are the main core of many natural and synthetic organic materials [11-13]. Phenazines are showing a variety of biological functions, including fungicidal [14], trypanocidal [15], antimalarial [16], antiplatelet [17] and antitumour [18] activities.
Also, quinoxaline derivatives are an important group of azapolycyclic compounds displaying a broad spectrum of biological activities which have made them privileged structures in pharmacologically active compounds [19, 20]. For example, they show very interesting pharmacological properties such as antibacterial [21], antifungal [22], antidepressant [23], and antitumor agents [24].
On the other hand, pyran annulated heterocyle derivatives are an important class of oxygen-containing heterocycles and are usually structural subunits in a variety of important natural compounds, including carbohydrates, alkaloids, polyether antibiotics, pheromones, and iridoids [25]. Pyarans are widely employed as cosmetics, pigments [26], and potential biodegradable agrochemicals [27] and that have various biological properties such as anti-leishmanial [28], anti-HIV [29], antioxidant [30], anti-tumor [31], and central nervous system (CNS) activities and effects [32]; they are also used for treatment of Alzheimer’s disease [33] and schizophrenia [34].
Moreover, spiro heterocycles are found in a number of natural or synthetic molecules [35, 36]. A spiro heterocycle compound in which the spiro carbon is part of cyclic ring has many unique properties [37, 38] and they are particularly interesting because the conformational restriction associated to the structural rigidity affects considerably their biological activity [39].
Furthermore, xanthine is a common structural component in medicinal chemistry and new chemical entities (NCEs) development [40]. Xanthine-based lead molecules have been exploited in numerous therapeutic areas, for instance, Alzheimer’s disease [41], asthma [42], diabetes [43], Parkinson’s disease [44] and cancer [45]. Furthermore, xanthine derivatives are one of the most abundant chemical classes of adenosine receptor antagonists [46]. Theophylline (Fig. 1) is a methylxanthine drug used in therapy for respiratory diseases such aschronic obstructive pulmonary disease (COPD) and asthma under a variety of brand names.
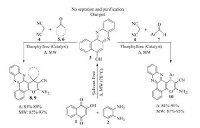
Considering the importance of phenazine, quinoxaline and pyran derivatives and in continuation of our research on multicomponent reactions and our ongoing program for the synthesis of complex organic compounds based on green chemistry protocols [47-52], herein we report a very green synthesis of functionalized spiro[benzo[a]pyrano[2, 3-c]phenazine] and benzo[a]pyrano[2, 3-c]phenazine derivatives catalyzed by theophylline as an efficient solid base catalyst under solvent-free conditions (Scheme 1).
|
Download:
|
| Scheme 1. One-pot, multi-component synthesis of novel spiro[benzo[a]pyrano [2,3-c]phenazine] and benzo[a]pyrano[2,3-c]phenazine derivatives in the presence of theophylline. | |
2. Results and discussion
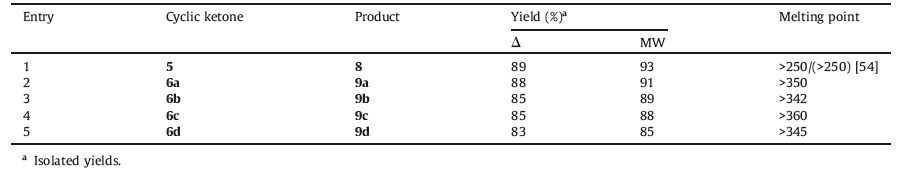
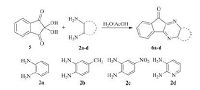
Theophylline is cheap and commercially available reagent, and its structure convinced us to accept that this reagent could potentially act as an effective, eco-friendly and basic catalyst in the synthesis of novel spiro[benzo[a]pyrano[2, 3-c]phenazine] and benzo[a]pyrano[2, 3-c]phenazine derivatives. For this purpose, at first, the aromatic ketones 6a-d were synthesized according to previous work [53], by means of reaction between ninhydrin (2, 2-dihydroxyindane-1, 3-dione) 5 and various aromatic 1, 2-diamines including benzene-1, 2-diamine 2a, 4-methylbenzene-1, 2-diamine 2b, 4-nitrobenzene-1, 2-diamine 2c and 2, 3-diaminopyridine 2d (Scheme 2). In the cases of benzene-1, 2-diamine and 4-methylbenzene-1, 2-diamine, higher yields of the products were obtained in shorter reaction time in comparison with 4-nitrobenzene-1, 2-diamine and 2, 3-diaminopyridine.
|
Download:
|
| Scheme 2. Synthesis of 11H-indeno[1,2-b]quinoxalin-11-one derivatives and 6H-indeno[1,2-b]pyrido[3,2-e]pyrazin-6-one. | |
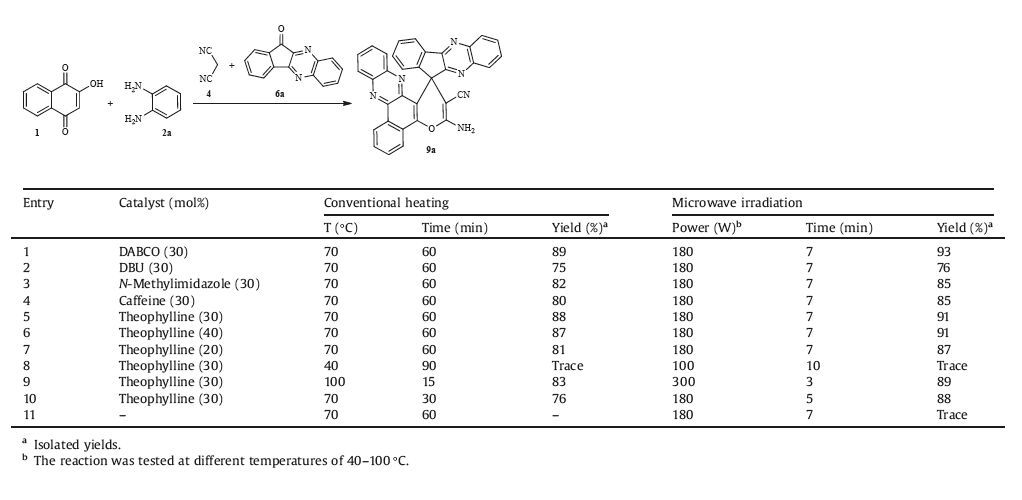
Then, benzo[a]phenazine 3 and malononitrile 4 were condensed with aromatic ketones 5 or 6a-d under optimized reaction conditions (Table 1, entry 5) to afford the related products (Scheme 3). Although the reaction showed better performance in the presence of DABCO, we used the theophylline as catalyst due to features such as non-toxicity, recoverability, and most importantly it was used as catalyst for the first time. The obtained results are summarized in Table 2. Using 11H-indeno[1, 2-b] quinoxalin-11-one led to a higher yield in comparison with other quinoxalin derivatives and 6H-indeno[1, 2-b]pyrido[3, 2-e]pyrazin-6-one.
|
|
Table 1 Optimization of the reaction conditions for the synthesis of 9a from 1,2a, 4, and 6a under various conditions. |

|
Download:
|
| Scheme 3. Synthesis of spiro[benzo[a]pyrano[2,3-c]phenazine] derivatives. | |
|
|
Table 2 Solvent-free synthesis of novel spiro[benzo[a]pyrano[2,3-c]phenazine]derivatives (8, 9a-d) from the reaction of 1, 2, 4 and cyclic ketones (5, 6a-d) in the presence of theophylline (30 mol%) as catalyst under thermal (70 ℃, 60 min) and microwave irradiation (180 W, max. 70 ℃, 7 min) conditions. |
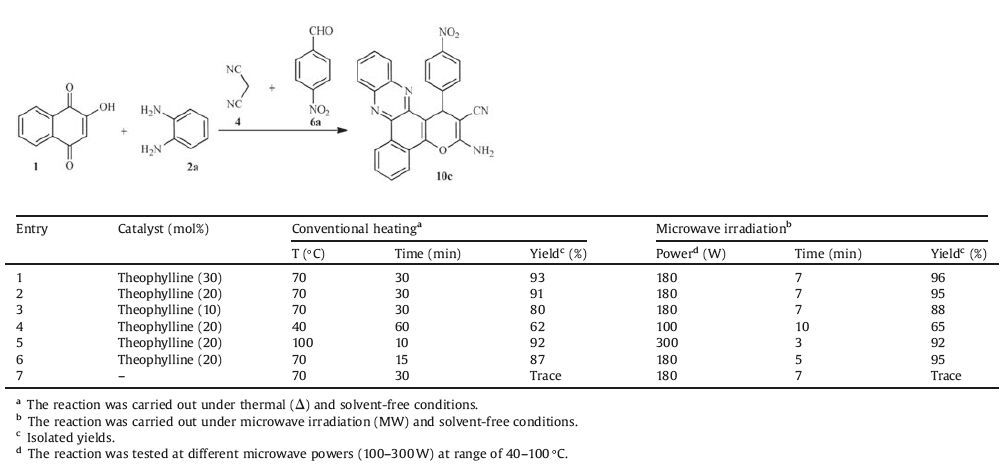
On the next step, to prepare 3-amino-2-cyano-1-aryl-1H-benzo-[a]pyrano[2, 3-c]phenazine derivatives in a more efficient way, and to minimize the reaction time and the amount of catalyst required, the reaction of 2-hydroxynaphthalene-1, 4-dione, benzene-1, 2-diamine, malononitrile and 4-nitrobenzaldehyde was selected as a model system. So, 2-hydroxynaphthalene-1, 4-dione (1 mmol) and benzene-1, 2-diamine (1 mmol) were mixed at 70 ℃ under thermal and solvent-free conditions until in less than 5 min an orange solid of benzo[a]phenazine was formed without using any catalyst. Then, 4-nitrobenzaldehyde (1 mmol), malononitrile (1 mmol), and theophylline as catalyst were added to the above reaction mixture which was heated further at same temperature. The use of different amounts of catalyst (10, 20, 30 mol%) at varieties temperatures (40, 70, 100 ℃) was investigated (Table 3). As it is shown in Table 3, the best result was obtained when the reaction was carried out in the presence of 20 mol% of the catalyst at 70 ℃ and afforded 3-amino-1-(4-nitrophenyl) -1H-benzo[a] pyrano[2, 3-c]phenazine-2-carbonitrile in 30 min with 91% of yield (Table 3, entry 2). The reaction was also examined under microwave irradiation. In order to select the appropriate microwave power, the model reaction was examined at different microwave powers (100-300 W) in the presence of theophylline. The best result was obtained with 20 mol% of theophylline at 180 W (Table 3, entry 6).
|
|
Table 3 Optimization of the reaction conditions for the synthesis of 10c from 1, 2a, 4 and 4-nitrobenzaldehyde under various conditions |
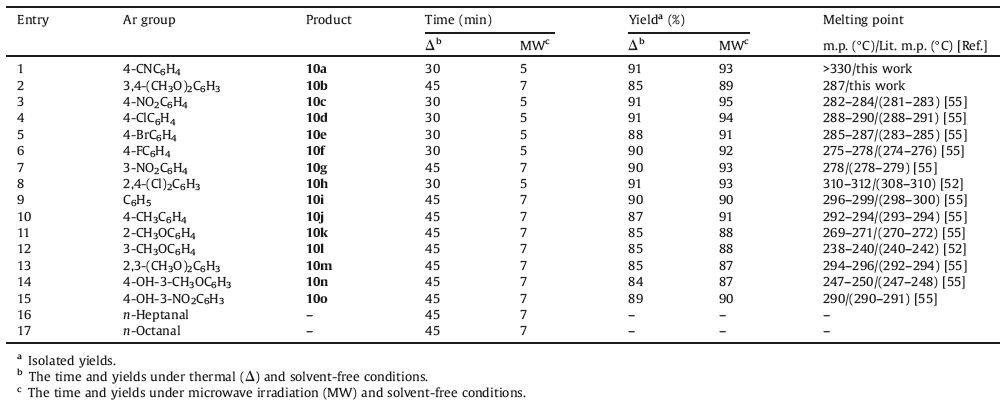
Using these optimized reaction conditions, the scope and efficiency of the reaction were explored for the synthesis of a wide variety of substituted benzo[a]chromeno[2, 3-c]phenazine derivatives. The results are summarized in Table 4. The desired pure products were characterized by comparison of their physical data (melting points, IR, and 1H NMR) with those of known compounds in the literature. The extensive ranges of substituted and structurally various aldehydes (ortho-, meta-, and para-substituted), afforded the corresponding products in high to excellent yields using the theophylline as environment-friendly catalyst (Table 4).As it was shown from Table 4, the reactions were efficiently promoted by microwave irradiation with increased yields and reduced reaction times rather than conventional heating and arylaldehydes with electron-withdrawing groups reacted rapidly and gave higher yields, while substitutions of electron-rich groups on the benzene ring required longer reaction times and got lower yields. Also, in the presence of aliphatic aldehydes such as n-heptanal and n-octanal the product expected was not obtained in this reaction conditions.
|
|
Table 4 Solvent-free synthesis of 3-amino-2-cyano-1-aryl-1H-benzo[a]pyrano[2,3-c]phenazine derivatives (10) from the reaction of 1, 2a, 4 and 7 in the presence of theophylline (20mol%) as catalyst under thermal (70 ℃) and microwave irradiation (180W, max. 70 ℃) conditions. |
Recovery of the catalysts is important in green organic synthesis. Thus, we also for recyclability of the catalysts, investigated the recycling of the theophylline under microwave irradiation and solvent-free conditions using a selected model reaction of 2-hydroxynaphthalene-1, 4-dione, benzene-1, 2-diamine, 4-nitrobenzaldehyde and malononitrile in the presence of theophylline as homogeneous catalyst (Table 4, entry 3). After completion of the reaction, the reaction mixture was cooled to room temperature. Then, 5 mL of water was added to the mixture. The theophylline was dissolved in water and filtered for separation of the crude product. The separated product was washed twice with water (2 × 5 mL). The resulting product subsequently recrystallized from hot ethanol to give the pure solid. In order to recover the catalyst, the filtrate was extracted with diethyl ether. The aqueous layer (including theophylline) was separated, and its solvent was evaporated under reduced pressure and theophylline was recovered and reused.
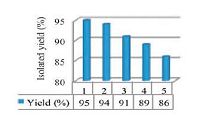
As shown in Fig. 2, we studied the reusability of theophylline as homogeneous catalyst for the same reactants. It was observed that the recovered catalyst works with the same performance up to 2nd run, while in the 3rd, 4th and 5th runs product yield gets reduced slightly that may be due to little weight loss of catalyst during each recovery process.
|
Download:
|
| Figure 2. The reusability of the catalyst (20mol%) in the synthesis of 10c from 1 (1mmol), 2a (1mmol), 4 (1mmol) and 4-nitrobenzaldehyde (1mmol) under microwave irradiation (180W, max. 70 ℃) conditions (5min). | |
In order to determine the catalytic behavior of theophylline, the suggested mechanism for the formation of the products is shown in Scheme 4. On the basis of this mechanism, at first, 2-hydroxynaphthalene-1, 4-dione 1 tautomrizes to intermediate 11. The primary condensation of 4-hydroxy-1, 2-naphthoquinone 11 with benzene-1, 2-diamine 2 obtain benzo[a]phenazin-5-ol 3. On this mechanism, theophylline is an efficient catalyst to form the olefin 12, which readily prepares in situ from Knoevenagel condensation of carbonyl groups of aldehyde or cyclic ketone 5-7 with malononitrile 4. The Michael addition of 6H-benzo[a] phenazin-5-ol 3 with olefin 12 in the presence of theophylline finally give intermediate 13, which then makes the inner molecular ring to be formed after a tautomeric proton shift to produce benzo [a]pyrano[2, 3-c]phenazine and novel spiro[benzo[a]pyrano[2, 3-c]-phenazine] derivatives 8-10.
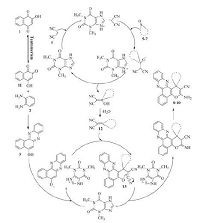
|
Download:
|
| Scheme 4. Proposed mechanism for the synthesis of novel spiro[benzo[a]pyrano[2,3-c]phenazine] and benzo[a]pyrano[2,3-c]phenazine derivatives. | |
3. Conclusion
In summary, we have described a simple, efficient, and environment-friendly one-pot procedure for the synthesis of novel spiro[benzo[a]pyrano-[2, 3-c]phenazine] and benzo[a]pyrano[2, 3-c]phenazine derivatives by using catalytic amount of theophylline under thermal or microwave irradiation and solventfree conditions. The catalytic system along with microwave heating was instrumental in reducing the reaction time and increasing yields. Moreover, our work was characterized the use of microwave irradiation as a partially reproducible energy source, avoidance of hazardous organic solvents. The methodology also offers several advantages such as easy work-up; i.e., the products can be isolated without chromatography, clean reaction profile, shorter reaction time, and use of theophylline as a non-toxic, inexpensive and easily obtained catalyst that make it a green, economically cost-effective and attractive process for the synthesis of these heterocycles.
4. Experimental 4.1. ChemistryAll melting points were determined on an Electrothermal 9100 apparatus and are uncorrected. IR spectra were recorded on a shimadzu IR-470 spectrometer. Elemental analyses for C, H, and N were performed using a Costech ECS 4010 CHNS-O analyser. Mass spectra were recorded on an Agilent Technology (HP) spectrometer operating at an ionization potential of 70 eV. The 1H NMR and 13C NMR spectra were recorded on Bruker DRX-400 Avance instruments with dimethyl sulfoxide (DMSO) as solvent. All reactions were carried out using a laboratorymicrowave oven (MicroSYNTH, Milestone Company, Italy). Thin-layer chromatography (TLC) was performed on silica-gel Polygram SILG/UV 254 plates. All reagents and solvent were purchased from Merck and Aldrich and used without further purification.
4.2. General procedure for the synthesis of novel spiro[benzo[a] pyrano-[2, 3-c]phenazine] and benzo[a]pyrano[2, 3-c]phenazine derivatives (8, 9, 10)Initially, 2-hydroxynaphthalene-1, 4-dione 1 (1 mmol) and benzene-1, 2-diamine 2 (1 mmol) were mixed at 70 ℃ (under thermal or microwave irradiation and solvent-free conditions) until in less than 5 min an orange solid of benzo[a]phenazine 3 was formed. Then, malononitrile 4 (1 mmol), cyclic ketones 5, 6 or aryl aldehydes 7 (1 mmol), and theophylline (30, 20 mol%, respectively) were added and this mixture was stirred under thermal conditions at 70 ℃ or it was irradiated in a microwave oven at 180 W for the appropriate time. The microwave was programmed to give a maximum internal temperature of 70 ℃. Upon completion of the reaction, monitored by TLC, the reaction mixture was allowed to cool to room temperature. Then, 5 mL of water was added to the mixture and filtered for separation of the crude product. The separated product was washed with water (2 × 5 mL). The solid crude product subsequently recrystallized from hot ethanol to give the pure solid 8/9/10. The analytical and spectroscopic data for selected products:
3-Amino-1', 3'-dioxo-1', 3'-dihydrospiro[benzo[a]pyrano[2, 3-c]-phenazine-1, 20-indene]-2-carbonitrile (8) : Yellow solid; yield 89% (under Δ, 0.404 g) and 93% (under MW, 0.422 g), mp > 250 ℃; IR (KBr, cm-1) : vmax 3500, 3383, 3275, 3142, 2246, 1705, 1682, 1580, 1522, 1469, 1383, 1359, 1306, 1261, 1211, 1155, 1130, 1071, 957, 840, 756; 1H NMR (400 MHz, DMSO-d6) : δ 7.21 (s, 1H, NH2), 7.80-7.96 (m, 5H, Ar-H), 8.17 (d, J = 8.0, 2H, Ar-H), 8.27-8.33 (m, 4H, Ar-H), 9.27 (t, J = 4.0, 1H, Ar-H), 11.56 (s, 1H, NH2); 13C NMR (100 MHz, DMSO-d6) : δ 59.4, 65.2, 104.0, 106.7, 121.8, 123.4, 125.30, 126.6, 128.6, 128.9, 129.3, 129.8, 130.4, 130.7, 131.4, 131.9, 139.7, 140.1, 143.2, 145.6, 145.8, 157.3, 157.4, 208.0.
3-Aminospiro[benzo[a]pyrano[2, 3-c]phenazine-1, 11'-indeno-[1, 2-b]quinoxaline]-2-carbonitrile (9a) : Yellow solid; yield 88% (under Δ, 0.463 g) and 91% (under MW, 0.479 g), mp > 350 ℃; IR (KBr, cm-1) : vmax 3450, 3395, 3230, 3050, 2195, 1662, 1616, 1589, 1510, 1467, 1395, 1367, 1326, 1287, 1219, 1168, 1132, 1060, 951, 844, 760; 1H NMR (400 MHz, DMSO-d6) : δ 6.95 (d, 1H, J = 8.4 Hz, Ar-H), 7.44 (t, 1H, J = 7.6 Hz, Ar-H), 7.52-7.59 (m, 3H, Ar-H), 7.61-7.65 (m, 2H, Ar-H), 7.69 (s, 2H, NH2), 7.78 (t, 1H, J = 8.0 Hz, Ar-H), 7.85-7.90 (m, 2H, Ar-H), 7.97-8.03 (m, 2H, Ar-H), 8.28 (d, 1H, J = 8.4 Hz, Ar-H), 8.33 (d, 1H, J = 7.6 Hz, Ar-H), 8.57 (d, 1H, J = 8.0 Hz, Ar-H), 9.02 (d, 1H, J = 8.0 Hz, Ar-H); 13C NMR (100 MHz, DMSO-d6) : δ 49.9, 58.8, 111.8, 118.5, 121.9, 123.0, 125.2, 125.5, 125.9, 127.3, 129.1, 129.2, 129.3, 129.4, 129.5, 130.1, 130.5, 130.6, 130.7, 131.1, 132.7, 137.3, 139.8, 139.9, 140.0, 140.7, 141.2, 142.1, 148.3, 153.3, 155.9, 159.9, 167.2; Anal. Calcd. for C34H18N6O: C, 77.5; H, 3.4; N, 15.9%. Found: C, 77.6; H, 3.4; N, 16.1%. MS (m/z, %) : 526 (M+, 3), 462 (13), 368 (44), 210 (48), 97 (53), 57 (100).
3-Aminospiro[benzo[a]pyrano[2, 3-c]phenazine-1, 6'-indeno-[1, 2-b]pyrido[3, 2-e]pyrazine]-2-carbonitrile (9d) : Brown solid; yield 83% (under Δ, 0.437 g) and 85% (under MW, 0.448 g), mp > 345 ℃; IR (KBr, cm-1) : vmax 3450, 3410, 3255, 3040, 2170, 1652, 1602, 1590, 1530, 1487, 1455, 1364, 1323, 1282, 1215, 1161, 1095, 1056, 946, 850, 760; 1H NMR (400 MHz, DMSO-d6) : δ 7.00 (d, 1H, J = 8.4 Hz, Ar-H), 7.50 (t, 1H, J = 8.4 Hz, Ar-H), 7.57-7.63 (m, 4H, Ar-H, NH2), 7.66-7.74 (m, 3H, Ar-H), 7.96 (t, 1H, J = 8.0 Hz, Ar-H), 8.04-8.08 (m, 2H, Ar-H), 8.33 (dd, 1H, J1 = 1.6 Hz, J2 = 8.4 Hz, Ar-H), 8.39 (d, 1H, J = 7.6 Hz, Ar-H), 8.60 (d, 1H, J = 8.4 Hz, Ar-H), 9.07-9.08 (m, 1H, Ar-H), 9.11 (d, 1H, J = 8.0 Hz, Ar-H); 13C NMR (100 MHz, DMSO-d6) : δ 45.0, 57.8, 111.0, 117.8, 122.0, 122.6, 124.4, 124.8, 125.1, 125.4, 126.8, 128.7, 129.2, 129.8, 130.3, 130.4, 130.9, 133.1, 135.8, 136.3, 137.8, 139.3, 139.5, 140.3, 147.9, 151.0, 152.9, 153.4, 158.3, 159.4, 167.6; Anal. Calcd. for C33H17N7O: C, 75.1; H, 3.2; N, 18.5%. Found: C, 75.3; H, 3.3; N, 18.5%. MS (m/z, %) : 527 (M+, 1), 471 (36), 368 (38), 252 (58), 105 (90), 57 (100).
3-Amino-1-(4-cyanophenyl) -1H-benzo[a]pyrano[2, 3-c]phenazine-2-carbonitrile (10a) : brown solid; yield 91% (under Δ, 0.387 g) and 93% (under MW, 0.395 g), mp > 330 ℃; IR (KBr, cm-1) : vmax 3350, 3310, 3185, 2420, 2225, 1658, 1624, 1591, 1548, 1464, 1397, 1375, 1345, 1292, 1239, 1178, 1115, 1078, 1048, 943, 845, 762; 1H NMR (400 MHz, DMSO-d6) : δ 5.57 (s, 1H, CH), 7.51 (s, 2H, NH2), 7.61 (d, 2H, J = 8.4 Hz, Ar-H), 7.70 (d, 2H, J = 8.4 Hz, Ar-H), 7.92-7.95 (m, 2H, Ar-H), 7.96-7.98 (m, 1H, Ar-H), 7.99-8.03 (m, 1H, Ar-H), 8.11-8.13 (m, 1H, Ar-H), 8.26-8.29 (m, 1H, Ar-H), 8.45 (d, 1H, J = 7.6 Hz, Ar-H), 9.24 (d, 1H, J = 7.2 Hz, Ar-H); 13C NMR (100 MHz, DMSO-d6) : δ 38.2, 57.3, 109.8, 112.9, 119.1, 120.3, 122.7, 125.3, 125.9, 129.1, 129.2, 129.5, 129.8, 130.6, 130.8, 131.0, 131.3, 132.8, 140.5, 140.9, 141.9, 146.9, 151.2, 160.2; Anal. Calcd. for C27H15N5O: C, 76.2; H, 3.5; N, 16.4%. Found: C, 76.2; H, 3.5; N, 16.3%. MS (m/z, %) : 425 (M+, 1), 323 (3), 180 (22), 121 (30), 101 (43), 86 (100).
AcknowledgmentsWe gratefully acknowledge financial support from the Research Council of the University of Sistan and Baluchestan and Islamic Azad University of Yazd.
| [1] | A. Döomling, I. Ugi, Multicomponent reactions with isocyanides. Angew. Chem. Int. Ed. 39 (2000) 3168–3210. DOI:10.1002/(ISSN)1521-3773 |
| [2] | A. Döomling, Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 106 (2006) 17–89. DOI:10.1021/cr0505728 |
| [3] | S.L. Schreiber, Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 287 (2000) 1964–1969. DOI:10.1126/science.287.5460.1964 |
| [4] | L.R. Wen, Z.R. Li, M. Li, H. Cao, Solvent-free and efficient synthesis of imidazo[1,2-a]pyridine derivatives via a one-pot three-component reaction. Green Chem. 14 (2012) 707–716. DOI:10.1039/c2gc16388h |
| [5] | D.M. D'souza, A. Kiel, D.P. Herten, T.J.J. Muller, Synthesis, structure and emission properties of spirocyclic benzofuranones and dihydroindolones:a domino insertion-coupling-isomerization-Diels-Alder approach to rigid fluorophores. Chem. Eur. J. 14 (2008) 529–547. DOI:10.1002/(ISSN)1521-3765 |
| [6] | D.M. D'souza, F. Rominger, T.J.J. Muller, A domino sequence consisting of insertion coupling, isomerization, and Diels-Alder steps yields highly fluorescent spirocycles. Angew. Chem. Int. Ed. 44 (2005) 153–158. DOI:10.1002/anie.200461489 |
| [7] | K. Tanaka, F. Toda, Solvent-free Organic Synthesis, WileyVCH, Weinheim, 2003. |
| [8] | J.G. Hernandez, E. Juaristi, Green synthesis of α,β-and β,β-dipeptides under solvent-free conditions. J. Org. Chem. 75 (2010) 7107–7111. DOI:10.1021/jo101159a |
| [9] | J.B. Bariwal, J.C. Trivedi, E.V. Van der Eycken, Microwave irradiation and multicomponent reactions. Top. Heterocycl. Chem. 25 (2010) 169–230. |
| [10] | D. Garella, E. Borretto, A. Di Stilo, K. Martina, G. Cravotto, P. Cintas, Microwaveassisted synthesis of N-heterocycles in medicinal chemistry. Med. Chem. Commun. 4 (2013) 1323–1343. DOI:10.1039/c3md00152k |
| [11] | N.V. De Witte, A.O. Stoppani, M. Dubin, 2-Phenyl-beta-lapachone can affect mitochondrial function by redox cycling mediated oxidation. Arch. Biochem. Biophys. 432 (2004) 129–135. DOI:10.1016/j.abb.2004.09.020 |
| [12] | A.L.B.S. Barreiros, J.M. David, J.P. David, Estresse oxidativo:relação entre geração de espécies reativas e defesa do organism. Quim. Nova 29 (2006) 113–123. DOI:10.1590/S0100-40422006000100021 |
| [13] | S.B. Ferreira, K. Salomão, F.C. da Silva, Synthesis and anti-trypanosoma cruzi activity of b-lapachone analogues. Eur. J. Med. Chem. 46 (2011) 3071–3077. DOI:10.1016/j.ejmech.2011.03.012 |
| [14] | J. Ligon, S. Dwight, P. Hammer, Natural products with antifungal activity from Pseudomonas biocontrol bacteria. Pest Manage. Sci. 56 (2000) 688–695. DOI:10.1002/(ISSN)1526-4998 |
| [15] | C. Neves-Pinto, V. Malta, M. Pinto, Structure-activity relationships for pyrido-, imidazo-, pyrazolo-, pyrazino-, and pyrrolophenazinecarboxamides as topoisomerasetargeted anticancer agents. J. Med. Chem. 45 (2002) 740–743. DOI:10.1021/jm010330+ |
| [16] | M. Makgatho, R. Anderson, J. O'sullivan, Tetramethylpiperidinesubstituted phenazines as novel anti-plasmodial agents. Drug Dev. Res. 50 (2000) 195–202. DOI:10.1002/(ISSN)1098-2299 |
| [17] | M. Muller, T. Sorrell, Inhibition of the human platelet cyclooxygenase response by the naturally occurring phenazine derivative, 1-hydroxyphenazine. Prostaglandins 50 (1995) 301–311. DOI:10.1016/0090-6980(95)00133-6 |
| [18] | G. Rewcastle, W. Denny, B. Baguley, Potential antitumor agents. 51. Synthesis and antitumor activity of substituted phenazine-1-carboxamides. J. Med. Chem. 30 (1987) 843–851. DOI:10.1021/jm00388a017 |
| [19] | G. Sakata, K. Makino, Y. Kurasama, Regent progress in the quinoxaline chemistry. Synthesis and biological activity. Heterocycles 27 (1988) 2481–2515. DOI:10.3987/REV-88-397 |
| [20] | L.E. Seitz, W.J. Suling, R.C. Reynolds, Synthesis and antimycobacterial activity of pyrazine and quinoxaline derivatives. J. Med. Chem. 45 (2002) 5604–5606. DOI:10.1021/jm020310n |
| [21] | M.M. Badran, A.A. Moneer, H.M. Refaat, A.A. El-Malah, Synthesis and antimicrobial activity of novel quinoxaline derivatives. J. Chin. Chem. Soc. 54 (2007) 469–478. DOI:10.1002/jccs.v54.2 |
| [22] | G. Aguirre, H. Cerecetto, R. Di Maio, Quinoxaline N,N'-dioxide derivatives and related compounds as growth inhibitors of Trypanosoma cruzi. Structure-activity relationships. Bioorg. Med. Chem. Lett. 14 (2004) 3835–3839. DOI:10.1016/j.bmcl.2004.04.088 |
| [23] | R. Sarges, H.R. Howard, R.G. Browne, 4-Amino[1,2,4] triazolo[4,3-a] quinoxalines. A novel class of potent adenosine receptor antagonists and potential rapid-onset antidepressants. J. Med. Chem. 33 (1990) 2240–2254. DOI:10.1021/jm00170a031 |
| [24] | S.T. Hazeldine, L. Polin, J. Kushner, Synthesis and biological evaluation of some bioisosteres and congeners of the antitumor agent, 2-(4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy)propionic acid (XK469). J. Med. Chem. 45 (2002) 3130–3137. DOI:10.1021/jm0200097 |
| [25] | L.F. Tietze, G. Kettschau, Hetero Diels-Alder reactions in organic chemistry. Top. Curr. Chem. 189 (1997) 1–120. DOI:10.1007/BFb0119239 |
| [26] | G.P. Ellis, in:A. Weissberger, E.C. Taylor (Eds.), The Chemistry of Heterocyclic Compounds Chromenes, Chromanes and Chromones, John Wiley, New York, NY, 1977, pp. 11-139(Chapter II). |
| [27] | E.A. Hafez, M.H. Elnagdi, A.G.A. Elagemey, F.M.A.A. El-Taweel, Nitriles in heterocyclic synthesis:novel synthesis of benzo. Heterocycles 26 (1987) 903–907. DOI:10.3987/R-1987-04-0903 |
| [28] | T. Narender, Shweta, S. Gupta, A convenient and biogenetic type synthesis of few naturally occurring chromeno dihydrochalcones and their in vitro anti leishmanial activity. Bioorg. Med. Chem. Lett. 14 (2004) 3913–3916. DOI:10.1016/j.bmcl.2004.05.071 |
| [29] | K. Asres, A. Seyoum, C. Veeresham, F. Bucar, S. Gibbons, Naturally derived antiHIV agents. Phytother. Res. 19 (2005) 557–581. DOI:10.1002/(ISSN)1099-1573 |
| [30] | A.J. Johnson, R.A. Kumar, S.A. Rasheed, Antipyretic analgesic, antiinflammatory and antioxidant activities of two major chromenes from melicope lunu-ankenda. J. Ethnopharmacol. 130 (2010) 267–271. DOI:10.1016/j.jep.2010.05.003 |
| [31] | H. Quan-Bin, Y. Nian-Yun, T. Hong-Lei, Xanthones with growth inhibition against heLa cells from garcinia xipshuanbannaensis. Phytochemistry 69 (2008) 2187–2192. DOI:10.1016/j.phytochem.2008.05.019 |
| [32] | J. Skommer, D. Wlodkowic, M. Matto, M. Eray, HA14-1 a small molecule Bcl-2 antagonist, induces apoptosis and modulates action of selected anticancer drugs in follicular lymphoma B cells. J. Leuk. Res. 30 (2006) 322–331. DOI:10.1016/j.leukres.2005.08.022 |
| [33] | C. Bruhlmann, F. Ooms, P.A. Carrupt, Coumarins derivatives as dual inhibitors of acetylcholinesterase and monoamine oxidase. J. Med. Chem. 44 (2001) 3195–3198. DOI:10.1021/jm010894d |
| [34] | S.R. Kesten, T.G. Heffner, S.J. Johnson, Design synthesis, and evaluation of chromen-2-ones as potent and selective human dopamine D4 antagonists. J. Med. Chem. 42 (1999) 3718–3725. DOI:10.1021/jm990266k |
| [35] | R. Rios, Enantioselective methodologies for the synthesis of spiro compounds. Chem. Soc. Rev. 41 (2012) 1060–1074. DOI:10.1039/C1CS15156H |
| [36] | T. Jin, M. Himuro, Y. Yamamoto, Triflic acid catalyzed synthesis of spirocycles via acetylene cations. Angew. Chem. Int. Ed. 48 (2009) 5893–5896. DOI:10.1002/anie.v48:32 |
| [37] | H. Diirr, R. Gleiter, Spiroconjugation. Angew. Chem. Int. Ed. 17 (1978) 559–569. DOI:10.1002/anie.197805591 |
| [38] | J. Sun, Y.J. Xie, C.G. Yan, Construction of dispirocyclopentanebisoxindoles via selfdomino michael-aldol reactions of 3-phenacylideneoxindoles. J. Org. Chem. 78 (2013) 8354–8365. DOI:10.1021/jo4010603 |
| [39] | H. Chen, D. Shi, Efficient one-pot synthesis of novel spirooxindole derivatives via three-component reaction in aqueous medium. J. Comb. Chem. 12 (2010) 571–576. DOI:10.1021/cc100056p |
| [40] | J.W. Daly, Caffeine analogs:biomedical impact. Cell. Mol. Life Sci. 64 (2007) 2153–2169. DOI:10.1007/s00018-007-7051-9 |
| [41] | L. Maia, A. De Mendonga, Does caffeine intake protect from Alzheimer's disease?. Eur. J. Neurol. 9 (2002) 377–382. DOI:10.1046/j.1468-1331.2002.00421.x |
| [42] | R.J. Stine, R.H. Marcus, C.A. Parvin, Aminophylline loading in asthmatic patients:a protocol trial. Ann. Emerg. Med. 18 (1989) 640–646. DOI:10.1016/S0196-0644(89)80518-9 |
| [43] | P. Cui, T.L. Macdonald, M. Chen, J.L. Nadler, Synthesis and biological evaluation of lisofylline (LSF) analogs as a potential treatment for type 1 diabetes. Bioorg. Med. Chem. Lett. 16 (2006) 3401–3405. DOI:10.1016/j.bmcl.2006.04.036 |
| [44] | B. Song, T. Xiao, X. Qi, Design and synthesis of 8-substituted benzamidophenylxanthine derivatives as MAO-B inhibitors. Bioorg. Med. Chem. Lett. 22 (2012) 1739–1742. DOI:10.1016/j.bmcl.2011.12.094 |
| [45] | A.C. Roy, F.A. Lunn, S.L. Bearne, Inhibition of CTP synthase from Escherichia coli by xanthines and uric acids. Bioorg. Med. Chem. Lett. 20 (2010) 141–144. DOI:10.1016/j.bmcl.2009.11.017 |
| [46] | A.J. Szentmiklosi, A. Cseppento, R. Gesztelyi, Xanthine derivatives in the heart:blessed or cursed?. Curr. Med. Chem. 18 (2011) 3695–3706. DOI:10.2174/092986711796642391 |
| [47] | M. Kangani, M.T. Maghsoodlou, N. Hazeri, Vitamin B12:an efficient type catalyst for the one-pot synthesis of 3,4,5-trisubstituted furan-2(5H)-ones and N-aryl-3-aminodihydropyrrol-2-one-4-carboxylates. Chin. Chem. Lett. 27 (2016) 66–70. DOI:10.1016/j.cclet.2015.07.025 |
| [48] | R. Doostmohammadi, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, An efficient one-pot multi-component synthesis of 3,4,5-substituted furan-2(5H)-onescatalyzedbytetra-n-butylammoniumbisulfate. Chin. Chem.Lett. 24 (2013) 901–903. DOI:10.1016/j.cclet.2013.06.004 |
| [49] | S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, A.C. Willis, One-pot five-component synthesis of highly functionalized piperidines using oxalic acid dihydrate as a homogenous catalyst. Chin. Chem. Lett. 23 (2012) 569–572. DOI:10.1016/j.cclet.2012.03.008 |
| [50] | P. Dastoorani, M.T. Maghsoodlou, M.A. Khalilzadeh, E. Sarina, Synthesis of new dibenzofuran derivatives via Diels-Alder reaction of euparin with activated acetylenic esters. Tetrahedron Lett. 57 (2016) 314–316. DOI:10.1016/j.tetlet.2015.12.021 |
| [51] | S. Salahi, M.T. Maghsoodlou, N. Hazeri, An efficient green synthesis of dispirohydroquinolines via a diastereoselective one-pot eight-component reaction. Chin. J. Catal. 36 (2015) 1023–1028. DOI:10.1016/S1872-2067(15)60846-4 |
| [52] | A. Yazdani Elah Abadi, M.T. Maghsoodlou, R. Heydari, R. Mohebat, An efficient four-component domino protocol for the rapid and green synthesis of functionalized benzo. Res. Chem. Intermed. 42 (2016) 1227–1235. DOI:10.1007/s11164-015-2083-5 |
| [53] | B.D. Pearson, R.A. Mitsch, N.H. Cromwell, Indenoquinolines. III. Derivatives of 11H-indeno[1,2-b]quinoxalineand related indenoquinolines. J. Org. Chem. 27 (1962) 1674–1678. DOI:10.1021/jo01052a046 |
| [54] | A. Hasaninejad, S. Firoozi, F. Mandegani, An efficient synthesis of novel spiro[benzo[c]pyrano[3,2-a]phenazines] via domino multi-component reactions using L-proline as a bifunctional organocatalyst. Tetrahedron Lett. 54 (2013) 2791–2794. DOI:10.1016/j.tetlet.2013.03.073 |
| [55] | S.L. Wang, F.Y. Wu, C. Cheng, Multicomponent synthesis of polysubstituted benzo[a]pyrano[2,3-c]phenazine derivatives under microwave heating. ACS Comb. Sci. 13 (2011) 135–139. DOI:10.1021/co1000376 |
 2017, Vol. 28
2017, Vol. 28