Multicomponent reactions (MCRs) [1] are progressive transformations [2] wherein the target is obtained from three or more different substrates by reacting in a well-defined manner. These coherent reactions are environmentally benign, versatile and atom economic. They offer significant advantages over conventional linear-step syntheses by reducing time, save money and rawmaterials; thus minimize the formation of waste [3]. MCRs minimize the feeding of auxiliary substances and energy, which can result in significant economic and environmental benefits. The outcome of MCRs has proved to be very influential and efficient bond-forming tools in organic, combinatorial and medicinal chemistry [4].
Pyridine containing scaffolds are ubiquitous heterocycles of paramount significance to organic and medicinal research since decades [5]. Amongst the vast scaffold, functionalized pyrazolo[3, 4-b]pyridine system are a promising class of compounds with intriguing biological properties [6] such as antitubercular, antibacterial, antioxidant, antiviral [7], antimalarial [8], antiinflammatory [9] and antileishmanial [10] activities. They are templates for drug discovery [11] and their applicability in the treatment of bipolar disorder, Alzheimer’s disease, schizophrenia, cancer, depression, diabetes, and dementia is well known [12]. These derivatives are potent inhibitors of glycogen synthase kinase-3 (GSK-3) [13], adenosine [14], blood platelet aggregation [15], HIV reverse transcriptase [16], protein kinase [17] and cyclin dependent kinase 1 (CDK1) [18]. In addition, they are often used as luminophores, fluorescence standards in organic light-emitting diodes [19] and corrosion inhibitors for metals and alloys [20].
Owing to the aforementioned facts, we were prompted to design these pharmacologically interesting class of motifs. A detailed literature survey reveals that, many efforts were put in by chemists towards their synthesis [21], but all these synthetic methodologies have one or more shortcomings such as low yield, drastic conditions, multistep reactions, cumbersome work up, use of toxic reagents and solvents, longer reaction duration and occurrence of several side reactions. Thus, the quest for green and efficient routes for the facile access to this fused heterocycle is highly desirable.
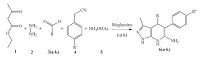
Today, considerable attention is directed towards the employment of harmless, safe, inexpensive and environmentally benign reagents like biodegradable materials as they possess high catalytic efficiency, nontoxic nature and recyclable properties [22]. Meglumine, an organic compound with the molecular formula C7H17NO5 and IUPAC name (2R, 3R, 4R, 5S) -6- (methylamino) hexane-1, 2, 3, 4, 5-pentol is an amino sugar derived from sorbitol. It offers many advantages due to noncorrosive nature, ready and commercial availability; it is inert, biodegradable, reusable and stable to moisture and air. It has served as an efficient catalyst in various organic transformations [23]. In view of the above considerations and our endeavours in developing novel onepot multicomponent reactions [24], we, herein, report a meglumine- catalyzed one-pot five-component reaction of substituted aromatic aldehydes, hydrazine, ethyl acetoacetate, substituted phenylacetonitriles and ammonium acetate for the synthesis of novel 4, 7-dihydro-1H-pyrazolo[3, 4-b]pyridin-6-amine derivatives in ethanol (EtOH) at room temperature as shown in Scheme 1.
2. Results and discussion
As a part of our growing interest in synthesizing heterocycles, we decided to carry out a systematic research study on the preparation of the target heterocyclic compounds under significantly milder conditions.
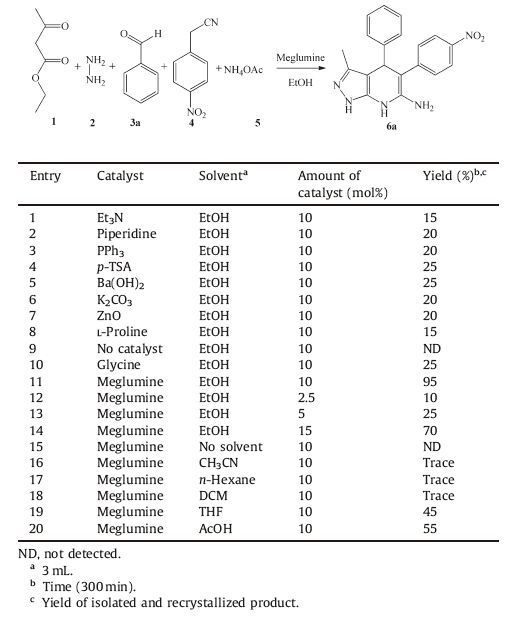
In order to optimize the reaction conditions, hydrazine, ethyl acetoacetate, benzaldehyde, 4-nitrophenylacetonitrile and ammonium acetate were considered as model substrates. In our initial study, to investigate the effect of the catalyst on the reaction rate and yield of the product, we first carried out the reaction in the presence of different catalysts and found that, the desired product was obtained to only about 15% with Et3N (Table 1, entry 1). Inadequate yields were obtained when piperidine, PPh3, p-TSA, Ba (OH)2, K2CO3, L-proline, glycine were used (Table 1, entries 2-8, 10). The reaction did not afford the desired product in absence of catalyst (Table 1, entry 9). To our pleasure, reaction with meglumine as catalyst afforded 6a in 95% yield (Table 1, entry 11).
|
|
Table 1 Optimization of conditions for the synthesis of 6a. |
Meglumine contains an ammonium ion, and an alkoxy group which can activate the nucleophilic as well as electrophilic sites of the ethyl acetoacetate and 4-nitrophenylacetonitrile through hydrogen bonding and by the donation of the lone pair of electrons present on the oxygen atom respectively. Hence, in our further studies, the reactions in the presence of meglumine catalyst at room temperature under mechanical stirring were considered as it was found to be a better catalyst when compared with the other catalysts (Table 1).
To optimize the amount of the catalyst, the model reaction was subjected to different quantities of the catalyst, and it was found that, the reaction did not produce desired yields with 2.5, 5, 15 mol% of the catalyst (Table 1, entries 12-14).
To analyze the effect of the solvent, various solvents such as CH3CN, n-hexane, DCM, THF, AcOH and EtOH were screened and was found that, under solvent-free condition the product was not detected (Table 1, entry 15). The use of CH3CN, n-hexane and DCM yielded trace quantities of the product (Table 1, entries 16-18) and poor yields were obtained in THF and AcOH (Table 1, entries 19, 20). Hence, it is clear that, the best solvent is EtOH (Table 1, entry 11), and has a significant effect in increasing the yield.
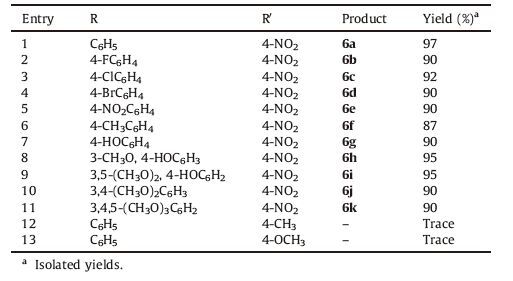
To broaden the scope of the designed protocol, we subjected hydrazine, ethyl acetoacetate, various aryl aldehydes bearing electron-donating or electron-withdrawing groups, substituted phenylacetonitriles (4-NO2, 4-CH3, 4-OCH3) and ammonium acetate to our optimized conditions and the results are presented in Table 2. Gratifyingly, in all the cases these five components congregated successfully into the corresponding 4, 7-dihydro-1H-pyrazolo[3, 4-b]pyridin-6-amine derivatives in excellent yields (Table 2, entries 1-11). It was also noted that, the electronic effects of the substituents on the aryl aldehydes did not have much impact on the product yields. To our dismay, the protocol could not be applicable for aliphatic aldehydes such as formaldehyde and acetaldehyde and also, it was found that, complex mixture of products were observed when electron donating groups were present on the phenylacetonitrile ring (Table 2, entries 12, 13). All the data are deposited in Supporting information.
|
|
Table 2 Synthesis of novel 4,7-dihydro-1H-pyrazolo[3,4-b]pyridin-6-amine derivatives. |
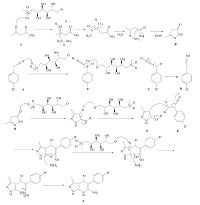
A plausible mechanism for the formation of the substituted 4, 7- dihydro-1H-pyrazolo[3, 4-b]pyridin-6-amine catalyzed by meglumine is envisaged. The initial step may involve the protonation of ethyl acetoacetate by the catalyst to give A, followed by intermolecular attack by hydrazine hydrate on A to yield B. The aryl acetonitrile (4) may then get activated by meglumine to give the intermediate C which may get stabilized by the nitro group of the aryl ring and further isomerize to give the adduct D, which may undergo a Knoevenagel condensation resulting in the formation of E. The enolic form of B which is F, may react with E by a Michael addition and an intramolecular nucleophilic attack by NH3 (generated from ammonium acetate) on the carbonyl carbon may result in the formation of the cyclized product 4, 7-dihydro- 1H-pyrazolo[3, 4-b]pyridin-6-amine (6) as depicted in Scheme 2.
|
Download:
|
| Scheme 2. A plausible mechanism for the formation of 4,7-dihydro-1H-pyrazolo[3, 4-b]pyridin-6-amines. | |
3. Conclusions
As described above, we have successfully designed, an elegant, efficient, easy and direct procedure and synthesized, eleven novel 4, 7-dihydro-1H-pyrazolo[3, 4-b]pyridin-6-amine derivatives at room temperature using hydrazine, ethyl acetoacetate, aryl aldehydes, substituted phenylacetonitriles, ammonium acetate in EtOH using meglumine as a catalyst. The effect of EtOH as solvent and the use of meglumine as a catalyst is significant in the preparation of 4, 7-dihydro-1H-pyrazolo[3, 4-b]pyridin-6-amines in excellent yields under the aspect of environmentally benign processes. This approach provides one of the easiest pathways for accessing this class of treasured compounds from effortlessly available starting materials, and a wide range of multi-substituted 4, 7-dihydro-1H-pyrazolo[3, 4-b]pyridin-6-amines can be prepared accordingly for the construction of a chemical library. This methodology has numerous and significant advantages such as use of simple procedure, milder conditions, shorter reaction duration and easier work-up procedure.
4. Experimental 4.1. Material and methodsReagents and solvents of commercial grade were used without further purification except liquid aldehydes which were distilled before use. The progress of the reactions and the purity of products were assessed by TLC [analytical silica gel plates (Merck60 F254) ]. Melting points were determined on a RAAGA, Indian make apparatus. The FT-IR (ATR) analyses were carried out on Cary 630 FT-IR spectrophotometer equipped with diffuse reflectance sampling interface (Agilent Technologies, USA). 1H NMR and 13C NMR spectra were recorded on an Advanced Bruker instrument operating at 400 MHz and 100 MHz in DMSO-d6 respectively. Chemical shifts are reported in δ (ppm). HRMS data were obtained on a Varian IonSpec QFT-MS spectrometer with the technique of electrospray ionization. Elemental analysis was carried out using vario MICRO CHN analyser.
4.2. Typical procedure for the synthesis of 4, 7-dihydro-1Hpyrazolo[3, 4-b]pyridin-6-aminesIn a dry 50 mL RB flask, a mixture of hydrazine (1, 1 mmol), ethyl acetoacetate (2, 1 mmol), aldehyde (3, 1 mmol), 4-nitrophenylacetonitrile (4, 1 mmol), ammonium acetate (5, 2 mmol) and EtOH (3 mL) were mixed along with meglumine (10 mol%) and then stirred at room temperature for 300 min. After completion of the reaction (TLC), the reaction mixture was quenched with crushed ice and the precipitate thus separated was filtered, washed with 10% EtOAc in light petrol to remove unreacted substrates, dried and recrystallized from ethanol.
AcknowledgmentThe authors gratefully acknowledge the financial assistance by the VGST, Dept. of IT, BT and Science & Technology, Government of Karnataka for the CESEM Award Grant No. 24 (2010-2011).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.09.013.
| [1] | (a) J. Zhu, H. Bienaymé, Multicomponent Reactions, John Wiley & Sons, 2006; (b) I. Ugi, Recent progress in the chemistry of multicomponent reactions, Pure Appl. Chem. 73(2001) 187-191; (c) V. Nair, C. Rajesh, A. Vinod, et al., Strategies for heterocyclic construction via novel multicomponent reactions based on isocyanides and nucleophilic carbenes, Acc. Chem. Res. 36(2003) 899-907; (d) D.J. Ramón, M. Yus, Asymmetric multicomponent reactions (AMCRs):the new frontier, Angew. Chem. Int. Ed. (2005) 1602-1634. |
| [2] | (a) S. Benetti, R. Romagnoli, C. De Risi, G. Spalluto, V. Zanirato, Mastering b-keto esters, Chem. Rev. 95(1995) 1065-1114; (b) P. Langer, Regio-and diastereoselective cyclization reactions of free and masked 1,3-dicarbonyl dianions with 1,2-dielectrophiles, Chem. Eur. J. 7(2001) 3858-3866; (c) C. Simon, T. Constantieux, J. Rodriguez, Utilisation of 1,3-dicarbonyl derivatives in multicomponent reactions, Eur. J. Org. Chem. 2004(2004) 4957-4980. |
| [3] | (a) B. Ganem, Strategies for innovation in multicomponent reaction design, Acc. Chem. Res. 42(2009) 463-472; (b) A. Dömling, Recent developments in isocyanide based multicomponent reactions in applied chemistry, Chem. Rev. 106(2006) 17-89. |
| [4] | (a) G.L. Adams, P.J. Carroll, A.B. Smith III, Access to the akuammiline family of alkaloids:totalsynthesisof(+)-scholarisineA,J.Am.Chem.Soc.135(2012)519-528; (b) Y. Inaba, T. Hasuda, Y. Hitotsuyanagi, et al., Abietane diterpenoids and a sesquiterpene pyridine alkaloid from Euonymus lutchuensis, J. Nat. Prod. 76(2013) 1085-1090; (c) J.A. Bull, J.J. Mousseau, G. Pelletier, A.B. Charette, Synthesis of pyridine and dihydropyridine derivatives by regio-and stereoselective addition to N-activated pyridines, Chem. Rev. 112(2012) 2642-2713; (d) P.W. Ondachi, D.L. Comins, Synthesis of fused-ring nicotine derivatives from (S) nicotine, J. Org. Chem. 75(2010) 1706-1716; (e) A.D. Melhado, W.E. Brenzovich Jr., A.D. Lackner, F.D. Toste, Gold-catalyzed threecomponentcoupling:oxidative oxyarylation of alkenes, J. Am. Chem. Soc.132(2010) 8885-8887; (f) H.E. Montenegro, P. Ramírez-López, M.C. de la Torre, M. Asenjo, M.A. Sierra, Two versatile and parallel approaches to highly symmetrical open and closed natural product-based structures, Chem. Eur. J. 16(2010) 3798-3814; (g) S.A. Snyder, S.P. Breazzano, A.G. Ross, Y. Lin, A.L. Zografos, Total synthesis of diverse carbogenic complexity within the resveratrol class from a common building block, J. Am. Chem. Soc. 131(2009) 1753-1765. |
| [5] | (a) K. Shekarrao, P.P. Kaishap, V. Saddanapu, et al., Microwave-assisted palladium mediated efficient synthesis of pyrazolo[3,4-b]pyridines, pyrazolo[3,4-b]quinolines, pyrazolo[1,5-a]pyrimidines and pyrazolo[1,5-a]quinazolines, RSC Adv. 4(2014) 24001-24006; (b) C. Kurumurthy, B. Veeraswamy, P.S. Rao, et al., Synthesis of novel 1,2,3-triazole tagged pyrazolo[3,4-b]pyridine derivatives and their cytotoxic activity, Bioorg. Med. Chem. Lett. 24(2014) 746-749; (c) D.K. Dodiya, A.R. Trivedi, V.B. Kataria, V.H. Shah, Advances in the synthesis of pyrazolo[3,4-b]pyridines, Curr. Org. Chem. 16(2012) 400-417; (d) M. El-Borai, H. Rizk, M. Abd-Aal, I. El-Deeb, Synthesis of pyrazolo[3,4-b]pyridines under microwave irradiation in multi-component reactions and their antitumor and antimicrobial activities-part 1, Eur. J. Med. Chem. 48(2012) 92-96; (e) Y. Hao, X.P. Xu, T. Chen, L.L. Zhao, S.J. Ji, Multicomponent approaches to 8-carboxylnaphthyl-functionalized pyrazolo[3,4-b]pyridinederivatives,Org.Biomol. Chem. 10(2012) 724-728; (f) D.B. Kendre, R.B. Toche, M.N. Jachak, Synthesis of pyrazolo[3,4-b]pyridines and attachment of amino acids and carbohydrate as linkers, J. Heterocycl. Chem. 45(2008) 1281-1286. |
| [6] | (a) S. Wenglowsky, K.A. Ahrendt, A.J. Buckmelter, et al., Pyrazolopyridine inhibitors of B-Raf V600E. Part 2:structure-activity relationships, Bioorg. Med. Chem. Lett. 21(2011) 5533-5537; (b) J. Quiroga, J. Portilla, B. Insuasty, et al., Solvent-free microwave synthesis of bis-pyrazolo[3,4-b:4',3'-e]-pyridines and study of their antifungal properties, J. Heterocycl. Chem. 42(2005) 61-66; (c) R.N. Misra, D.B. Rawlins, H.Y. Xiao, et al., 1H-Pyrazolo[3,4-b]pyridine inhibitors of cyclin-dependent kinases, Bioorg. Med. Chem. Lett. 13(2003) 1133-1136; (d) A. Straub, J. Benet-Buckholz, R. Fröde, et al., Metabolites of orally active NOindependent pyrazolopyridine stimulators of soluble guanylate cyclase, Bioorg. Med. Chem. Lett. 10(2002) 1711-1717. |
| [7] | E. De Clercq, Recent highlights in the development of new antiviral drugs. Curr. Opin. Microbiol. 8 (2005) 552–560. DOI:10.1016/j.mib.2005.08.010 |
| [8] | R.G. Stein, J.H. Biel, T. Singh, Antimalarials. 4-Substituted 1H-pyrazolo[3,4-b]quinolines. J. Med. Chem. 13 (1970) 153–155. DOI:10.1021/jm00295a049 |
| [9] | T. Sado, A. Inoue, Preparation of 1H-pyrazolo[3,4-b]pyrazines as blood platelet. Chem. Abstr. (1990) 78422k. |
| [10] | H. de Mello, A. Echevarria, A.M. Bernardino, M. Canto-Cavalheiro, L.L. Leon, Antileishmanial pyrazolopyridine derivatives:synthesis and structure-activity relationship analysis. J. Med. Chem. 47 (2004) 5427–5432. DOI:10.1021/jm0401006 |
| [11] | (a) S.A. Thompson, P.B. Wingrove, L. Connelly, P.J. Whiting, K.A. Wafford, Tracazolate reveals a novel type of allosteric interaction with recombinant γ-aminobutyric acid A receptors, Mol. Pharmacol., 2002,61: 861-869;(b) J.P. Stasch, E.M. Becker, C. Alonso-Alija, et al., NO-independent regulatory site on soluble guanylate cyclase, Nature, 2001,410: 212-215;(c) J. Zezula, A. Slany, W. Sieghart, Interaction of allosteric ligands with GABA A receptors containing one, two, or three different subunits. Eur. J. Pharmacol. 301 (1996) 207–214. DOI:10.1016/0014-2999(96)00066-0 |
| [12] | (a) T. Fong, S. Heymsfield, Cannabinoid-1 receptor inverse agonists:current understanding of mechanism of action and unanswered questions, Int. J. Obes., 2009,33: 947-955;(b) C.S. Stika, G.A. Gross, G. Leguizamon, et al., A prospective randomized safety trial of celecoxib for treatment of preterm labor, Am. J. Obstet. Gynecol., 2002,187: 653-660;(c) K. Dilger, C. Herrlinger, J. Peters, et al., Effects of celecoxib and diclofenac on blood pressure, renal function, and vasoactive prostanoids in young and elderly subjects, J. Clin. Pharmacol., 2002,42: 985-994;(d) P. Chavatte, S. Yous, C. Marot, N. Baurin, D. Lesieur, Three-dimensional quantitative structure-activity relationships of cyclo-oxygenase-2(COX-2) inhibitors:a comparative molecular field analysis. J. Med. Chem. 44 (2001) 3223–3230. DOI:10.1021/jm0101343 |
| [13] | J. Witherington, V. Bordas, A. Gaiba, 6-Aryl-pyrazolo[3,4-b]pyridines:potent inhibitors of glycogen synthase kinase-3(GSK-3). Bioorg. Med. Chem. Lett. 13 (2003) 3055–3057. DOI:10.1016/S0960-894X(03)00645-0 |
| [14] | A. Akahane, A. Tanaka, WO 2002100864 A1, 2003, Chem. Abstr. (2003) 24732a. |
| [15] | K. Imaizumi, T. Sado, Jpn Kokai Tokkyo Koho JP 0680570[9480570] (Cl. A61K31/495) (22 Mar. 1994), Chem. Abstr. (1994) 91797w. |
| [16] | S.A. Saggar, J.T. Sisko, T.J. Tucker, HIV reverse transcriptase inhibitors. Google Patents (2010) . |
| [17] | G. Chiu, S. Li, P. Connolly, 3-(Benzimidazolyl)-pyrazolopyridines as protein kinase inhibitors, their preparation, pharmaceutical compositions, and use in therapy. PCT Int. Appl. WO (2006) 2006130673. |
| [18] | S. Huang, R. Lin, Y. Yu, Synthesis of 3-(1H-benzimidazol-2-yl)-5-isoquinolin-4-ylpyrazolo[1,2-b]pyridine, a potent cyclin dependent kinase 1(CDK1) inhibitor. Bioorg. Med. Chem. Lett. 17 (2007) 1243–1245. DOI:10.1016/j.bmcl.2006.12.031 |
| [19] | D.B. Kendre, R.B. Toche, M.N. Jachak, Synthesis of novel dipyrazolo[3,4-b:3,4-d]pyridines and study of their fluorescence behavior. Tetrahedron 63 (2007) 11000–11004. DOI:10.1016/j.tet.2007.08.052 |
| [20] | M.A. El Mhammedi, A. Chtaini, Investigation of the inhibitive effect of pyrazolo[3,4-b]pyridine on corrosion of stainless steel in 1 M HCl solutions. Leonardo Electron. J. Pract. Technol. (2007) 37–46. |
| [21] | (a) K. Balamurugan, S. Perumal, J.C. Menéndez, New four-component reactions in water:a convergent approach to the metal-free synthesis of spiro[indoline/acenaphthylene-3,4'-pyrazolo[3,4-b]pyridine derivatives, Tetrahedron 67(2011) 3201-3208; (b) A. Soliman, Synthesis of novel pyrazolopyridine and pyridopyrimidine derivatives, J. Heterocycl. Chem. 48(2011) 592-596; (c) A. Rahmati, Synthesis of 4-aryl-3-methyl-6-oxo-4,5,6,7-tetrahydro-2H-pyrazolo[3,4-b]pyridine-5-carbonitrile via a one-pot, three-component reaction, Tetrahedron Lett. 51(2010) 2967-2970; (d) A. Díaz-Ortiz, J.R. Carrillo, F.P. Cossío, et al., Synthesis of pyrazolo[3,4-b]pyridines by cycloaddition reactions under microwave irradiation, Tetrahedron 56(2000) 1569-1577. |
| [22] | (a) C.S. Hege, S.M. Schiller, Non-toxic catalysts for ring-opening polymerizations of biodegradable polymers at room temperature for biohybrid materials, Green Chem. 16(2014) 1410-1416; (b) P.K. Sahu, P.K. Sahu, S.K. Gupta, D.D. Agarwal, Chitosan:an efficient, reusable, and biodegradable catalyst for green synthesis of heterocycles, Ind. Eng. Chem. Res. 53(2014) 2085-2091; (c) I. Siddiqui, P. Rai, A. Srivastava, Chitosan:an efficient promoter for the synthesis of 2-aminopyrimidine-5-carbonitrile derivatives in solvent free conditions, New J. Chem. 38(2014) 3791-3795; (d) E. Mosaddegh, A. Hassankhani, Application and characterization of eggshell as a new biodegradable and heterogeneous catalyst in green synthesis of 7,8-dihydro-4H-chromen-5(6H)-ones, Catal. Commun. 33(2013) 70-75; (e) Z.N. Siddiqui, K. Khan, Friedlander synthesis of novel benzopyranopyridines in the presence of chitosan as heterogeneous, efficient and biodegradable catalyst under solvent-free conditions, New J. Chem. 37(2013) 1595-1602; (f) G.Y. Sun, J.T. Hou, J.J. Dou, et al., Xanthan sulfuric acid as an efficient biodegradable and recyclable catalyst for the one-pot synthesis of α-amino phosphonates, J. Chin. Chem. Soc. 57(2010) 1315-1320. |
| [23] | (a) R.Y. Guo, Z.M. An, L.P. Mo, et al., Meglumine:a novel and efficient catalyst for one-pot, three-component combinatorial synthesis of functionalized 2-amino-4H-pyrans, ACS Comb. Sci. 15(2013) 557-563; (b) J. Yang, H. Li, M. Li, J. Peng, Y. Gu, Multicomponent reactions of β-ketosulfones and formaldehyde in a bio-based binary mixture solvent system composed of meglumine and gluconic acid aqueous solution, Adv. Synth. Catal. 354(2012) 688-700. |
| [24] | (a) S. Tabassum, S. Govindaraju, R.R. Khan, M.A. Pasha, Ultrasound mediated, iodine catalyzed green synthesis of novel 2-amino-3-cyano-4H-pyran derivatives, Ultrason. Sonochem. 24(2015) 1-7; (b) S. Tabassum, S. Govindaraju, R.R. Khan, M.A. Pasha, Ultrasound mediated, green innovation for the synthesis of polysubstituted 1,4-dihydropyridines, RSC Adv. 6(2016) 29802-29810; (c) K.B. Ramesh, M.A. Pasha, Study on one-pot four-component synthesis of 9-aryl-hexahydro-acridine-1,8-diones using SiO2-I as a new heterogeneous catalyst and their anticancer activity, Bioorg. Med. Chem. Lett. 24(2014) 3907-3913; (d) S.H.S.Azzam,A.Siddekha,M.A.Pasha,One-potfour-componentsynthesisof some novel octahydroquinolindiones using ZnO as an efficient catalyst in water, Tetrahedron Lett. 53(2012) 6306-6309. |
 2017, Vol. 28
2017, Vol. 28