Organic pollutants not only put a huge pressure on the environment but also represent a serious threat to human health . Among numerous organic pollutants, nitrophenols have been listed in the top 114 by the United States Environmental Protection Agency [2]. Due to their high stability and large solubility, nitrophenols can remain in rivers and soil for a long time without degradation which causes harmful effects to agricultural plants, animals and human beings [3, 4]. It is well known that catalytic reduction of 4-nitrophenol (4-NP) to 4- aminophenol (4-AP) is the most efficient and eco-friendly approach to convert harmful organic waste into valuable intermediate chemicals [5]. 4-AP is an important category of intermediate for synthesizing analgesic antipyretic drugs and an ideal reducing agent for photographic developers, corrosion inhibitor, anticorrosion-lubricant, and the dye industry [6]. Therefore, development of low-cost and highly active catalysts for nitro-compound reduction is highly desirable considering the environmental and economic benefits.
Due to their unprecedented activities, noble metal nanoparticles have attracted significant interest in catalysis science and engineering [7-11]. Some of these nanocatalysts were found effective for environmental remediation applications, particularly in catalytic decomposition or conversion of organic pollutants such as nitrophenols, dyes, etc. [11] Compared with other noble metals such as platinum, gold and palladium, silver is relatively inexpensive and has found wide applications in catalytic reduction of 4-NP [2, 12-15]. Ag2S has not been studied as extensively as Ag, however we find that the catalytic activity toward the reduction of 4-NP for nanostructured Ag2S appear to be much higher than those of comparable Ag systems recently.
Graphene, a new emerging star of nanocarbon family, has attracted wide and intense scientific interest because it comprises one atom-thick, two-dimensional (2D) atomic layer of sp2-hybridized carbon atoms [16, 17]. Moreover, graphene features high specific surface area, good transparency, super stability and excellent electrical conductivity and has been widely used to support nanoparticles for improved catalytic performance [18, 19]. Here, we successfully synthesized the Ag2S nanoparticles on reduced graphene oxide (Ag2S NPs/RGO) nanocomposites through a simple hydrothermal sulfurization reaction with Ag NPs/RGO nanocomposites as precursors. The catalytic behavior of Ag2S/RGO nanocomposites was investigated and it was found that Ag2S NPs/ RGO nanocomposites can work as extraordinary catalysts for the reduction of 4-NP to 4-AP in the presence of NaBH4 as a reducing agent. It only takes 5 min for Ag2S NPs/RGO to reduce 98% of 4-NP, and the rate constant of the composites is almost 13 times higher than that of Ag NPs/RGO composites. To the best of our knowledge, Ag2S NPs/RGO nanocomposites have never been examined as efficient catalyst for the reduction of 4-NP.
2. Results and discussionThe phases of Ag NPs/RGO and Ag2S NPs/RGO nanocomposites were investigated by X-ray diffraction (XRD), as shown in Fig. 1. The four strong peaks at 2θ values at around 38.1°, 44.4°, 64.4° and 77.5° in Ag NPs/RGO spectra can be respectively indexed to (111), (200), (220) and (311) crystal planes of Ag with the cubic structure (PDF#65-2871). As for Ag2S NPs/RGO composites, all the peaks match well with the standard diffraction pattern of Ag2S (PDF#65- 2356). The strong and sharp diffraction peaks showed that the obtained products were well crystallized and no peak shift was observed when GO was introduced into the Ag and Ag2S NPs, meaning that the formation of the composites has a negligible effect on the crystal phase of Ag and Ag2S NPs. Similar to the previous TiO2/RGO composites [20] and α-Fe2O3/RGO composites [21] using GO as the precursor of RGO, no typical diffraction peaks of RGO were detected, which can be explained by the destroyed regular stack of RGO by the intercalation of Ag or Ag2S.

|
Download:
|
| Figure 1. XRD spectra of the Ag2S NPs/RGO and Ag NPs/RGO nanocomposites. | |
The morphologies of as-obtained samples are observed using TEM. The typical TEM image of Ag NPs/RGO nanocomposite is presented in Fig. 2a. It is clearly visualized that Ag nanoparticles with an average size of 10-15 nm are well deposited on RGO without agglomeration. The high-resolution TEM (HRTEM) (Fig. 2b) image taken from one nanoparticle reveals a lattice parameter of 0.235 nm, which corresponds to the (111) diffraction plane of Ag. Structural modification of Ag NPs/RGO nanocomposite after sulfurization was probed by TEM and HRTEM. As shown in Fig. 2c, after sulfurization, the Ag2S nanoparticles were still well distributed on RGO and preserved the uniform spherical shape while the size of such NPs become bigger. The HRTEM image in Fig. 2d reveals a highly crystalline characteristic of Ag2S NPs, the lattice fringes of 0.315 nm match well with the atomic plane of (021) of Ag2S. Note that the same preparation without GO gives bulk Ag2S (Fig. 2e), suggesting GO is essential to the generation of small Ag2S nanoparticles. Fig. 2f presents the scanning TEM (STEM) image and the corresponding EDX elemental mapping images of Ag, S and C for Ag2S NPs/RGO nanocomposites, revealing both Ag and S elements are uniformly distributed throughout the Ag2S NPs. All these results strongly support the formation of Ag2S NPs/RGO from Ag NPs/RGO by sulfurization reaction.
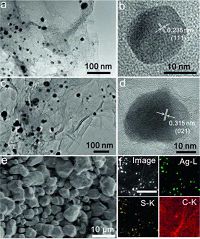
|
Download:
|
| Figure 2. (a) TEM image, (b) HRTEM image of Ag NPs/RGO nanocomposites. (c) TEM image, (d) HRTEM image of Ag2S NPs/RGO nanocomposites. (e) SEM image of bulk Ag2S. (f) STEM and corresponding EDX elemental mapping of Ag, S and C for Ag2S NPs/RGO (scale bar: 250 nm). | |
Typical Raman spectra of GO, Ag/RGO and Ag2S/RGO nanocomposite are shown in Fig. 3. Two broad characteristic peaks attributing to the well-documented G and D bands can be obviously observed in the three samples. The G band, at around 1598 cm-1, stands for the existence of sp2 hybridized carbon atoms, while the D band, at approximately 1356 cm-1, demonstrates structural defects and disorders that break the symmetry. In Ag/RGO and Ag2S/RGO spectra, the G and D bands were shifted to lower wave number (red-shifted) compared with GO spectra [22]. The shift of the G band suggests there is a chemical doping for carbonaceous materials in general. The intensity ratios of D/G peak (ID/IG) for Ag2S/RGO, Ag/RGO, GO are 1.43, 1.22 and 0.95, respectively. Compared with GO, an increased ID/IG value was observed for Ag/RGO nanocomposites, demonstrating that more disordered carbon structure was produced after the reduction of GO and the Ag NPs on RGO stressed its surface and induced more disorder [23]. Generally, the graphitization degree of carbon materials can be represented by using ID/IG obtained from Raman spectra [24]. For Ag2S/RGO nanocomposites, the further increased ID/IG value as compared with Ag/RGO nanocomposites can be explained that the RGO sheets were further reduced after hydrothermal sulfurization reaction.

|
Download:
|
| Figure 3. Raman spectra of GO, Ag NPs/RGO and Ag2S NPs/RGO nanocomposites. | |
The reduction progress of GO into RGO was monitored by UV-vis spectroscopy. As shown in Fig. 4a, the spectrum of GO shows a C=C aromatic peak at 228 nm corresponding to the π-π* transition [25, 26] and a shoulder band at 300 nm which should be attributed to the n-π* transitions of the carbonyl groups [27]. Upon reduced by NaBH4 via hydrothermal reduction, the UV-vis absorption spectra of Ag NPs/RGO suspension indicated that the adsorption peak of GO gradually shifted from 228 nm to 270 nm [28] and the adsorption peak of Ag NPs was located at 434 nm [29]. This result revealed the successful reduction of GO into RGO owning to the increased π-electron density and the formation of Ag NPs/RGO nanocomposites. After a simple hydrothermal sulfurization reaction with Ag NPs/RGO the peak of RGO gradually shifted from 270 nm to 279 nm which indicates the further reduction of RGO [26]. While the disappearance of the adsorption peak of Ag NPs strongly support the formation of Ag2S NPs/RGO from Ag NPs/RGO by sulfurization reaction.

|
Download:
|
| Figure 4. (a) UV-vis absorption spectra and (b) FTIR spectra of GO, Ag NPs/RGO and Ag2S NPs/RGO nanocomposites. | |
The reduction of GO was also monitored by FTIR spectroscopy. Fig. 4b represents the FTIR spectra of GO, Ag NPs/RGO and Ag2S NPs/RGO nanocomposites. The spectrum of GO included a broad and intense peak centered around 3400 cm-1, which should be assigned to O—H groups. The strong peaks at 1740 cm-1, 1420 cm-1, 1220 cm-1 and 1050 cm-1 should be attributed to C=O stretching vibration, O—H deformation peak, C—OH stretching vibration peak and C—O stretching vibration peak, respectively [26]. After the reduction, these functional groups were mostly eliminated or reduced evidenced by the disappearance of the peaks at 3400 cm-1, 1740 cm-1, 1220 cm-1 and 1050 cm-1 as well as the decreased intensity at 1630 cm-1 and 1420 cm-1. The foregoing results indicated that most oxygenous groups of Ag NPs/RGO and Ag2S NPs/RGO nanocomposites have been successfully reduced.
The Ag2S NPs/RGO nanocomposites show excellent catalytic activity for reduction of nitro to amino groups of 4-NP in the presence of NaBH4. For comparison, Ag NPs/RGO nanocomposites were also examined. The UV-vis absorption spectra of the aqueous mixture of 4-NP and NaBH4 had an absorption maximum at 400 nm due to the nitro compound [30]. With the Ag2S NPs/RGO catalyst added, the reduction reaction did proceed. The reaction kinetics could be monitored easily from the time-dependent absorption spectra, which showed the successive intensity decrease of the absorption peak at 400 nm, ascribed to nitro compounds, and the concomitant development of a new peak at 300 nm corresponding to 4-AP [31, 32], the reduction product of 4-NP (Fig. 5a). After the reaction finished (5 min), the peak due to the nitro compound was no longer observed, meaning that the catalytic reduction of 4-NP had proceeded successfully. While the concentration of 4-NP only decreases by 23% as the reductive reaction with Ag NPs/RGO nanocomposites proceed to 6 min (Fig. 5b). Therefore, it can be confirmed that the Ag2S NPs/RGO nanocomposites exhibit remarkably improved catalytic performance compared to Ag NPs/RGO. In addition, the catalytic activity for Ag2S NPs with different size were also studied (Fig. S1 in Supporting information). It can been seen from Fig. S1 that Ag2S NPs with bigger size (100-500 nm) have much lower activity and bulk Ag2S NPs (around 10 mm) have very limited activity for the reduction of 4-nitrophenol. This can be explained that the small size (10-15 nm) of Ag2S NPs favors the exposure of more active sites for the reduction of 4-nitrophenol.

|
Download:
|
| Figure 5. UV-vis spectra of 4-NP reduction using (a) Ag2S NPs/RGO nanocomposites and (b) Ag NPs/RGO nanocomposites, respectively. | |
Since the concentration of NaBH4 largely exceeds that of 4-NP, the reduction with regard to 4-NP only which was considered as a pseudo-first-order reaction. In this system the absorbance was proportional to the concentration of 4-NP and the reaction rate constant k was calculated from the rate equation, ln[Ct/C0] = kt, where C0 and Ct are the concentrations of 4-NP at time 0 and t, respectively. To follow the kinetics of the reaction, UV-vis spectra of the reaction mixture were monitored at 1 min intervals regularly. Fig. 6 shows the time-dependent UV-vis spectra of 4-NP catalyzed by the Ag2S NPs/RGO (black line) and Ag NPs/RGO (red line) with slopes of -0.55 and -0.041, respectively. According to the slope of the fitted line the rate constant k for Ag2S NPs/RGO was calculated to be -0.55 min-1 which is almost 13-fold higher than that of Ag NPs/RGO (-0.041 min-1). Such excellent catalytic performance of the Ag2S NPs/RGO could be mainly attributed to the following three reasons: (1) Like metal complex catalysts, the Ag2S NPs is also rich with metal center Ag (δ+), with pendant base S (δ-) close to it, and thus the Ag and basic S function as the electronacceptor and proton-acceptor centers, respectively, which facilitates the catalyst reaction; (2) RGO features the high adsorption ability toward 4-NP which provides a high concentration of 4-NP near the Ag2S NPs; and (3) electron transfer from RGO to Ag2S NPs, facilitating the uptake of electrons by 4-NP molecules.

|
Download:
|
| Figure 6. Plots of ln(C/C0) versus time of Ag2S NPs/RGO and Ag NPs/RGO nanocomposites, respectively. | |
In consideration of the practical applications, reusability of the products has been studied. As shown in Fig. 7a, the conversion of 4-NP could still reach 79% over eight cycles in 5 min, which exhibits super recyclability of the samples. The structure of Ag2S NPs/RGO has no change after the catalytic reaction. The Ag2S NPs with the same size distribution compared with the catalyst before the reaction were uniformly supported on RGO sheets (Fig. 7b), indicating that the attachment between Ag2S NPs and RGO is sufficiently strong.

|
Download:
|
| Figure 7. (a) Recyclability measurement of Ag2S NPs/RGO nanocomposites during eight successive cycles in 5 min, (b) TEM image of Ag2S NPs/RGO nanocomposites after eight successive cycles. | |
3. Conclusion
In summary, we have first demonstrated that Ag2S NPs/RGO shows a high catalytic performance toward the reduction of 4-NP. As an novel catalyst for the reduction of 4-nitrophenol (4-NP), it only takes 5 min for Ag2S NPs/RGO to reduce 98% of 4-NP, and the rate constant of the composites is almost 13 times higher than that of comparable Ag systems. The high performance could be considered to arise from the unique structure of Ag2S nanoparticles. This work is believed to be significantly beneficial to the design and development of active Ag2S catalysts as advanced catalytic systems for practical applications.
4. Experimental 4.1. Chemicals4-Nitrophenol (AR), silver nitrate (AR), ammonium hydroxide (AR), sodium borohydride (AR) and Na2S (AR) were purchased from Beijing Chemical Corp. Natural graphite powders with an average diameter of 300 mm were supplied from Qingdao Graphite Company. Sulfuric acid (98%, AR), phosphorus pentoxide (98%, AR), potassium permanganate (AR), hydrogen peroxide (30%, AR) and ethanol (AR) were purchased from Shanghai Jinlu Chemical Co., Ltd. All the reagents were used as received. The ultrapure water used throughout all experiments was made by the Flom ultrapure water system.
4.2. Preparation of Ag NPs/RGO nanocompositesGraphite oxide (GO) was first prepared according to the modified Hummers method [33]. GO (50 mg) was dispersed in distilled water (50 mL) with ultrasonication for 1 h to form a uniform suspension. Then 1 mL freshly prepared [Ag (NH3) 2]+ aqueous solution (0.1176 mol/L) was added and stirred for 30 min. Subsequently, 100 mg NaBH4 as the reductant was put into the above suspension under stirring. The well-mixed precursor suspension was then transferred into a stainless steel autoclave (100 mL) after 30 min. The autoclave was heated and maintained at 80 ℃ for 24 h. Finally, the Ag/RGO nanocomposites were washed and dried for further use.
4.3. Preparation of Ag2S NPs/RGO nanocomposites and bulk Ag2SAg2S NPs/RGO nanocomposites were prepared by using the as-synthesized Ag NPs/RGO nanocomposite as the precursors. Briefly, 56.45 mL freshly prepared Na2S aqueous solution (25 mmol/L) was mixed with 10 mg above well-prepared Ag NPs/RGO nanocomposites and the mixture was sonicated for 30 min to form a uniform suspension. Similarly, the suspension was put into an autoclave of 100 mL and maintained at 120 ℃ for 6 h. Finally, the as-prepared Ag2S NPs/RGO nanocomposites were washed with copious water and dried prior to the subsequent utilization. The bulk Ag2S was prepared by the same procedure without introduction of GO. The Ag2S NPs with bigger size (Fig. S2 in Supporting information) were prepared by the same procedure but maintained at 120 ℃ for 12 h.
4.4. Catalytic reduction of 4-NPTo study the catalyst activity, 10 mL freshly prepared NaBH4 aqueous solution (0.1 mol/L) was put into 40 mL 4-NP aqueous solution (0.3 mmol/L) by magnetic stirring. Then, 2 mg of the corresponding catalyst was added to the mixture solution. At a given reaction time, a 1.0 mL solution was sampled through a 0.1 mm membrane filter. Ultraviolet-visible (UV-vis) spectroscopy was used to monitor the process of the conversion of 4-NP to 4-AP. The absorption spectra were recorded within the wavelength range of 250-500 nm. The rate constants of the reduction reaction were calculated by measuring the peak intensity evolution every minute at wavelengths of 400 nm for 4-NP.
4.5. CharacterizationsX-ray diffraction (XRD) patterns were recorded on a RigakuD/ MAX 2550 diffractometer with Cu Ka radiation (λ = 1.5418 Å). Scanning electron microscopy (SEM) images were taken on a XL30 ESEM FEG scanning electron microscope at an accelerating voltage of 20 kV. Transmission electron microscopy (TEM) measurements were made on a Hitachi H-8100 electron microscopy (Hitachi, Tokyo) with an accelerating voltage of 200 kV. Raman spectra were obtained on a Jobin-Yvon HR800 Raman spectrometer with 633 nm wavelength incident laser. Ultraviolet- visible (UV-vis) spectroscopy measurements were recorded with a UV-1800PC UV-vis spectrophotometer. Fourier transform infrared (FTIR) spectra were recorded on a HYPERION 2000 spectrometer with ATR mode.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.10.019.
| [1] | R. Lamba, A. Umar, S.K. Mehta, S.K. Kansal, Well-crystalline porous ZnO-SnO2 nanosheets:an effective visible-light driven photocatalyst and highly sensitive smart sensor material. Talanta 131 (2015) 490–498. DOI:10.1016/j.talanta.2014.07.096 |
| [2] | T. Ji, L. Chen, M. Schmitz, F.S. Bao, J.H. Zhu, Hierarchical macrotube/mesopore carbon decorated with mono-dispersed Ag nanoparticles as a highly active catalyst. Green Chem. 17 (2015) 2515–2523. DOI:10.1039/C5GC00123D |
| [3] | H.G. Lee, G. Sai-Anand, S. Komathi, Efficient visible-light-driven photocatalytic degradation of nitrophenol by using graphene-encapsulated TiO2 nanowires. J. Hazard. Mater. 283 (2015) 400–409. DOI:10.1016/j.jhazmat.2014.09.014 |
| [4] | S.H. Zhang, W.J. Liu, C. Wang, Improving the NOx decomposition and storage activity through co-incorporating ammonium and copper ions into Mg/Al hydrotalcites. RSC Adv. 6 (2016) 45127–45134. DOI:10.1039/C6RA06665H |
| [5] | S. Saha, A. Pal, S. Kundu, S. Basu, T. Pal, Photochemical green synthesis of calcium-alginate-stabilized Ag and Au nanoparticles and their catalytic application to 4-nitrophenol reduction. Langmuir 26 (2010) 2885–2893. DOI:10.1021/la902950x |
| [6] | J. Li, C.Y. Liu, Y. Liu, Au/graphene hydrogel:synthesis, characterization and its use for catalytic reduction of 4-nitrophenol. J. Mater. Chem. 22 (2012) 8426–8430. DOI:10.1039/c2jm16386a |
| [7] | S. Aguado, S. El-Jamal, F. Meunier, J. Canivet, D. Farrusseng, A Pt/Al2O3-supported metal-organic framework film as the size-selective core-shell hydrogenation catalyst. Chem. Commun. 52 (2016) 7161–7163. DOI:10.1039/C6CC03096C |
| [8] | T. Kaito, H. Tanaka, H. Mitsumoto, PtCu, and PtNi alloy electrocatalysts:the correlation of enhanced oxygen reduction reaction activity and structure. J. Phys. Chem. C 120 (2016) 11519–11527. DOI:10.1021/acs.jpcc.6b01736 |
| [9] | C.L. Li, B. Jiang, H.R. Chen, Superior electrocatalytic activity of mesoporous Au film templated from diblock copolymer micelles. Nano Res. 9 (2016) 1752–1762. DOI:10.1007/s12274-016-1068-z |
| [10] | S. Shrestha, M.P. Harold, K. Kamasamudram, Selective oxidation of ammonia to nitrogen on bi-functional Cu-SSZ-13 and Pt/Al2O3 monolith catalyst. Catal. Today 267 (2016) 130–144. DOI:10.1016/j.cattod.2015.11.035 |
| [11] | H.T. Qi, P. Yu, Y.X. Wang, Graphdiyne oxides as excellent substrate for electroless deposition of Pd clusters with high catalytic activity. J. Am. Chem. Soc. 137 (2015) 5260–5263. DOI:10.1021/ja5131337 |
| [12] | Z.M. Wang, C.L. Xu, X. Li, Z.H. Liu, In situ green synthesis of Ag nanoparticles on tea polyphenols-modified graphene and their catalytic reduction activity of 4-nitrophenol. Colloids Surf. A 485 (2015) 102–110. DOI:10.1016/j.colsurfa.2015.09.015 |
| [13] | G.H. Chang, Y.L. Luo, W.B. Lu, Ag nanoparticles decorated polyaniline nanofibers:synthesis, characterization, and applications toward catalytic reduction of 4-nitrophenol and electrochemical detection of H2O2 and glucose. Catal. Sci. Technol. 2 (2012) 800–806. DOI:10.1039/c2cy00454b |
| [14] | Y.W. Zhang, S. Liu, W.B. Lu, In situ green synthesis of Au nanostructures on graphene oxide and their application for catalytic reduction of 4-nitrophenol. Catal. Sci. Technol. 1 (2011) 1142–1144. DOI:10.1039/c1cy00205h |
| [15] | W.B. Lu, R. Ning, X.Y. Qin, Synthesis of Au nanoparticles decorated graphene oxide nanosheets:noncovalent functionalization by TWEEN 20 in situ reduction of aqueous chloroaurate ions for hydrazine detection and catalytic reduction of 4-nitrophenol. J. Hazard. Mater. 197 (2011) 320–326. DOI:10.1016/j.jhazmat.2011.09.092 |
| [16] | J.M. Wang, P. Yang, M.M. Cao, A novel graphene nanodots inlaid porous gold electrode for electrochemically controlled drug release. Talanta 147 (2016) 184–192. DOI:10.1016/j.talanta.2015.09.020 |
| [17] | J.M. Wang, H.H. Zhu, Y.H. Xu, Graphene nanodots encaged 3-D gold substrate as enzyme loading platform for the fabrication of high performance biosensors. Sens. Actuators B 220 (2015) 1186–1195. DOI:10.1016/j.snb.2015.06.044 |
| [18] | D.A. Reddy, J. Choi, S. Lee, R. Ma, T.K. Kim, Green synthesis of AgI nanoparticlefunctionalized reduced graphene oxide aerogels with enhanced catalytic performance and facile recycling. RSC Adv. 5 (2015) 67394–67404. DOI:10.1039/C5RA07267K |
| [19] | J.M. Wang, W.R. Yang, J.Q. Liu, CoP2 nanoparticles on reduced graphene oxide sheets as a super-efficient bifunctional electrocatalyst for full water splitting. J. Mater. Chem. A 4 (2016) 4686–4690. DOI:10.1039/C6TA00596A |
| [20] | Y.H. Zhang, Z.R. Tang, X.Z. Fu, Y.J. Xu, TiO2-graphene nanocomposites for gasphase photocatalytic degradation of volatile aromatic pollutant:is TiO2-graphene truly different from other TiO2-carbon composite materials. ACS Nano 4 (2010) 7303–7314. DOI:10.1021/nn1024219 |
| [21] | S.C. Han, L.F. Hu, Z.Q. Liang, One-step hydrothermal synthesis of 2D hexagonal nanoplates of α-Fe2O3/graphene composites with enhanced photocatalytic activity. Adv. Funct. Mater. 24 (2014) 5719–5727. DOI:10.1002/adfm.v24.36 |
| [22] | D. Graf, F. Molitor, K. Ensslin, Spatially resolved Raman spectroscopy of single-and few-layer graphene. Nano Lett. 7 (2007) 238–242. DOI:10.1021/nl061702a |
| [23] | H.J. Yan, C.G. Tian, L. Wang, Phosphorus-modified tungsten nitride/reduced graphene oxide as a high-performance, non-noble-metal electrocatalyst for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 54 (2015) 6325–6329. DOI:10.1002/anie.201501419 |
| [24] | Z.H. Ni, Y.Y. Wang, T. Yu, Z.X. Shen, Raman spectroscopy and imaging of grapheme. Nano Res. 1 (2008) 273–291. DOI:10.1007/s12274-008-8036-1 |
| [25] | J.I. Paredes, S. Villar-Rodil, A. Martínez-Alonso, J.M.D. Tascón, Graphene oxide dispersions in organic solvents. Langmuir 24 (2008) 10560–10564. DOI:10.1021/la801744a |
| [26] | J.Z. Zhang, Y.H. Xu, Z. Liu, W.R. Yang, J.Q. Liu, A highly conductive porous graphene electrode prepared via in situ reduction of graphene oxide using Cu nanoparticles for the fabrication of high performance supercapacitors. RSC Adv. 5 (2015) 54275–54282. DOI:10.1039/C5RA07857A |
| [27] | D.C. Marcano, D.V. Kosynkin, J.M. Berlin, Improved synthesis of graphene oxide. ACS Nano 4 (2010) 4806–4814. DOI:10.1021/nn1006368 |
| [28] | Q.Q. Zhuo, J. Gao, M.F. Peng, Large-scale synthesis of graphene by the reduction of graphene oxide at room temperature using metal nanoparticles as catalyst. Carbon 52 (2013) 559–564. DOI:10.1016/j.carbon.2012.10.014 |
| [29] | W.P. Xu, L.C. Zhang, J.P. Li, Facile synthesis of silver@graphene oxide nanocomposites and their enhanced antibacterial properties. J. Mater. Chem. 21 (2011) 4593–4597. DOI:10.1039/c0jm03376f |
| [30] | A.Y. Li, Q.J. Luo, S.J. Park, R.G. Cooks, Synthesis and catalytic reactions of nanoparticles formed by electrospray ionization of coinage metals. Angew. Chem. Int. Ed. 53 (2014) 3147–3150. DOI:10.1002/anie.201309193 |
| [31] | Y. Dai, S.J. Liu, N.F. Zheng, C2H2 treatment as a facile method to boost the catalysis of Pd nanoparticulate catalysts. J. Am. Chem. Soc. 136 (2014) 5583–5586. DOI:10.1021/ja501530n |
| [32] | N. Lu, W. Chen, G.Y. Fang, 5-fold twinned nanowires and single twinned right bipyramids of Pd:utilizing small organic molecules to tune the etching degree of O2/halides. Chem. Mater. 26 (2014) 2453–2459. DOI:10.1021/cm4042204 |
| [33] | G.Q. Lv, H.Q. Wang, Y.X. Yang, Aerobic selective oxidation of 5-hydroxymethyl-furfural over nitrogen-doped graphene materials with 2,2,6,6-tetramethylpiperidin-oxyl as co-catalyst. Catal. Sci. Technol. 6 (2016) 2377–2386. DOI:10.1039/C5CY01149C |
 2017, Vol. 28
2017, Vol. 28 


