Lithium sulfur (Li-S) battery is regarded as one of the most promising energy storage systems for next generation electric vehicles, due to the high theoretical specific capacity (1672 mAh g-1) and high energy density (2600 Wh kg-1) of sulfur, which are several times higher than those of the commercial lithium-ion batteries [1-4]. However, the sulfur cathode are facing some big challenges for its practical application. The insulating characteristic of sulfur (σ = 5 × 10-30 S cm-1 at 25 ℃) results in low sulfur utilization and poor rate capability, the dissolution and shuttling effect of the long chain lithium polysulfides (Li2Sn, 4 ≤ n < 8), which are generated during charge and discharge process in liquid electrolytes, lead to the active material loss and deteriorate the cycling performance [1, 5].
To address these problems, integrating sulfur with carbonbased materials including micro/mesoporous carbon [6-8], carbon nanofibers [9-11], carbon nanotubes [12-14] and graphene [15-27] have been widely adopted to enhance the electrochemical performance of Li-S batteries. Among these carbon materials, graphene is regarded as an ideal substrate to host sulfur, due to its superior conductivity, high theoretical specific surface area, and excellent mechanical flexibility [17]. In recent years, many efforts have been made to tailor the pore structure [20-22] or modify the surface chemistry [23-26] of the graphene to host sulfur for enhancing lithium sulfur battery performance. For example, Ding et al . [22] reported a highly porous chemical activated graphene as sulfur host, the existence of nanopores suppressed the polysulfides diffusion and accommodate the volume expansion, resulting in high specific capacity and good cycling stability. Yang et al . [20] and Chen et al . [21] designed a graphene-based layered porous nanostructure and a sandwich-type carbon nanosheets consisting of graphene and micro/mesoporous carbon layer to encapsulate sulfur, respectively. The hierarchical porous carbon layers on graphene sheets could minimize the polysulfide dissolution and shuttling in the electrolyte, thus significantly improve the cycling performance and rate capabilities. Qiu et al . [25] prepared an Ndoped graphene using thermal nitridation process in NH3 atmosphere to wrap S nanoparticles, the as-prepared S@NG demonstrates excellent rate performance and ultralong cycle life up to 2000 cycles, indicating N-doping in graphene could effectively trap lithium polysulfides species and result in stable cycle life.
These results suggest the combination of pore forming and heteroatom doping of graphene is a promising way to develop advanced graphene/S cathode for Li-S batteries. Herein, we present the preparation of a nanoporous N-doped reduced graphene oxide (p-N-rGO) through carbothermal reaction between graphene oxide and ammonium-containing oxometalates as sulfur host for Li-S battery. The nanopores on the graphene sheets were estimated to have diameters of 10-40 nm and the nitrogen content in the p-N-rGO was 2.65 at%. When used as sulfur cathode, the obtained p-N-rGO/S composite demonstrates a better electrochemical performance compared with those of the rGO/S composite. The rational combination of nanopores and N-doping enables a high reversible capacity of 1110 mAh g-1 at 1C rate and stable cycling performance with 781.8 mAh g-1 retained after 110 cycles.
2. Results and discussionFig. 1 shows the typical preparation process of the p-N-rGO/S composite. The p-N-rGO composite was prepared by a previously reported method [28]. First, the graphene oxide (GO) was prepared from graphite flakes by an improved Hummers method [29], then the GO aqueous solution was mixed well with an ammonium molybdate solution, the dispersed mixture was freeze dried and then annealed at 900 ℃ under Ar/5%H2 reduction atmosphere to form the MoO2@N-rGO composite (Fig. S1a in Supporting information). From Fig. S1a, we could see that large amounts of nanoparticles with diameters much less than 100 nm were anchored on the surface of the wrinkled rGO sheets. The X-ray diffraction patterns prove that the metal oxide nanoparticles are MoO2 (Fig. S2 in Supporting information). Second, the MoO2 nanoparticles in the composite were etched in the aqueous acid solution to generate p-N-rGO composite (Fig. S1b), on which lots of nanopores are observed. Finally, the p-N-rGO/S composite was synthesized by a solution deposition method.

|
Download:
|
| Figure 1. Schematic illustration for the preparation of p-N-rGO/S composite. | |
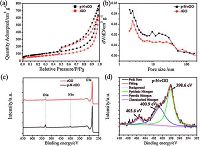
The microstructure of the p-N-rGO was characterized by transmission electron microscopy (TEM). For comparison, the rGO sample was also prepared by the same method except without adding the ammonium molybdate. Fig. 2 shows the TEM images of the rGO and the p-N-rGO composite. The rGO sample (Fig. 2a) has a smooth morphology without nanopores on its surface, while the pN-rGO sample (Fig. 2b) shows abundant nano-scaled pores of 10- 40 nm on the wrinkled sheets, indicating the nanoporous structure of the p-N-rGO. The pore structures were further analyzed by nitrogen adsorption/desorption isotherm at 77 K. Fig. 3a, b shows the nitrogen adsorption-desorption isotherms and pore size distribution curves of the rGO and p-N-rGO samples. The p-NrGO shows a high specific surface area of 321.7 m2 g-1 and a pore volume of 1.187 cm3 g-1, while the rGO has a lower specific surfaces area (217.3 m2 g-1) and a small pore volume (0.963 cm3 g-1). From the pore size distribution curves, we could see that there is a remarkable difference ranging from 2 nm to 40 nm in Fig. 3b. This difference is caused by the etching of the MoO2 nanoparticles during the preparation of the p-N-rGO, after etched by acid solution, large amounts of nanopores emerge on the graphene sheets, resulting in a dramatic increase of the specific surface area and pore volume (Fig. S3 in Supporting information). The nanoporous structure is beneficial to providing more space for sulfur and volume expansion as well as better accessibility to the electrolyte [22].

|
Download:
|
| Figure 2. TEM images of the materials (a, rGO; b, p-N-rGO). | |
|
Download:
|
| Figure 3. (a) N2 adsorption-desorption isotherm profiles and (b) pore size distributions of the rGO and p-N-rGO; (c) XPS survey scan of the rGO and p-N-rGO, (d) N 1s XPS spectrum of the p-N-rGO. | |
To examine surface chemical composition of the rGO and p-NrGO samples, X-ray photoelectron spectroscopy (XPS) (Fig. 3c) were measured. The rGO sample only shows the C 1s and O 1s signals in the XPS survey spectra at 284.5 eV and 532.5 eV, respectively [30]. As for the p-N-rGO sample, a new peak at 399.5 eV corresponding to N 1s is observed [25, 30], indicating the efficient N-doping in the p-N-rGO sample. During the carbothermal reaction, the ammonium molybdate could be thermally decomposed and generate ammonia gas, the ammonia further react with graphene and produce with N functional groups. The atomic ratio of nitrogen in the p-N-rGO sample is estimated to be 2.65% from the peak areas of C 1s, N 1s and O 1s. In the N 1s spectrum (Fig. 3d), the three different peaks at 398.6 eV, 400.9 eV and 405.6 eV are ascribed to pyridinic N, pyrrolic N and chemicalsorbed N, respectively [31]. The pyridinic N and pyrrolic N types are dominant in the p-N-rGO sample, which are believed to be beneficial to improve the affinity and binding energy of the nonpolar carbon atoms with polar polysulphides/Li2S, thus alleviating dissolution and shuttle of lithium polysulfides [25, 32].
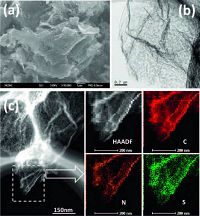
Fig. 4 shows the SEM and TEM images of the p-N-rGO/S composite. From Fig. 4a and b, we could see that after sulfur encapulation, the p-N-rGO/S composite almost keeps it original morphology without large aggregated sulfur particles observed on the surface of the p-N-rGO sheets. The STEM-EDS mapping (Fig. 4c) further verifies the homogeneous distribution of sulfur and nitrogen throughout the p-N-rGO nanosheets [32]. The sulfur content in the p-N-rGO/S composite is determined through thermogravimetric analysis (TGA) at a heating rate of 10 ℃ in nitrogen atmosphere. Fig. S4 indicates the p-N-rGO/S composite has a high sulfur content of 69.3 wt%. The XRD pattern in Fig. S5 in Supporting information confirms S in the p-N-rGO/S composite belongs to the orthorhombic sulfur phase (JCPDS card No. 24- 0733), which is in agree with previous report [25].
|
Download:
|
| Figure 4. Morphology and microstructure of the p-N-rGO/S (a, SEM image; b, TEM image; c, STEM-EDS mappings). | |
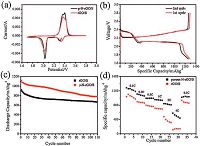
2016 type of coin cells were fabricated to test the electrochemical performance of the p-N-rGO/S and rGO/S electrodes. The cathode electrodes were consisted of 80 wt% S cathodes, 12 wt% carbon Super P and 8 wt% water-soluble binder LA133. The cyclic voltammetry (CV) of the p-N-rGO/S and rGO/S composites were tested at a scan rate of 0.1 mV s-1, the results are shown in Fig. 5a. Both electrodes show two well-defined cathodic peaks at ~2.3 V and ~2.0 V during the first cathodic reduction process, which are attributed to the transformation of cyclo-S8 to long-chain soluble lithium polysulfides and the further reduction of those polysulfide species (Li2Sn, 4 ≤ n < 8) to insoluble short-chain lithium sulfides (Li2Sn, n ≤ 2), respectively. In the following anodic oxidation process, two peaks at 2.35 V and 2.40 V can be observed, corresponding to the oxidation of the lithium sulfides into longchain polysulfide and eventually to elemental sulfur [31, 34]. Though the positions of the cathodic and anodic peaks of the p-N-rGO/S electrode are similar to those of the rGO/S electrode, their intensities are obviously stronger than those of the p-N-rGO/S electrode, indicating the enhanced kinetics of the p-N-rGO/S electrode.
Fig. 5b shows the first and second discharge/charge curves of the p-N-rGO/S electrode at 0.1C rate, the initial discharge and charge capacities were 1286.6 and 1288.9 mAh g-1, corresponding to a columbic efficiency of ~100%. The high initial discharge capacity attests the high sulfur utilization of the p-N-rGO/S composite, which may be attributed to the nanoporous structure and better dispersion and chemical contact of sulfur, providing easy and sufficient access to electrolyte and fast electrochemical kinetics [20, 22]. The second discharge/charge curves overlap well with the initial ones, except for small capacity loss. Fig. 4c shows the cycling performance of the p-N-rGO/S electrode at 1C rate. The p-N-rGO/S electrode delivers a high discharge capacity of 1110 mAh g-1 even at a high current density of 1672 mA g-1, and a discharge capacity of 781.8 mAh g-1 can be still remained after 110 cycles. In contrast, the rGO/S electrode shows lower initial discharge capacity of 924 mAh g-1 and faster capacity decay especially at initial cycles. The enhanced cycling performance of the the p-N-rGO/S electrode is ascribed to the introduced N functional groups and abundant nanopores of the graphene sheets. The N functional groups could effectively suppress the lithium polysulfide shuttling while the nanopores of the p-N-rGO could accommodate volume expansion and immobilize polysulfides during cycling, both results in better cycling performance.
The rate performance of the p-N-rGO/S electrode was investigated as well. Fig. 5 shows the discharge/charge curves from 0.1C to 4C rate. The curves show similar figures at different C rates, indicating low over potentials at high C rate. Fig. 5d demonstrates the rate capacities of the p-N-rGO/S and rGO/S electrodes, the discharge specific capacities of the p-N-rGO/S electrode are around 1280, 1100, 990, 950, 840, and 570 mAh g-1 when cycled at 0.1, 0.2, 0.5, 1, 2 and 4C rates, respectively. When the C rate was reduced back to 0.1C, the p-N-rGO/S electrode recovers most of its initial capacity, reaching a capacity of 1020 mAh g-1. For comparison, the rGO/S electrode exhibits poorer rate capabilities than the p-N-rGO/S electrode, it only delivers discharge capacities of 1100, 890, 790, 710, 390 and 140 mAh g-1 at 0.1C, 0.2C, 0.5C, 1C, 2C and 4C rates, respectively. The enhanced rate performance of the p-N-rGO electrode is mainly ascribed to the nanoporous structural features, the thin graphene sheets with large amounts of nanopores not only afford rapid electron transfer but also provide efficient contact and wetting with the electrolyte.
|
Download:
|
| Figure 5. Electrochemical performance of the rGO/S and p-N-rGO/S electrodes (a, CV curves; b, the first and second discharge/charge curves of the p-N-rGO/S electrode at 0.1C rate; c, cycling performance; d rate capabilities). | |
3. Conclusion
In summary, a nanoporous N-doped rGO nanostructure was synthesized to host sulfur as cathode material. The obtained p-NrGO/S composite delivers high reversible capacities of 1286.6 and 1110 mAh g-1 at 0.1C and 1C rate, respectively. The excellent electrochemical performances are ascribed to the combination of the introduced N functional groups and nanopores: the N functional groups could effectively suppress the lithium polysulfide shuttling, the nanopores of the p-N-rGO could accommodate volume expansion and immobilize polysulfides during cycling as well as provide efficient contact and wetting with the electrolyte. These results demonstrate the nanoporous N-doped graphene is a promising material to host sulfur for lithium sulfur batteries.
4. Experimental 4.1. Synthesis of p-N-rGO and rGOGO was prepared from graphite flakes by an improved Hummers method [29]. The p-N-rGO sample was prepared by a previous report [28]. A homogeneous graphene oxide aqueous dispersion (10.0 mg mL-1) was sonicated for 2 h before use. 1.2 g ammonium molybdate ( (NH4) 6Mo7O24·4H2O) was dissolved into 40 mL water, then the solution was added into 60 mL GO dispersion, and the mixture was sonicated for 0.5 h and was stirred 2 h. The homogeneous mixture was freeze-dried and then heated to 900 ℃ at a rate of 5 ℃ min-1 and stayed for 2 h in the atmosphere of Ar/5%H2. The obtained black product was immersed in aqua regia to remove the metal oxide species. Finally, the p-NrGO sample was collected by filtration, washing with water and ethanol, and drying at 60 ℃ in vacuum. The rGO sample was prepared by the same method except without adding ammonium molybdate.
4.2. Preparation of p-N-rGO/S and rGO/SThe p-N-rGO/S nanocomposite was fabricated via an in-situ solution deposition method [33, 34]. Breifly, 0.05 g p-N-rGO was added into aqueous-ethanol (80 mL:20 mL) solution and sonicated for 1 h, then 1.0 g Na2S·9H2O and 0.81 g Na2SO3 were dissovled in 50 mL DI water and poured into the above solution. Then 20 mL 1 mol L-1 hydrochloric acid solution was added in under magnetic stir for 1 h. Finally, the reaction solution was ultrasonicated for 30 min and centrifuged 3 times with DI water, after vacuum drying at 65 ℃ for 12 h, the p-N-rGO/S nanocomposite was obtained. The rGO/S nanocomposite was prepared following the same procedure, but replacing the p-N-rGO with rGO. The sulfur contents in the asprepared composites were also calculated by the mass change before and after the formation of the composite, which almost agrees with TG analysis (Fig. S4 in Supporting information). The sulfur contents in the p-N-rGO/S and rGO/S nanocomposite were 69.3 wt% and 70 wt%, respectively.
4.3. Materials characterizationThe structure of the prepared samples were measured by XRD (SIEMENS D-500) using Cu Ka1 irradiation, ranging from 10° to 60° at a step of 8° min-1. The micro morphologies of the nanocomposites were studied using field emission scanning electron microscope (HITACHI S4800, Japan). TEM, HRTEM images, and STEM-EDS elemental mapping were recorded with a FEI Tecnai 2100 instrument. The nitrogen adsorption-desorption analysis was done at 77.3 K on a V-Sorb 2800 equipment. The thermogravimetric analysis (TGA) was measured with a TGA-600 with a heating rate of 10 ℃ min-1 under N2 atmosphere to confirm the sulfur content in the composite.
4.4. Electrochemical characterizationsElectrochemical experiments were performed using 2016 type coin cells. The working electrode was prepared by mixing 80 wt% sulfur cathode materials with 12 wt% Super P and 8 wt% LA133 aqueous binder using water and isopropanol as the solvent. The sulfur mass loading is 1.0-1.5 mg cm-2. After mixing well, the slurry was pasted on Al foil and dried overnight at 65 ℃ in a vacuum oven. 0.5 mol L-1 lithium bis-trifluoromethanesulfonylimide (LiTFSI) in a mixed solvent of 1, 3-dioxolane and 1, 2- dimethoxyethane (DOL/DME, 1:1, v/v) with 0.5 mol L-1 LiNO3, purchased from Fosai New Material Co., Ltd. (Suzhou), was used as electrolyte, lithium metal foil was used as the anode and the polypropylene membranes from Celgard Inc. were used as the separators. Galvanstatic charge-discharge measurements were performed using a battery tester (LAND CT-2001A, Wuhan, China) at room temperature in a potential range of 1.7-2.8 V (vs. Li+/Li) at current densities of 0.1C, 0.2C, 0.5C, 1C, 2C, 4C (1C = 1672 mA h g-1). Cyclic voltammetry (CV) was performed with an electrochemical workstation (CHI 660C) between 1.7 and 2.8 V at a sweep rate of 0.1 mV s-1. Electrochemical Impedance (EIS) analyses were conducted using the same equipment from 100 mHz to 1 MHz.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.10.038.
Acknowledgment Financial support from the Research Project of National University of Defense Technology (No. ZDYYjcYj20140701)| [1] | Y. Yang, G.Y. Zheng, Y. Cui, Nanostructured sulfur cathodes. Chem. Soc. Rev. 42 (2013) 3018–3032. DOI:10.1039/c2cs35256g |
| [2] | A. Manthiram, Y.Z. Fu, Y.S. Su, Challenges and prospects of lithium-sulfur batteries. Acc. Chem. Res. 46 (2013) 1125–1134. DOI:10.1021/ar300179v |
| [3] | S. Evers, L.F. Nazar, New approaches for high energy density lithium-sulfur battery cathodes. Acc. Chem. Res. 46 (2013) 1135–1143. DOI:10.1021/ar3001348 |
| [4] | Y.X. Yin, S. Xin, Y.G. Guo, L.J. Wan, Lithium-sulfur batteries:electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. 52 (2013) 13186–13200. DOI:10.1002/anie.201304762 |
| [5] | D.W. Wang, Q.C. Zeng, G.M. Zhou, Carbon-sulfur composites for Li-S batteries:status and prospects. J. Mater. Chem. A 1 (2013) 9382–9394. DOI:10.1039/c3ta11045a |
| [6] | J.Z. Chen, D.X. Wu, E. Walter, Molecular-confinement of polysulfides within mesoscale electrodes for the practical application of lithium sulfur batteries. Nano Energy 13 (2015) 267–274. DOI:10.1016/j.nanoen.2015.01.031 |
| [7] | G.Y. Xu, B. Ding, P. Nie, Hierarchically porous carbon encapsulating sulfur as a superior cathode material for high performance lithium-sulfur batteries. ACS Appl. Mater. Interfaces 6 (2014) 194–199. DOI:10.1021/am4038728 |
| [8] | Z. Li, Y. Jiang, L.X. Yuan, A highly ordered meso@microporous carbonsupported sulfur@smaller sulfur core-shell structured cathode for Li-S batteries. ACS Nano 8 (2014) 9295–9303. DOI:10.1021/nn503220h |
| [9] | G.Y. Zheng, Y. Yang, J.J. Cha, S.S. Hong, Y. Cui, Hollow carbon nanofiberencapsulated sulfur cathodes for high specific capacity rechargeable lithium batteries. Nano Lett. 11 (2011) 4462–4467. DOI:10.1021/nl2027684 |
| [10] | L.W. Ji, M.M. Rao, S. Aloni, Porous carbon nanofiber-sulfur composite electrodes for lithium/sulfur cells. Energy Environ. Sci. 4 (2011) 5053–5059. DOI:10.1039/c1ee02256c |
| [11] | M.M. Rao, X.Y. Geng, X.P. Li, S.J. Hu, W.S. Li, Lithium-sulfur cell with combining carbon nanofibers-sulfur cathode and gel polymer electrolyte. J. Power Sources 212 (2012) 179–185. DOI:10.1016/j.jpowsour.2012.03.111 |
| [12] | Y. Zhao, W.L. Wu, J.X. Li, Z.C. Xu, L.H. Guan, Encapsulating MWNTs into hollow porous carbon nanotubes:a tube-in-tube carbon nanostructure for highperformance lithium-sulfur batteries. Adv. Mater. 26 (2014) 5113–5118. DOI:10.1002/adma.201401191 |
| [13] | J.C. Guo, Y.H. Xu, C.S. Wang, Sulfur-impregnated disordered carbon nanotubes cathode for lithium-sulfur batteries. Nano Lett. 11 (2011) 4288–4294. DOI:10.1021/nl202297p |
| [14] | L.X. Yuan, H.P. Yuan, X.P. Qiu, L.Q. Chen, W.T. Zhu, Improvement of cycle property of sulfur-coated multi-walled carbon nanotubes composite cathode for lithium/sulfur batteries. J. Power Sources 189 (2009) 1141–1146. DOI:10.1016/j.jpowsour.2008.12.149 |
| [15] | J.Z. Wang, L. Lu, M. Choucair, Sulfur-graphene composite for rechargeable lithium batteries. J. Power Sources 196 (2011) 7030–7034. DOI:10.1016/j.jpowsour.2010.09.106 |
| [16] | L.C. Yin, J.L. Wang, F.J. Lin, J. Yang, Y. Nuli, Polyacrylonitrile/graphene composite as a precursor to a sulfur-based cathode material for high-rate rechargeable Li-S batteries. Energy Environ. Sci. 5 (2012) 6966–6972. DOI:10.1039/c2ee03495f |
| [17] | M.Q. Zhao, Q. Zhang, J.Q. Huang, Unstacked double-layer templated graphene for high-rate lithium-sulphur batteries. Nat. Commun. 5 (2014) 3410. |
| [18] | C.X. Zu, A. Manthiram, Hydroxylated graphene-sulfur nanocomposites for high-rate lithium-sulfur batteries. Adv. Energy Mater. 3 (2013) 1008–1012. DOI:10.1002/aenm.v3.8 |
| [19] | S.T. Lu, Y. Chen, X.H. Wu, Z.D. Wang, Y. Li, Three-dimensional sulfur/graphene multifunctional hybrid sponges for lithium-sulfur batteries with large areal mass loading. Sci. Rep. 4 (2014) 4629. |
| [20] | X. Yang, L. Zhang, F. Zhang, Y. Huang, Y.S. Chen, Sulfur-infiltrated graphenebased layered porous carbon cathodes for high-performance lithium-sulfur batteries. ACS Nano 8 (2014) 5208–5215. DOI:10.1021/nn501284q |
| [21] | X.A. Chen, Z.B. Xiao, X.T. Ning, Sulfur-impregnated, sandwich-type, hybrid carbon nanosheets with hierarchical porous structure for highperformance lithium-sulfur batteries. Adv. Energy Mater. (2014) 1301988. |
| [22] | B. Ding, C.Z. Yuan, L.F. Shen, Chemically tailoring the nanostructure of graphene nanosheets to confine sulfur for high-performance lithium-sulfur batteries. J. Mater. Chem. A 1 (2013) 1096–1101. DOI:10.1039/C2TA00396A |
| [23] | C. Wang, K. Su, W. Wan, High sulfur loading composite wrapped by 3D nitrogen-doped graphene as a cathode material for lithium-sulfur batteries. J. Mater. Chem. A 2 (2014) 5018–5023. DOI:10.1039/c3ta14921h |
| [24] | X.W. Wang, Z.A. Zhang, Y.H. Qu, Y.Q. Lai, J. Li, Nitrogen-doped graphene/sulfur composite as cathode material for high capacity lithium-sulfur batteries. J. Power Sources 256 (2014) 361–368. DOI:10.1016/j.jpowsour.2014.01.093 |
| [25] | Y.C. Qiu, W.F. Li, W. Zhao, High-rate, ultralong cycle-life lithium/sulfur batteries enabled by nitrogen-doped graphene. Nano Lett. 14 (2014) 4821–4827. DOI:10.1021/nl5020475 |
| [26] | G.M. Zhou, E. Paek, G.S. Hwang, A. Manthiram, Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge. Nat. Commun. 6 (2015) 7760. DOI:10.1038/ncomms8760 |
| [27] | H.W. Chen, C.H. Wang, W.L. Dong, Monodispersed sulfur nanoparticles for lithium-sulfur batteries with theoretical performance. Nano Lett. 15 (2015) 798–802. DOI:10.1021/nl504963e |
| [28] | D. Zhou, Y. Cui, P.W. Xiao, M.Y. Jiang, B.H. Han, A general and scalable synthesis approach to porous graphene. Nat. Commun. 5 (2014) 4716. DOI:10.1038/ncomms5716 |
| [29] | D.C. Marcano, D.V. Kosynkin, J.M. Berlin, Improved synthesis of graphene oxide. ACS Nano 4 (2010) 4806–4814. DOI:10.1021/nn1006368 |
| [30] | Z.S. Wu, A. Winter, L. Chen, Three-dimensional nitrogen and boron codoped graphene for high-performance all-solid-state supercapacitors. Adv. Mater. 24 (2012) 5130–5135. DOI:10.1002/adma.201201948 |
| [31] | C. Tang, Q. Zhang, M.Q. Zhao, Nitrogen-doped aligned carbon nanotube/graphene sandwiches:facile catalytic growth on bifunctional natural catalysts and their applications as scaffolds for high-rate lithium-sulfur batteries. Adv. Mater. 26 (2014) 6100–6105. DOI:10.1002/adma.201401243 |
| [32] | L.F. Xiao, Y.L. Cao, J. Xiao, A soft approach to encapsulate sulfur:polyaniline nanotubes for lithium-sulfur batteries with long cycle life,. Adv. Mater. 24 (2012) 1176–1181. DOI:10.1002/adma.v24.9 |
| [33] | S.Q. Chen, X.D. Huang, B. Sun, Multi-shelled hollow carbon nanospheres for lithium-sulfur batteries with superior performances. J. Mater. Chem. A 2 (2014) 16199–16207. DOI:10.1039/C4TA03877K |
| [34] | S.K. Liu, K. Xie, Z.X. Chen, A 3D nanostructure of graphene interconnected with hollow carbon spheres for high performance lithium-sulfur batteries. J. Mater. Chem. A 3 (2015) 11395–11402. DOI:10.1039/C5TA00897B |
 2017, Vol. 28
2017, Vol. 28 


