Owing to rich physical properties, functionalities, and potential applications in switchable dielectric devices, signal processing, sensing, etc., phase transition materials have attracted intense interest for several decades [1-4]. Phase transition in materials is always accompanied by changed thermal capacity, motion of molecules or ions, as well as structural and electric physical properties [5-11]. It is crucial to design and to synthesize new molecule-based phase transition materials, not only for searching technologically useful materials, but also for studying physical properties and structure-property relationships [12-15]. Recently, much attention has been devoted to simple molecular-ionic crystals containing organic cations and acid radicals, due to the tenability of their special structural features and interesting physical properties [16-18]. As an organic cation, 1, 4-diazabicy- clo-[2.2.2]octane (DABCO) has recently been highlighted due to the designing of molecule-based phase transition materials in the crystal lattice upon ‘frozen' ordering or molecular rotation [19-23]. Interaction of the cations with monovalent tetrahedral counter anions (e.g. BF4-, PF6-) is expected to generate the phase transition materials, not only for the proneness to position changes with varying temperature, but also for the possibility of forming weak hydrogen bonds between N and F atoms [24-26]. 1-Isopropyl-1, 4- diazabicyclo[2.2.2]octan-1-ium, as a derivative of DABCO, has the possibility of disorder. Taking all these into consideration, as a continuation of our systematic studies of phase transition materials, we reported herein two novel phase transition materials, [C9H20N2)][Na(BF4)3] (1) and [C9H20N2][(PF6)2] (2). The synthesis and structures of low-temperature phase (LTP) and high-temperature phase (HTP) showed that they underwent reversible phase transitions at low temperature, which were characterized by variable-temperature single crystal X-ray diffraction (XRD), differential scanning calorimetry (DSC) and dielectric constant measurement.
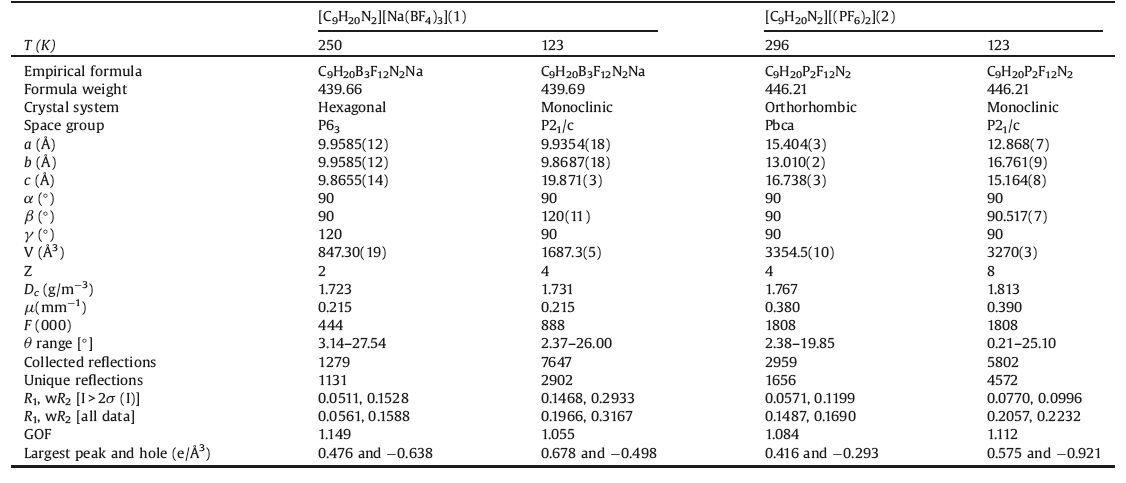
2. Results and discussion 2.1. DSCIt is well-known that DSC measurement is one of the useful thermodynamic methods to detect the dependence of reversible phase transition on temperature. When a compound undergoes structural phase transition accompanied by thermal entropy change, heat anomalies can be observed during heating and cooling. When the compound is subjected to disorder-order transition of ions or protons, reversible heat anomalies can be detected in DSC measurement upon heating and cooling. Heating the crystalline sample of 1 shows a main endothermic peak at 186.9 K and a main exothermic peak on cooling at 176.4 K (Fig. 1). The shapes of these two main peaks and the thermal hysteresis of 10.5K reveal discontinuous character of the transition, as a first- order phase transition. Meanwhile, the crystalline sample of 2 exhibits a main endothermic peak at 135.4K and a main exothermic peak on cooling at 130.9K (Fig. 2). The shapes of these two main peaks and the thermal hysteresis of 4.5 K also reveal that 2 was a first-order phase transition.
|
Download:
|
| Figure 1. DSC curves of 1 obtained in a heating-cooling mode. | |

|
Download:
|
| Figure 2. DSC curves of 2 obtained in a heating–cooling mode. | |
2.2. Crystal structure of 1
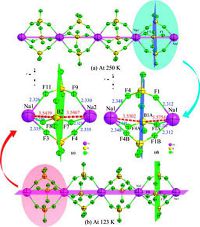
The phase transition of 1 was further confirmed by determining the crystal structures at 250 K and 123 K, respectively (Fig. 3a-b). In HTP (250K), the crystals are in the hexagonal space group P63 (No.173), with a = 9.9585(12) Å, b = 9.9585(12) Å, c = 9.8655(14) Å, α = β = 90°,γ=120°,V=847.30(19) Å3 and Z = 2. When cooled to 123K, the crystals become monoclinic space group P21/c (No.14), with a = 9.9354(18) Å, b = 9.8687(18) Å, c = 19.871(3) Å, α= γ = 90°, β = 120°, V=1687.3(5) Å3 and Z = 4. The phase transition can be elucidated by doubling of the cell volume and c-axis without changing a-axis or b-axis. Molecular (ionpair) volume nearly doubles from 847.30(19) Å3 to 1687.3(5) Å3.
|
Download:
|
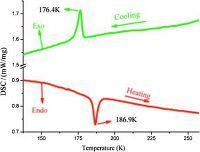
| Figure 3. View of coordination environment of 1 with atomic numbering scheme at (a) 250 K, symmetry codes: A:1 - y, -1 + x - y, z; B:2 - x + y,1 - x, -z; C:x - y, -1 + x, -0.5 + z; D:2 - x, -y, -0.5 + z; (b) 123 K, symmetry codes: A:2 - x, -1 - y, 1 - z; B:x, -1 + y, 1 - z; C:2 - x, -1 - y, 1 - z; D:2 - x, -y, 1 - z. | |
In HTP (250K) (Fig. 3a), the crystal structure of 1 contains one independent [C9H20N2]2+ cation and one [BF4]- anion with one sodium ion in different geometries. Sodium ions are connected with tetrafluoroborate by F atoms, each of which is connected with six fluorine ions. BF4- anion in the asymmetric unit adopts an ideal tetrahedron geometry, with the bond distances of B-F ranging from 1.356(3)Å to 1.391(4) Å and the bond angles ofF-B-F ranging from 108.0(3)° to111.2(3)°, being in good agreement with those in similar compounds [27-29]. Meanwhile, the bond distances of Na- F are from 2.313(3) Å to 2.349(3) Å, and the bond angles ofF-Na-F are from 85.81° to 173.73°.
In the crystal structure of LTP (123 K) (Fig. 3b), an asymmetric unitconsistsofone[C9H20N2]2+cation, three[BF4]- anionsandtwo sodium ions. BF4- anion in the asymmetric unit also adopts an ideal tetrahedron geometry, with the B-F distances from 1.359(11) Å to1.408(11)Å and the F-B-F angles from 107.9(9)° to 111.9(11)°, which are comparable with those in HTP. Meanwhile, the bond distances of Na-F are from 2.327(7) Å to 2.336(8) Å, and the bond angles of F-Na-F are from 89.6° to 180.0°, which are slightly different from those in HTP. The torsion angles of N-C-C-N are 24.5° at 250 K. Moreover, N-C-C-N in DABCO rings exhibits large twisting conformations with the torsion angles of 26°, 24° and 28° at 123 K, suggesting that the rings are seriously distorted compared with those in HTP.
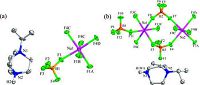
It is interesting that simple molecular-ionic crystals contain one-dimensional chains which are better shown by light pink planes. At different temperatures, the anionic group forms two similar one-dimensional chain structures with different bond angles of F-B-Na (Fig. 4). Na atoms in [Na(BF4)3]n are six- coordinated by F atoms from tetrafluoroborate, with the F4-B1 - Na1 and F1-B1-Na1A bond angles of 25.18° and 18.22° respectively. In LTP, the bond angles of F9-B1 -Na2 and F11 -B1 -Na1 are 21.65° and 21.52° respectively, being different from those in HTP. Tetrafluoroborate is stabilized by being frozen, accompanied bythe changed angles from HTP to LTP.
|
Download:
|
| Figure 4. One-dimensional chain structures of 1 at (a) (d) 250K, (b) (c) 123K. | |
In the HTP crystal structure of 1, the central F3, F2, F2A, F3A, F2B and F3B atoms deviate from the plane through B1, B1A and B1B atoms. Differently, in the LTP crystal structure of 1, B1A, B2 and B3 atoms also lie on a light green plane on which central F12, F5, F6, F1 and F2 atoms are located. Consequently, the diagrams and details of bond lengths are outlined by red or blue numbers in Fig. 4c and d respectively, which differ at different temperature.
It is easier to understand the overall structure by focusing on the extended acplane. A central snowflake-like anion is surrounded by six cationic groups, each of which is adjacent to anions (on b-axis). This gives an overall hexagonal arrangement as shown in Fig. 5a. As a weak base, DABCO is an excellent electron- donating species for hydrogen bonding. The crystal structure exhibits hydrogen-bonding between the tertiary amine of monocationic DABCO and F atoms (Fig. 5b), and the distances of hydrogen bonds range from 2.274Å to 2.777Å (Table S3 in Supporting information). As a result, compound 1 forms a similar 3D structure, with the topology structure like a honeycomb.
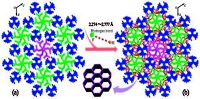
|
Download:
|
| Figure 5. The molecular lattice of 1, the view is of the extended ac plane. | |
Phase transition is often accompanied by Landau symmetry change. Compound 1 undergoes a phase transition at 176.4 K from HTP with a space group of P63 to LTP with a space group of P21/c. As shown in Fig. 6, the four kinds of symmetric elements (E, C2, 2C6, 2C3) in HTP (above 176.4 K) have changed two kinds of symmetric elements (E, C2, i, σh) in LTP (below 176.4 K), which suggested the occurrence of phase transition.
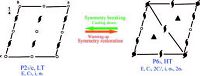
|
Download:
|
| Figure 6. Changes of symmetry operations of 1 from P63 in HTP to P21/c in LTP. | |
2.3. Crystal structure of 2
The phase transition of 2 was further confirmed by determining its crystal structures at 296K and 123K, respectively. In HTP (296K), the crystals are in the orthorhombic space group Pbca (No.61), with a=15.404(3) Å, b = 13.010(2) Å, c = 16.738(3) Å, α = β = γ = 90°, V = 3354.5(10) Å3 and Z = 4. When cooled to 123K, the crystals transform into monoclinic space group P21/c (No.14), with a = 12.868(7) Å, b= 16.761(9) Å, c= 15.164(8) Å, β = 90.517(7)°, V = 3270(3) Å3 and Z = 8. At the two temperatures, the crystallographic data are obviously different.
In the crystal structure of 2 in HTP (Fig. 7a), both unit cells contain independent [C9H20N2]2+ cations and two discrete [PF6]- anions in different geometries. One of the anions is seriously disordered, with each F atom over two positions, which may result in the formation of a higher symmetry (Pbca). The other PF6- anions in the asymmetric unit adopt an ideal octahedral geometry, with the bond distances of P-F from 1.553(4) Å to 1.584(3) Å and the bond angles of F-P-F from 89.3(2)°to 178.3(2)°(Table S2 in Supporting information), being in good agreement with those in similar compounds [30-32]. There is also one hydrogen bond N2- H2…F12#1, with the bond length and bond angle of 2.9247(99) Å and 147.64°, respectively.

|
Download:
|
| Figure 7. View of coordination environment of 1 with atomic numbering scheme at (a) 296 K, (b) 123 K, symmetry codes: #1 1/2 - x,y + 1/2,z. | |
In LTP (123 K) (Fig. 7b), an asymmetric unit consists of two [C9H20N2]2+ cations and four discrete [PF6]- anions, but one of the anions remains disordered as mentioned above. Other PF6- anions in the asymmetric unit adopt an ideal octahedral geometry respectively, with the bond distances of P-F from 1.586(4) Å to 1.632(4)Å and the bond angles of F-P-F from 88.4(2)°to 179.8(3)°,which are comparable with those in HTP. Moreover, there are two kinds of hydrogen bonds in LTP, N-H - F and C-H - F (Fig. 8). Hexafluorophosphate acts as a bis-bidentate bridging mode that gives rise to a one-dimensional chain by hydrogen bonds. The bond lengths of N-H-…F are from 2.920 Å to 3.052 Å and the bond angles are from 123° to 139° (Table S4 in Supporting information). The bond lengths of C-H-…F are from 3.398 Åto 3.4729Å and the bond angles are from 121° to 136°.

|
Download:
|
| Figure 8. One-dimensional chain structures of 2 are formed by hydrogen bonds at 123 K. | |
Meanwhile, conformations of DABCO rings show significant differences between HTP (296K) and LTP (123K). The torsion angles of N-C-C-N are 0.483-2.146° at 296 K. N-C-C-N in the two DABCO rings exhibits large twisting conformations, with the torsion angles of 19.463°, 18.552° and 19.144° as well as 21.249°, 21.008°and 23.171°respectively at 123 K, suggesting that the rings are seriously distorted compared with those in HTP. Such phase transition may be driven by the seriously distorted DABCO and the increase of hydrogen bonds from HTP to LTP.
X-ray crystal structures of 2 were measured at 260K, 240K, 220 K, 200 K, 180 K, 160 K, 140 K and 120 K. The cell parameters of 2 measured from 260K to 140K are slightly different from those d at room temperature, i.e.the effects of thermal m and contraction can be excluded. Thus, there is no ansition at 140K. Particularly, the cell parameters of 2 at iange dramatically vs. those at 140K. Besides, the cell parameters of 2 measured from 260K to 140K are almost identical, indicating the absence of phase transition . β angle in the LTP structure moderately changes compared to that in HTP. However, the lengths of a-axis, b-axis and c-axis change distinctly. Therefore, the phase transition between 140K and 120K is consistent with that observed by DSC.
Compound 2 undergoes a phase transition from HTP with a space group of Pbca to LTP with a space group of P2i/cat 130.9 K, accompanied by Landau symmetry breaking with an Aizu notation of mmm F2/m [33]. As shown in Fig. 9, the eight symmetric elements (E, C2, C2',C2'',i, σh,σv, σd) in HTP (above 130.9K) are halved into four (E, C2, i, σh) in LTP (below 130.9 K) owing to the loss of C2', C2'',σv and σd.

|
Download:
|
| Figure 9. Changes of symmetry operations of 2 from Pbca at HTP to P21/c at LTP. | |
2.4. Dielectric properties
Dielectric property undergoes abrupt changes in the vicinity of phase transition, while the magnitude of the variations is related to the characteristics of such transition [34]. We measured the dielectric changes of 1 and 2 at different frequencies.
The dielectric permittivity of 1 was measured in the frequency range between 1 kHz and 1 MHz (Fig. 10a). Unexpectedly, there is no obvious dielectric anomaly in the measured frequency range, probably because large molecules rotate slowly in the electric field at low temperature [35-37].

|
Download:
|
| Figure 10. Temperature-dependent dielectric constant of 1 (a) and 2 (b) at different frequencies. | |
The dielectric permittivity of 2, which is temperature-dependent, was measured at 100 kHz and 1 MHz (Fig. 10b). As expected, a clear dielectric anomaly occurs at about 141 K upon cooling and again at about 144 K upon heating. Therefore, the slight dielectric anomaly reveals the phase transition character [38-40], being consistent with the DSC result and changes of cell parameters at approximately 140 K.
|
|
Table 1 Crystallographic data for 1 and 2 at different temperature. |
3. Conclusion
In summary, variable-temperature structural analysis, DSC and dielectric measurements revealed that 1 and 2 underwent reversible phase transitions. The ordering of twisting motions of DABCO rings along with the formation of hydrogen bonds probably drove the transitions.
4. Experimental 4.1. Materials and methodsAll reagent-grade chemicals and solvents were obtained from commercial sources and used without further purification. Infrared (IR) spectra were recorded on a SHIMADZU IR prestige-21 FTIR-8400S spectrometer in the range of 4000- 500cm-1 with samples in the form of potassium bromide pellets (Figs. S1 and S2 in Supporting information). Elemental analyses were taken on a PerkinElmer 240C elemental analyzer. Thermo gravimetric analyses (TGA) were conducted on a NETZSCH TG 209 F3 thermo gravimeter with the heating rate of 10 K/min in a N2 atmosphere (Figs. S3 and S4 in Supporting information). PowderX- ray diffraction (PXRD) patterns of 1 and 2 at room temperature match very well those simulated from the single-crystal structures, indicating the samples are single-phase (Figs. S6 and S7 in Supporting information).
4.2. Preparation of compound 11-Isopropyl-1, 4-diazabicyclo[2.2.2]octan-1-ium (3.105 g, 20 mmol) and sodium tetrafluoroborate (2.199 g, 20 mmol) were mixed in aqueous solution (20 mL) (Scheme 1). After being stirred for 30 min in air, the solution of reaction mixture was evaporated slowly at room temperature for 7 days, and yellow bulk crystals were obtained in 69% yield (based on sodium tetrafluoroborate). IR data of 1 (KBr pellet, cm-1): n 3421 (s), 3226 (s), 2522 (w), 2366 (w), 2266 (w), 1476 (s), 1417 (s), 1190 (m), 1055 (vs), 827 (m), 788 (m), 634 (w), 530 (m). Anal. (%) calcd. for C9H20B3F12N2Na: C, 24.64; H, 4.37; N, 6.39; Found: C, 24.52; H, 4.58; N, 6.17. From the TG measurement, compound 1 was stable up to 100 ℃.
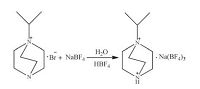
|
Download:
|
| Scheme1. Synthesis of compound 1. | |
4.3. Preparation of compound 2
1-Isopropyl-1, 4-diazabicyclo[2.2.2]octan-1-ium (3.105 g, 20 mmol) and hexafluorophosphoric acid (2.919 g, 20 mmol) were mixed in aqueous solution (20mL) (Scheme 2). After being stirred for 30 min in air, the solution of reaction mixture was evaporated slowly at room temperature for 2days, and colorless bulk crystals were obtained in 74% yield (based on hexafluorophos- phoric acid). IR data of 2 (KBr pellet, cm-1): n 3406 (s), 3240 (w), 3014 (m), 2819 (w), 1633 (m), 1479 (m), 1411 (m), 999 (w), 840 (vs), 553 (m). Anal. (%) calcd. for C9H20P2F12N2: C, 24.23; H, 4.52; N, 6.28; Found: C, 24.19; H, 4.47; N, 6.17. From the TG measurement, compound 2 was stable up to 200℃. (Caution! Although no problems have been encountered herein, sodium tetrafluoroborate and hexafluorophosphoric acid are potentially corrosive and should be handled with care and only in small quantities).

|
Download:
|
| Scheme2. Synthesis of compound 2. | |
4.4. X-ray structure determination
Crystallographic data for 1 and 2 with appropriate dimensions were collected at different temperatures on a Bruker SMART APEX-II CCD diffractometer equipped with Mo-Ka radiation (λ = 0.71073 Å). Absorption corrections were applied by using SADABS. The structures were solved by direct methods and refined with full-matrix least-squares technique using SHELXTL-97 software package [41, 42]. All non-hydrogen atoms were refined anisotropically, and hydrogen atoms were located and included at their geometrically idealized positions. Distances and angles between some atoms were calculated using DIAMOND and other calculations were carried out using SHELXTL. Crystallographic data and details of collection and refinement of two compounds at different temperatures are given in Table 1. Nos. CCDC-1423809, 1423810, 1423811 and 1423812 contain the supplementary crystallographic data of two compounds for this article. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_re-quest/cif.
4.5. Dielectric measurementsTemperature dependences of the dielectric constants of two compounds were measured on Tonghui TH2828A analyzer in the temperature ranges of 120-280 K and 100-270 K, respectively, within the frequency range of 1 kHz-1 MHz at the AC voltage of 1V. Pellet samples were prepared at 20 MPa and the pressed powder pellets deposited with silver-conductive glue were used for dielectric studies.
4.6. DSC measurementDSC runs of crystals 1 (2.67 mg) and 2 (8.17 mg) were recorded using a PerkinElmer Diamond DSC instrument in the ranges of 140 K to 260 K and 120 K to 180 K on cooling/heating under nitrogen with a heating rate of 10 K/min at atmospheric pressure in aluminum crucibles, respectively.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 21671084), NSF of Jiangsu Province (No. BK20131244), Six talent peaks project in Jiangsu Province (No. 2014-XCL-008); the Qing Lan Project of Jiangsu Province, the Innovation Program of Graduate Students in Jiangsu Province (No. KYLX16-0508), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institution; Innovation Program for Graduate Student from Jiangsu University of Science and Technology (No.YCX15S-19) and the Foundation of Jiangsu Educational Committee (No.16KJB430011).
| [1] | J.F. Scott, C.A.P. de Araujo, Ferroelectric memories. Science 246 (1989) 1400–1405. DOI:10.1126/science.246.4936.1400 |
| [2] | L.Z. Chen, D.D. Huang, J.Z. Ge, F.M. Wang, Temperature-induced reversible structural phase transition of 1-(chloromethyl)-1,4-diazoniabicyclo[2.2.2] octane bis(perchlorate). CrystEngComm 16 (2014) 2944–2949. DOI:10.1039/c3ce42190b |
| [3] | H.M. Zheng, J.B. Rivest, T.A. Miller, Observation of transient structuraltransformation dynamics in a Cu2S nanorod. Science 333 (2011) 206–209. DOI:10.1126/science.1204713 |
| [4] | L.Z. Chen, H. Zhao, J.Z. Ge, R.G. Xiong, H.W. Hu, Observation of deuteration effect in co-crystal system:hexamethylenetetraminium 3,5-dinitrobenzoate hemideuterated water. Cryst. Growth Des. 9 (2009) 3828–3831. DOI:10.1021/cg900348x |
| [5] | M. Wojta s, R. Jakubas, J. Zaleski, G. Bator, J. Baran, The phase situation and ferroelectric properties in the mixed crystals[4-NH2 PyH] [SbCl4(1-x)Br4x]. J. Mol. Struct. 887 (2008) 262–268. DOI:10.1016/j.molstruc.2008.02.050 |
| [6] | O. Czupinski, R. Jakubas, A. Pietraszko, On structural phase transitions in 4-aminopyridinium fluoroborate,[4-NH2C5H5N] [BF4]:differential scanning calorimetry, dielectric and infrared studies. J. Mol. Struct. 704 (2004) 177–187. DOI:10.1016/j.molstruc.2004.01.066 |
| [7] | B. Kulicka, T. Lis, V. Kinzhybalo, R. Jakubas, A. Piecha, Novel anionic watercontaining inorganic fragment in[4-NH2PyH]8[Bi2Cl11] [Bi2Cl9(H2O)2]:structural characterization, thermal, dielectric and vibrational properties. Polyhedron 29 (2010) 2014–2022. DOI:10.1016/j.poly.2010.03.017 |
| [8] | D.W. Fu, W. Zhang, H.L. Cai, Diisopropylammonium chloride:a ferroelectric organic salt with a high phase transition temperature and practical utilization level of spontaneous polarization. Adv. Mater. 23 (2011) 5658–5662. DOI:10.1002/adma.v23.47 |
| [9] | A. Piecha, R. Jakubas, A. Pietraszko, Phase transitions and electric properties of imidazolium chlorobismuthate(III):[C3H5N2]6[Bi4Cl18]. J. Mol. Struct. 829 (2007) 149–154. DOI:10.1016/j.molstruc.2006.06.017 |
| [10] | L.Z. Chen, D.D. Huang, J.Z. Ge, Q.J. Pan, Reversible ferroelastic phase transition of N-chloromethyl-1,4-diazabicyclo[2.2.2] octonium trichlorobromoaquo copper(II). Inorg. Chem. Commun. 45 (2014) 5–9. DOI:10.1016/j.inoche.2014.03.037 |
| [11] | D. Paliwoda, K.F. Dziubek, A. Katrusiak, Imidazole hidden polar phase. Cryst. Growth Des. 12 (2012) 4302–4305. DOI:10.1021/cg300852t |
| [12] | J.Z. Ge, X.Q. Fu, T. Hang, Q. Ye, R.G. Xiong, Reversible phase transition of the 1:1 complexes of 18-crown-6 with 4-ethoxyanilinium perchlorate. Cryst. Growth Des. 10 (2010) 3632–3637. DOI:10.1021/cg100523b |
| [13] | Y. Zhang, W.Q. Liao, H.Y. Ye, D.W. Fu, R.G. Xiong, Reversible phase transition of 1,4-diazoniabicyclo[2.2.2] octane-1-acetate-4-acetic acid chloride trihydrate. Cryst. Growth Des. 13 (2013) 4025–4030. DOI:10.1021/cg400829d |
| [14] | B. Rodríguez-Molina, N. Farfa'n, M. Romero, Anisochronous dynamics in a crystalline array of steroidal molecular rotors:evidence of correlated motion within 1D helical domains. J. Am. Chem. Soc. 133 (2011) 7280–7283. DOI:10.1021/ja2006274 |
| [15] | S. Yahyaoui, W. Rekik, H. Naïli, T. Mhiri, T. Bataille, Synthesis, crystal structures, phase transition characterization and thermal decomposition of a new dabcodiium hexaaquairon(II) bis(sulfate):(C6H14N2)[Fe(H2O)6] (SO4)2. J. Solid State Chem. 180 (2007) 3560–3570. DOI:10.1016/j.jssc.2007.10.019 |
| [16] | M.L. Liu, Tetrakis(4-methoxyanilinium) hexachloridobismuthate(III) chloride monohydrate. Acta Crystalllogr. Sect. E Struct. Rep. 68 (2012) m652. DOI:10.1107/S1600536812017096 |
| [17] | L.Z. Chen, D.D. Huang, Q.J. Pan, J.Z. Ge, Novel pure Pnma-P212121 ferroelastic phase transition of 1,4-diisopropyl-1,4-diazonia-bicyclo[2.2.2] octane tetrachlorobromo-M(Ii) (M=Mn and Co). RSC Adv. 5 (2015) 13488–13494. DOI:10.1039/C4RA12690D |
| [18] | L.Z. Chen, X.X. Cao, D.D. Huang, Q.J. Pan, Switchable dielectric phase transition in tris(1-(chloromethyl)-1,4-diazabicyclo[2.2.2] octane) tetra(tetrafluoroborate) dichloride. Inorg. Chem. Commun. 61 (2015) 93–96. DOI:10.1016/j.inoche.2015.09.001 |
| [19] | Y. Zhang, W. Zhang, S.H. Li, Ferroelectricity induced by ordering of twisting motion in a molecular rotor. J. Am. Chem. Soc. 134 (2012) 11044–11049. DOI:10.1021/ja3047427 |
| [20] | N.L. Nkhili, W. Rekik, H. Naïli, T. Mhiri, T. Bataille, Crystal structure phase transition and thermal behaviour of dabcodiium hexaaquacopper(II) bis (selenate/sulfate), (C6H14N2)[Cu (H2O)6] (S0.66Se0.34O4)2. J. Chem. Crystallogr. 41 (2011) 1680–1687. DOI:10.1007/s10870-011-0157-9 |
| [21] | W. Rekik, H. Naïli, T. Bataille, T. Mhiri, Synthesis crystal structure, phase transition and thermal behaviour of a new dabcodiium hexaaquanickel(II) bis (sulphate), (C6H14N2)[Ni(H2O)] 6(SO4)2. J. Organomet. Chem. 691 (2006) 4725–4732. DOI:10.1016/j.jorganchem.2006.07.019 |
| [22] | W. Zhang, H.Y. Ye, H.L. Cai, Discovery of new ferroelectrics:[H2dbco]2·[Cl3]·[CuCl3(H2O)2]·H2O (dbco=1,4-diaza-bicyclo[2.2.2] octane). J. Am. Chem. Soc. 132 (2010) 7300–7302. DOI:10.1021/ja102573h |
| [23] | L.Z. Chen, D.D. Huang, Q.J. Pan, L. Zhang, Temperature-induced isosymmetric reversible structural phase transition in[Cl2Cd(dabco-CH2Cl)]2(μ-Cl)2. J. Mol. Struct. 1078 (2014) 68–73. DOI:10.1016/j.molstruc.2014.03.030 |
| [24] | L.Z. Chen, X.X. Cao, D.D. Huang, Q.J. Pan, Temperature-induced reversible structural phase transition of 1,4-dimethyl-1,4-diazabicyclo[2.2.2] octane bis (perchlorate). RSC Adv. 5 (2015) 55914–55919. DOI:10.1039/C5RA07526B |
| [25] | L.Z. Chen, Q.J. Pan, X.X. Cao, F.M. Wang, Crystal structure, magnetism, and dielectric properties based on the axially chiral ligand 2,2'-dinitro-4,4'-biphenyldicarboxylic acid. CrystEngComm 18 (2016) 1944–1952. DOI:10.1039/C5CE02426A |
| [26] | H.Y. Ye, H.L. Cai, J.Z. Ge, R.G. Xiong, Reversible structural phase transition of pyridinium-4-carboxylic acid perchlorate. J. Appl. Crystallogr. 43 (2010) 1031–1035. DOI:10.1107/S0021889810024039 |
| [27] | L. Alvarado, C. Brewer, G. Brewer, Supramolecular assemblies prepared from an iron(II) tripodal complex, tetrafluoroborate, and alkali metal cations. The effect of cation size on coordination number anion disorder and hydrogen bonding. CrystEngComm 11 (2009) 2297–2307. DOI:10.1039/b905061b |
| [28] | R.E. Banks, I. Sharif, R.G. Pritchard, Structure of the new electrophilic fluorinating agent 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2] octane bis(tetrafluoroborate) and two of its monoquaternary analogues. Acta Crystallogr. Scet. C Cryst. Sturct. Commun. 49 (1993) 492–495. DOI:10.1107/S0108270192008588 |
| [29] | A. Budzianowski, A. Katrusiak, M. Szafrański, Anomalous protonic-glass evolution from ordered phase in NH N hydrogen-bonded DabcoHBF4 ferroelectric. J. Phys. Chem. B 112 (2008) 16619–16625. DOI:10.1021/jp801316a |
| [30] | A. Peuronen, A. Valkonen, M. Kortelainen, K. Rissanen, M. Lahtinen, Halogen bonding-based catch and release:reversible solid-state entrapment of elemental iodine with monoalkylated DABCO salts. Cryst. Growth Des. 12 (2012) 4157–4169. DOI:10.1021/cg300669t |
| [31] | M. Albrecht, M. Müller, O. Mergel, K. Rissanen, A. Valkonen, CH-directed anion-π interactions in the crystals of pentafluorobenzyl-substituted ammonium and pyridinium salts. Chem. Eur. J. 16 (2010) 5062–5069. DOI:10.1002/chem.v16:17 |
| [32] | R.Q. Zhu, Bis(μ-nitrito-к2O:O)bis[bis(1-methyl-1H-imidazole-кN3)(nitrito-кO)copper(II)]. Acta Crystallogr. Sect. E Struct. Rep. 68 (2012) m398. DOI:10.1107/S1600536812009804 |
| [33] | K. Aizu, Possible species of ferromagnetic ferroelectric, and ferroelastic crystals. Phys. Rev. B 2 (1970) 754–772. DOI:10.1103/PhysRevB.2.754 |
| [34] | Y. Zhang, K. Awaga, H. Yoshikawa, R.G. Xiong, Ferroelastic phase transition and dielectric anomalies in 2,4,6-trimethylanilinium perchlorate. J. Mater. Chem. 22 (2012) 9841–9845. |
| [35] | D.H. Wu, L. Jin, Y. Zhang, Temperature-triggered reversible ferroelastic phase transition in an 1:1 inclusion complex of 18-crown[6]with 4-ethylanilinium tetrafluoroborate. Inorg. Chem. Commun. 23 (2012) 117–122. DOI:10.1016/j.inoche.2012.06.021 |
| [36] | L.Z. Chen, D.D. Huang, J.Z. Ge, Q.J. Pan, Temperature-induced reversible structural phase transition of N-chloromethyl-1,4-diazabicyclo[2.2.2] octonium trichloroaquo-manganese(II). J. Mol. Struct. 1072 (2014) 307–312. DOI:10.1016/j.molstruc.2014.05.041 |
| [37] | W.Q. Liao, Q.Q. Zhou, Y. Zhang, L. Jin, Synthesis structures and dielectric properties of two five-coordinate copper (II) complexes based on Nchloromethyl-1,4-diazabicyclo[2.2.2] octane. Inorg. Chem. Commun. 33 (2013) 161–164. DOI:10.1016/j.inoche.2013.04.031 |
| [38] | L.Z. Chen, D.D. Huang, Q.J. Pan, F.M. Wang, Ferroelectricity based on coordination compound:transition metal Co(II) sulfates templated by homochiral 2-methylpiperazine (C5H14N2)[Co(H2O)6] (SO4)2. Chin. Chem. Lett. 25 (2014) 967–972. DOI:10.1016/j.cclet.2014.05.038 |
| [39] | X.M. Zhang, S.S. Yu, H. Zhang, H.B. Duan, Magnetic and relax-like dielectric response behavior in a charge-transfer crystal. Chin. J. Inorg. Chem. 32 (2016) 25–33. |
| [40] | L.Z. Chen, D.D. Huang, Synthesis structure and dielectric properties of a novel Gd coordination polymer based on 2-(pyridin-4-yl)-1H-imidazole-4,5-dicarboxylate. Chin. Chem. Lett. 25 (2014) 279–282. DOI:10.1016/j.cclet.2013.11.031 |
| [41] | G.M. Sheldrick, SHELXS97, Program for Crystal Structure Solution, University of Göttingen, Germany, 1997. |
| [42] | G.M. Sheldrick, SHELXL97, Program for Crystal Structure Refinement, University of Göttingen, Germany, 1997. |
 2017, Vol. 28
2017, Vol. 28