b Key Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China
The solar spectrum consists mostly of ultraviolet, visible and infrared light. UV radiation from the sun is conventionally divided into three categories depending on its wavelength, UVC (100280nm), UVB (280-320nm) and UVA (320-400nm). Short wavelengths UVC radiation is completely absorbed in the ozone layer. The remaining UVB together with UVA reaching the earth surface, cause accelerated photodegradation of organic materials as well as damage our skin (such as sunburn, suntan, acceleration of aging, skin cancer, etc.)[1, 2]. Ultraviolet radiation has increased dramatically in recent years as a result of the deterioration of ozone depletion [3]. Therefore, the development of UV-shielding materials has attracted considerable attention in the field of coatings and sunscreens, so as to reduce the destruction of UV irradiation.
Sunscreens are used to protect the skin against the harmful effects of solar UV radiation. Based on their protection mechanism, sunscreens are classified as chemical absorbers and inorganic UV blocking materials. Inorganic sunscreens reflect and scatter UV and visible radiation, while organic sunscreens absorb UV radiation and then release energy as heat or light [4]. Organic absorbers possess excellent UV absorption ability. However, they may pose safety problems when used at higher concentrations such as allergies, photoallergic reactions etc.[5]. Generally, inorganic UV blocking materials (TiO2and ZnO ultrafine particles) have been widely used, owing to their optical and thermal stability as well as nontoxicity. However, their photocatalytic activity as a side-effect would induce photodegradation of organic substances and damage the living organism simultaneously, which may be a serious problem because it takes place on the skin. Therefore, it is necessary to search for novel anti-ultraviolet materials with high safety stability and excellent light shielding behavior [6].
Layered double hydroxides (LDHs), are a well-known type of anionic clay with the general formula [M1-xIIMxIII(OH)2](An-)x/n·mH2O, MII and MIII are divalent and trivalent metals respectively, An- symbolizes various interlamellar anions, m is the moles of water molecules located in the interlayer galleries together with the anions [7]. The identities of the di- and trivalent cations and the interlayer anion together with the value of the stoichiometric coefficient (x) may be varied over a wide range, giving rise to a large class of isostructural materials, which have been widely explored in the fields of catalysis, separation, electrode materials, polymer additives and functional additives [8-12]. Intercalation of UV absorbers into the interlayer galleries of LDHs for sunscreen materials has been reported a lot (e.g., Perioli et al. [13] studied the safety and photostability properties of ZnAl-LDH, MgAl-LDH intercalated with PABA; Sun et al. [14] studied ZnAl-LDH intercalated with cinnamic acid series organic UV ray absorbents, etc.). However, LDH-based UV-shielding materials mostly are MgAl-LDH, ZnAl-LDH, the reported of ZnTi-LDH is seldom. Shi et al. [15] have studied the UV-shielding mechanism of layered double hydroxide materials. Both the experimental and theoretical results show that the introduction of Zn element is an effective way to tune the electron structure, band gap, transition mode and resulting UV-shielding properties. In an attempt to optimize the UV shielding properties of LDHs, in the present work, we have systematically explored the UV absorbing and screening properties of LDHs with different amounts of titanium cations in the layer. The UV shielding properties of the materials have been investigated experimentally and theoretically (by density functional theory calculations). Besides, compared with TiO2and ZnO, the ability of ZnTi-LDHs to generate free radicals was also evaluated by means of electron spin resonance (EPR).
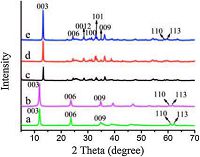
2. Results and discussion 2.1. Structural and morphological characterization of LDH 2.1.1. Powder X-ray diffractionFig. 1 illustrates the powder XRD patterns of MgAl-LDH, ZnAl- LDH and ZnTi-LDHs with different initial molar ratio of Zn2+/Ti4+ (2:1, 3:1 and 4:1). The MgAl-LDH and ZnAl-LDH exhibit well- developed layer structure with narrow, symmetric, strong peaks at low 2θ values (the basal (0 0 3) reflection and higher harmonics (0 0 6) and (0 0 9)) and weaker, less symmetric lines at high 2θ values, including the two (110) and (113) reflections between 60 and 63°. ZnTi-LDHs with different molar ratio show a strong(0 0 3) peak reflection appear at 13.22°, the reflections of (0 0 6), (0 0 9), (110) and (113) which can be indexed to typical LDH materials were also observed [16]. It should be noted that several weak reflections at 2θ = 22, 31, and 36° for ZnTi-LDH were observed, which can be assigned to a small amount of zinc hydroxide impurity (PDF card no. 20-1437). It was found that the basal reflections of ZnTi-LDHs (2θ = 13.22°, d003 = 0.669nm), in contrast to MgAl-LDH (2θ=11.91°, d003= 0.742 nm) and ZnAl-LDH (2θ = 12.03°, d003 = 0.73 5 nm) display a little shift to higher scattering angle, the results indicate that the basal spacing decreases with the incorporation of tetravalent metal (Ti) in hydrotalcite materials. This is in accordance with the previous report on Ti-containing LDHs [17, 18]. In the case of ZnxTi-LDHs, both the intensity and half-peak width show no significant change with various Zn2+/Ti4+ ratios, demonstrating chemical composition imposes no obvious effect on the crystallinity of LDHs.
|
Download:
|
| Figure 1. X-ray powder diffraction patterns of (a) MgAl-LDH, (b) ZnAl-LDH and ZnTi-LDH with initial molar ratio of (c) Zn2+/Ti4+ = 2:1, (d) Zn2+/Ti4+ = 3:1, (e) Zn2+/Ti4+ = 4:1. | |
2.1.2. FT-IR spectroscopy
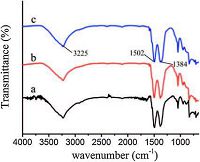
The FT-IR spectra of ZnTi-LDH with different initial molar ratio of Zn2+/Ti4+ are shown in Fig. 2. The spectrum of ZnTi-LDH (Fig. 2) shows a strong broad absorption band between 3500 and 3100 cm-1 due to the OH stretching mode of layer hydroxyl groups and of interlayer water molecules. The band at 1502 cm-1 together with its accompanying band at 1384cm-1 is ascribed to mode ν3 of interlayer carbonate species [19].
|
Download:
|
| Figure 2. FT-IR spectra of ZnTi-LDH with different initial molar ratio of (a) Zn2+/ Ti4+ = 2:1, (b)Zn2+/Ti4+ = 3:1and(c)Zn2+/Ti4+ = 4:1. | |
2.1.3. Elemental analysis
The results of chemical compositions of the ZnTi-LDH with different molar ratio of Zn2+/Ti4+ are listed in Table 1. The number of water molecule in the LDHs was determined by TG analysis (Figs. S1 -S3 in Supporting information). The molar ratio of metal cations in the layers is lower than the values in the nominal ratio. There are two reasons lead to the results. Firstly, the emergency of zinc hydroxide impurity in the synthesis process. Secondly, TiCU is easy to decompose in the air. Nevertheless, the changing tendency of metal cations in the layers is same to the nominal ratio.
|
|
Table 1 Chemical compositions of the ZnTi-LDH with different molar ratio of Zn2+/Ti4+. |
2.1.4. SEM and TEM
It has been reported in the literature that the UV blocking properties of LDH particles depend on both the physical morphology and chemical composition [20, 15]. For comparison, MgAl-LDH and ZnAl-LDH with the similar particle size distribution were synthesized under the same conditions as for the ZnTi-LDH. SEM and TEM study were carried out to study the micromorphology characteristics of MgAl-LDH, ZnAl-LDH and ZnTi-LDH (molar ratio Zn2+/Ti4+ of 2:1 to 4:1). As shown in Fig. 3A, the expected plate-like nature of the crystallites is clearly apparent in the micrograph of MgAl-LDH. ZnAl-LDH (Fig. 3B) appears as aggregates of sheet-like crystal. The particle size of MgAl-LDH and ZnAl-LDH is in the range ca. 0.5-2 μm. The synthesized ZnTi-LDH (Fig. 3C-E) displays clearly sheet-like morphology with a diameter of ca. 300nm-2 μm. The HRTEM micrographs (Fig. 3F) indicate the single-crystalline nature of the ZnTi-LDH material. The lattice with interplanar spacing of 0.38 nm was observed, corresponding to the (0 0 6) plane of ZnTi-LDH, which is in accordance with the XRD results.

|
Download:
|
| Figure 3. SEM images of (A) MgAl-LDH, (B) ZnAl-LDH, ZnTi-LDH with Zn2+/Ti4+ initial synthesis molar ratio of (C) 2:1, (D) 3:1, (E) 4:1; (F) HRTEM images of ZnTi-LDH with initial Zn2+/Ti4+ molar ratio of 3:1 | |
2.2. UV-shielding properties of ZnTi-LDHs
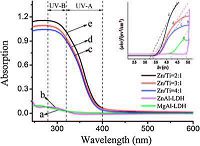
UV absorption curves of MgAl-LDH, ZnAl-LDH and ZnTi-LDH with different Zn2+/Ti4+ratio are shown in Fig. 4. As a result ofshort wavelengths UVC radiation is completely absorbed by the ozone layer in the upper atmosphere, UV radiation that reach the ground are the focus of our attention. The absorption spectra of MgAl-LDH, ZnAl-LDH and ZnTi-LDH are measured by samples with the same particle size distribution but different chemical composition and Zn2+/Ti4+ ratios. On account of the UV shielding effect of the LDH layers, the UV absorption intensity increases significantly below ~400nm. The UV absorption intensity of ZnTi-LDH almost ten times stronger than that of MgAl-LDH and ZnAl-LDH between 280 nm and 400 nm due to the presence oftitanium element in the layers. The results show that the UV absorption intensity enhances along with the increase of the Ti4+ content, indicating the Ti element facilitates the absorption of photons and the resulting electron transition. Moreover, UV-vis diffuse reflectance spectra of the samples were also used to determine the band gap (Fig. 4, inset), from which the band gap of ~4.15, ~4.86, ~3.20, ~3.27, -3.33 for (a) MgAl-LDH, (b) ZnAl-LDH, ZnTi-LDH with Zn2+/Ti4+ initial molar ratio of (c) 2:1, (d) 3:1, and (e) 4:1 were obtained.
|
Download:
|
| Figure 4. UV-visible absorbance spectra of (a) MgAl-LDH, (b) ZnAl-LDH, (c) ZnTi-LDH with initial molar ratio of Zn2+/Ti4+ = 2:1, (d) 3:1 and (e) 4:1. | |
To obtain the electronic structure information for the effect of the Ti element on the UV absorption capacity, the structures of ZnTi-LDH after geometry optimization are displayed in Fig. 5. Obviously, the matrix of each ZnTi-LDH model is uniform and carbonate anions are well distributed in the interlayer space of ZnTi-LDH. The band structures of ZnTi-LDH with different initial molar ratio are shown in Fig. S4 in Supporting information. The band gap energy of ZnTi-LDH with initial molar ratio of 2:1 (2.94eV), 3:1 (3.02eV), and 4:1 (3.04eV) increases with a decreasing amount of titanium in the layers, approximately matching with the value determined by the UV-vis diffuse reflectance spectra. Compared with MgAl-LDH and ZnAl-LDH (4.36eV and 3.50eV) [21], the decrease in band gap (2.94-3.04 eV) of the ZnTi-LDHs testifies the increase in photon absorption efficiency.

|
Download:
|
| Figure 5. The optimized geometry of ZnTi-LDH with Zn2+/Ti4+ initial synthesis molar ratio of (A) 2:1, (B) 3:1 and (C) 4:1. The color for each element is as follows: violet for Zn, silver for Ti, gray for C, red for O, and white for H, respectively. | |
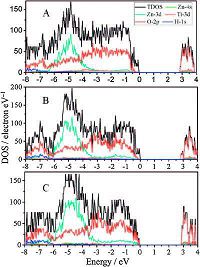
To better understand why the band gap energy of ZnTi-LDH decreases when Al3+ is substituted by Ti4+, the total density of states (TDOS) and partial densities of states (PDOS) of every element for each ZnTi-LDH matrix was analyzed and the results are displayed in Fig. 6. For Al-containing LDHs, such as ZnAl-LDH, the location of the conduction band minimum (CBM) is determined by the 4 s orbitals of zinc (from 3.2 eV to 5 eV) while the location of the valence band maximum (VBM) is determined by the 2p orbitals of oxygen (from -8 eV to 0 eV) and 3d orbitals of zinc (from -8eV to 0 eV) [15, 20, 21]. In contrast, the ZnTi-LDH samples possess a new peak originating from Ti-3d orbitals (from 2.75 to 4 eV) in the CBM, which is lower than that of Zn-4s. The lower energy of the CBM results in the lower band gap energies of Ti containin compared to Al-containing LDHs. Furthermore, this peak ii rises slightly with the amount of Ti4+ in the layers combi (Fig. S4), implying the enhancement of UV absorption c Therefore, both the experimental and theoretical results sh the introduction of Ti is an effective way to tune the el structure, band gap and UV-shielding property.
2.3. Photoactivity properties of ZnTi-LDHThe highly photocatalytic activity of TiO2 and ZnO re their application as inorganic UV absorbers. As a no shielding material, the photocatalytic performance of Zn with solar light response attracted our research interest [2 order to understand the redox ability of the ZnTi-LDH, th trapping electron spin resonance (EPR) technique was employed to monitor the presence of active radicals formed in the ZnTi-LDH, TiO2 and ZnO. Hydroxyl radicals (OH·) and superoxide anion radicals (O2·-) are the primary reactive oxygen species. The intensity of active radicals (OH· and O2·-) generated by the ZnTi- LDH with Zn2+/Ti4+ molar ratio of 2:1 is shown in Figs. 7 and 8. As shown in Fig. 7, the six typical peaks for DMPO-O2·- were observed in methanol. Besides, the four characteristic peaks for DMPO-OH· with intensity ratio of 1:2:2:1 were obviously observed in Fig. 8. The intensity of the samples corresponds to the generation of active radicals. Compared with TiO2 and ZnO, the EPR signal of ZnTi-LDH is remarkably weak. The results indicate that ZnTi-LDHs generated a relatively lower active radicals, which implied ZnTi- LDH is more safely as a UV-shielding material.
|
Download:
|
| Figure 6. The total density of states and partial densities of states for ZnTi- Zn2+/Ti4+ initial synthesis molar ratio of (A) 2:1, (B) 3:1 and (C) 4:1. | |
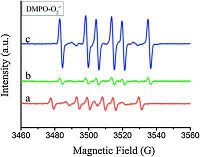
|
Download:
|
| Figure 7. DMPO spin-trapping EPR spectra recorded for (a) ZnTi-LDH with Zn2+/Ti4+ initial molar ratio of 2:1, (b) TiO2 and (c) ZnO at ambient temperature in methanol dispersion for DMPO-O2·- ([DMPO] = 0.20M, mcat = 2.5 mg, Vsolvent = 0.5 mL). | |

|
Download:
|
| Figure 8. DMPO spin-trapping EPR spectra recorded for (a) ZnTi-LDH with Zn2+/Ti4+ initial molar ratio of 2:1, (b) TiO2 and (c) ZnO at ambient temperature in aqueous dispersion for DMPO-OH· ([DMPO] = 0.20M, mcat = 2.5mg, Vsolvent = 0.5mL). | |
3. Conclusion
ZnTi-LDHs with varying Zn2+/Ti4+ molar ratio in the layers were successfully synthesized by the method of co-precipitation. Both the experimental and theoretical results show that the introduction of Ti element is an effective way to tune the electron structure, band gap, and the resulting UV-shielding properties. The remarkable UV shielding property of ZnTi-LDH is attributed to the decreasing band gap energy, as confirmed by density functional theory calculations. Besides, compared with common inorganic UV blocking materials TiO2 and ZnO, ZnTi-LDH generates remarkably lower active radicals, implying ZnTi-LDH is more suitable as a safe UV-shielding material. In addition, anion exchange properties of LDH materials provide the chances of incorporating organic UV absorbing materials in the interlayer galleries of ZnTi-LDH to avoid direct contact of organic molecules with human skin and enhance the UV ray resistance ability. Therefore, it is expected that the Zn-Ti LDH has an expansive application prospects in the field of sunscreens.
4. Experimental 4.1. MaterialsAnalyticalgradeZn(NO3)2·6H2O, TiCl4, urea,Mg(NO3)2·6H2O,Al (NO3)3·9H2O were purchased from Sinopharm Chemical Reagent Co., Ltd. and used without further purification. Deionized water was used in all the experimental processes.
4.2. Preparation of ZnTi-LDHZnTi-LDH was prepared by the co-precipitation of zinc and titanium salts from a homogeneous solution. TiCU (0.44mL), Zn (NO3)2·6H2O (2.38g) and urea (6.0g) were dissolved in deionized water (200 mL) under vigorous stirring. The resulting reactant was aged in an autoclave at 130 ℃ for 48h. The solid product was centrifuged, washed thoroughly with deionized water, and finally dried at 60℃ for 12 h. The ZnTi-LDHs with initial Zn2+/Ti4+ ratio of 3:1, 4:1 were prepared using the same procedure by varying Ti4+ dosage.
4.3. Preparation of MgAl-LDH and ZnAl-LDHAs a contrast, MgAl-LDH and ZnAl-LDH were prepared and investigated. MgAl-LDH was prepared as follows, 0.04mol/L Mg (NO3)2·6H2O, 0.02mol/L Al(NO3)3·9H2O and 0.24mol/L urea were dissolved in 100mL deionized water with vigorous stirring at room temperature. The resulting slurry was transferred in an autoclave and aged at 100 ℃ for 24 h. The precipitate was centrifuged, washed thoroughly with deionized water, and finally dried at 60 ℃ for 12 h.
ZnAl-LDH was synthesized by coprecipitation method, sufficient amountsofZn(NO3)2·6H2O, Al(NO3)3·9H2O and urea were dissolved in 100 mL deionized water to yield a solution containing 20 mmol/L Zn2+, 10mmol/L Al3+ and 70mmol/L urea. The mixed solution was heated at the refluxing temperature under continuous magnetic stirring for 24 h. The precipitate was centrifuged, washed thoroughly with deionized water, and finally dried at 60℃ for 12 h.
4.4. Characterization of samplesX-ray diffraction (XRD) measurements were performed on an X-ray powder diffractometer (Bruker D2, Karlsruhe, Germany), using Cu IKa radiation (λ = 1.5406Å) at 30kV, 10 mA, with a scanning rate of 5°/min in the 2θ range from 5° to 70°. The FT-IR spectra were recorded on a Thermo Scientific Nicolet IS 10 instrument equipped in attenuated total reflectance (ATR) mode. Elemental analyses were conducted by inductivelycoupled plasma (ICP) emission spectroscopy using solutions prepared bydissolving the samples in hot concentrated H2SO4. Thermogravimetry and differential thermal analysis (TG-DTA) curves were obtained on TGA Q5000IR instrument in the temperature range 20-650 ℃ with a heating rate of 10 ℃/min in air. Scanning electron microscopy (SEM) images were obtained using a Hitachi S-4700 scanning electron microscope operating at 20 kV. High resolution TEM (HRTEM) observations were carried out on a JEOL JEM- 2100 transmission electron microscope. Diffuse reflectance UV- vis absorbance spectra were recorded by a Shimadzu UV- 3700 spectrometer equipped with an integrating sphere attachment using BaSO4 as reference. The generation of radical species was monitored by electron spin resonance (EPR) spectroscopy (Bruker E500 EPR spectrometer) with dimethylpyridine N-oxide (DMPO) as a spin-trapping reagent. The samples are irradiated with a lamp simulating solar light for 1 min.
4.5. Model construction and computational methods 4.5.1. Model constructionAccording to the results of elemental analysis, three kinds ofZnTi- LDHs with different initial molar ratio of Zn/Ti were constructed. The chemical compositions of ZnTi-LDH with Zn2+/Ti4+ initial molar ratio of 2:1, 3:1 and 4:1 are Zn13Ti14(OH)54(CO32-)14, Zn18Ti9(OH)54(CO32-)9 and Zn20Ti7(OH)54(CO32-)7, respectively. The space group of these three models is p3m1, meaning that the lattice parameters α, β, and γ is 90°, 90° and 120°, respectively. The other three lattice parameters a, b, and c were referred to the powder X-ray diffraction observation.
4.5.2. Computational methodsAll the calculations were performed using the CASTEP code in the Materials Studio, version 6.1 software package (Accelrys software Inc., San Diego, CA) [24]. The DFT calculations were performed using a plane wave implementation [25] at the generalized gradient approximation (GGA) Perdew-Burke-Ern- zerhof (PBE) level [26]. Spin-polarized DFT + U theory is applied. In this work, the value of U-J (Ueff) is 6.0eV for Ti [27]. The structure optimization is based on the following points: (1) an energy tolerance of 1 × 10-5eV per atom; (2) a maximum force tolerance of 0.03 eV/Å; and (3) a maximum displacement tolerance of 1 × 10-3 Å. For the calculation of band structure and density of states (DOS) of ZnTi-LDHs, the T-point-centered k- point meshes used for the Brillouin zone integrations are 3 × 3 × 1 k-points.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21301012), the Development of High- Caliber Talents Project of Beijing Municipal Institutions (No. CIT & TCD 201504009), China Cosmetic Collaborative Innovation Center, BTBU and the Open Research Fund Program of Beijing Key Lab of Plant Resource Research and Development, BTBU. We are grateful to Prof. Zheshuai Lin (Chinese Academy of Sciences, Technical Institute of Physics and Chemistry) for his help in the DFT analysis.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j. cclet.2016.09.002.
| [1] | A.L. Andrady, H. Hamid, A. Torikai, Effects of solar UV and climate change on materials. Photochem. Photobiol. Sci. 10 (2011) 292–300. DOI:10.1039/c0pp90038a |
| [2] | I.A. Siddiquey, E. Ukaji, T. Furusawa, M. Sato, N. Suzuki, The effects of organic surface treatment by methacryloxypropyltrimethoxysilane on the photostability of TiO2. Mater. Chem. Phys. 105 (2007) 162–168. DOI:10.1016/j.matchemphys.2007.04.017 |
| [3] | R.L. McKenzie, P.J. Aucamp, A.F. Bais, L.O. Björn, M. Llyas, Changes in biologically-active ultraviolet radiation reaching the Earth's surface. Photochem. Photobiol. Sci. 6 (2007) 218–231. DOI:10.1039/B700017K |
| [4] | T.G. Smijs, S. Pavel, Titanium dioxide and zinc oxide nanoparticles in sunscreens:focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 4 (2011) 95–112. |
| [5] | T. Wong, D. Orton, Sunscreen allergy and its investigation. Clin. Dermatol. 29 (2011) 306–310. DOI:10.1016/j.clindermatol.2010.11.002 |
| [6] | J.C. Yu, J.G. Yu, W. Ho, Z.T. Jiang, L.Z. Zhang, Effects of F- doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 14 (2002) 3808–3816. DOI:10.1021/cm020027c |
| [7] | (a) P.J. Sideris, U.G. Nislsen, Z.H. Gan, C.P. Grey. Mg/Al ordering in layered double hydroxides revealed by multinuclear NMR spectroscopy. Science, 2008,321: 113-117;(b) R. Gao, M.J. Zhao, Y. Guan, et al., Ordered and flexible lanthanide complex thin films showing up-conversion and color-tunable luminescence. J. Mater. Chem. C, 2014,2: 9579-9586;(c) J.B. Han, Y.B. Dou, M. Wei, D.G. Evans, X. Duan. Erasable nanoporous antireflection coatings based on the reconstruction effect of layered double hydroxides. Angew. Chem. Int. Ed., 2010,49: 2171-2174;(d) D.P. Yan, M. Wei. Photofunctional Layered Materials (Structure and Bonding), Springer International Publishing, Switzerland. 2015. |
| [8] | D.P. Yan, J. Lu, M. Wei, Luminescent ultrathin film of anionic styrylbiphenyl derivative-layered double hydroxide and its reversible sensing for heavy metal ions.. Phys. Chem. Chem. Phys. 14 (2012) 8591–8598. DOI:10.1039/c2cp40350a |
| [9] | Q. Wang, D. O'Hare, Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 112 (2012) 4124–4155. |
| [10] | (a) H. Zhang, J. Zhang, R.P. Yun, et al., Nanohybrids of organo-modified layered double hydroxides and polyurethanes with enhanced mechanical, damping and UV absorption properties. RSC Adv., 2016,6: 34288;(b) G.R. Wang, S.M. Xu, C.H. Xia, et al., Fabrication of host-guest UV-blocking materials by intercalation of fluorescent anions into layered double hydroxides. RSC Adv., 2015,5: 23708-23714. |
| [11] | (a) R. Gao, X.D. Lei, M.X. Chen, D.P. Yan, M. Wei. Two-color polarized emission and angle-dependent luminescence based on layer-by-layer assembly of binary chromophores/layered double hydroxide thin films. New J. Chem., 2013,37: 4110-4118;(b) H.Y. Ma, R. Gao, D.P. Yan, J.W. Zhao, M. Wei. Organic-inorganic hybrid fluorescent ultrathin films and their sensor application for nitroaromatic explosives. J. Mater. Chem. C, 2013,1: 4128-4137;(c) Y.B. Zhao, H.Y. Lin, M.X. Chen, D.P. Yan. Niflumic anion intercalated layered double hydroxides with mechano-induced and solvent-responsive luminescence. Ind. Eng. Chem. Res., 2014,53: 3140-3147. |
| [12] | C.M. Li, M. Wei, D.G. Evans, X. Duan, Layered double hydroxide-based nanomaterials as highly efficient catalysts and adsorbents. Small 10 (2014) 4469–4486. DOI:10.1002/smll.v10.22 |
| [13] | L. Periolia, V. Ambrogia, B. Bertinia, Anionic clays for sunscreen agent safe use:photoprotection, photostability and prevention of their skin penetration. Eur. J. Pharm. Biopharm. 62 (2006) 185–193. DOI:10.1016/j.ejpb.2005.08.001 |
| [14] | W.L. Sun, Q.L. He, Y. Luo, Synthesis and properties of cinnamic acid series organic UV ray absorbents-interleaved layered double hydroxides. Mater. Lett. 61 (2007) 1881–1884. DOI:10.1016/j.matlet.2006.07.148 |
| [15] | W.Y. Shi, Y.J. Lin, S.T. Zhang, Study on UV-shielding mechanism of layered double hydroxide materials. Phys. Chem. Chem. Phys. 15 (2013) 18217–18222. DOI:10.1039/c3cp52819g |
| [16] | O. Saber, H. Tagaya, New layered double hydroxide, Zn-Ti LDH:preparation and intercalation reactions. J. Incl. Phenom. Macrocycl. Chem. 45 (2003) 107–115. DOI:10.1023/A:1023078728942 |
| [17] | C.G. Silva, Y. Bouizi, V. Fornés, H. García, Layered double hydroxides as highly efficient photocatalysts for visible light oxygen generation from water. J. Am. Chem. Soc. 131 (2009) 13833–13839. DOI:10.1021/ja905467v |
| [18] | M.F. Shao, J.B. Han, M. Wei, D.G. Evans, X. Duan, The synthesis of hierarchical Zn-Ti layered double hydroxide for efficient visible-light photocatalysis. Chem. Eng. J. 168 (2011) 519–524. DOI:10.1016/j.cej.2011.01.016 |
| [19] | X. Duan, D.G. Evans. Layered Double Hydroxides (Structure and Bonding), Springer-Verlag, Berlin, Heidelberg. 2006. |
| [20] | G.R. Wang, D.M. Rao, K.T. Li, Y.J. Lin, UV blocking by Mg-Zn-Al layered double hydroxides for the protection of asphalt road surfaces. Ind. Eng. Chem. Res. 53 (2014) 4165–4172. DOI:10.1021/ie403901n |
| [21] | S.M. Xu, T. Pan, Y.B. Dou, Theoretical and experimental study on MIIMIIIlayered double hydroxides as efficient photocatalysts toward oxygen evolution from water. J. Phys. Chem. C 119 (2015) 18823–18834. DOI:10.1021/acs.jpcc.5b01819 |
| [22] | Y.B. Dou, S.T. Zhang, T. Pan, TiO2@layered double hydroxide core-shell nanospheres with largely enhanced photocatalytic activity toward O2 generation. Adv. Funct. Mater. 25 (2015) 2243–2249. DOI:10.1002/adfm.201404496 |
| [23] | Y.B. Dou, J.B. Han, T.L. Wang, Fabrication of MMO-TiO2 one-dimensional photonic crystal and its application as a colorimetric sensor. J. Mater. Chem. 22 (2012) 14001–14007. DOI:10.1039/c2jm31560b |
| [24] | M.D. Segall, P.J.D. Lindan, M.J. Probert, First-principles simulation:ideas, illustrations and the CASTEP code. J. Phys.:Condens. Matter 14 (2002) 2717–2744. DOI:10.1088/0953-8984/14/11/301 |
| [25] | M.C. Payne, M.P. Teter, D.C. Allan, T.A. Arias, J.D. Joannopoulos, Iterative minimization techniques for ab initio total-energy calculations:molecular dynamics and conjugate gradients. Rev. Mod. Phys. 64 (1992) 1045–1097. DOI:10.1103/RevModPhys.64.1045 |
| [26] | J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77 (1996) 3865–3868. DOI:10.1103/PhysRevLett.77.3865 |
| [27] | N.A. Deskins, M. Dupuis, Intrinsic hole migration rates in TiO2 from density functional theory. J. Phys. Chem. C 113 (2009) 346–358. DOI:10.1021/jp802903c |
 2017, Vol. 28
2017, Vol. 28 



