b Department of Green Chemistry, National Research Centre, Dokki 12622, Cairo, Egypt;
c Department of Mathematics and Natural Science, Faculty of Science and Technology, University of Stavanger, N-4036 Stavanger, Norway;
d Department of Tanning Materials and Leather Technology, National Research Centre, Dokki 12622, Cairo, Egypt
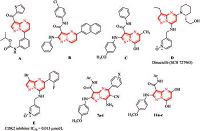
Recently, there is an urgent need to give much attention for design, synthesis and production of more potent and effective human therapeutic agents to treat cancer diseases, which is responsible for major deaths worldwide [1]. On the other hand, pyrazolo[1, 5-a]pyrimidines are purine analogue [2] of particular significance in medicinal chemistry due to their broad scope of remarkable antitumor [3] and antibacterial [4] activities. In addition, certain substituted pyrazolo[1, 5-a]pyrimidines appeared as promising antitumor agents. For example, compound A, isopropyl pyrazolo[1, 5-a]pyrimidine carbamate, showed good potency in the colon (HCT116) and p21 deficient cell line [5] and compound B, 5-(naphthalen-2-yl)pyrazolo[1, 5-a]pyrimidine- 3-carboxamide, is effective against HCT116(colon) and HeLa (cervix) cell lines [6]. Furthermore, we have reported the synthesis of compound C, N-(phenyl)-7-hydroxy-5-methylpyrazolo[1, 5-a] pyrimidine-3-carboxamide, as a potent antitumor agent against liver HepG2 cell lines [7] (Fig. 1).
|
Download:
|
| Figure 1. Structures of the anticancer pyrazolo[1, 5-a]pyrimidine derivatives A-C, Dinaciclib D, enzyme inhibitor E and the target pyrazolo[1, 5-a]pyrimidines 7a-i and 11a-c. | |
During the last two decades, there is a growing interest in the synthesis of pyrazolo[1, 5-a]pyrimidine derivatives as promising drugs fortreatment ofcancerdiseases. For example, Dinaciclib (SCH 727965) D (Fig. 1) was synthesized by Paruch et al., as a potent and selective cyclin-dependent kinase (CDK) inhibitor that is currently undergoing clinical evaluation [8]. Also, compound E (Fig. 1) is a potent and selective CDK2 inhibitor with IC50 of 0.013 mmol/L and showed efficacy in a mouse A2780 xenograft model [9].
From the previous findings of biological effectiveness of pyrazolo[1, 5-a]pyrimidine derivatives and in continuation of our research program on the synthesis of new compounds exhibiting biological activities [10-16], we have herein synthesized a new series of pyrazolo[1, 5-a]pyrimidine derivatives 7a-i and 11a-c (Fig. 1) for evaluation of their anticancer properties against three human carcinoma cell lines (HCT-116 "colon",PC-3 "prostate" and HepG-2 "liver")using MTT assay.
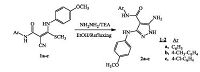
2. Results and discussion 2.1. ChemistryThe synthesized target compounds, pyrazolo[1, 5-a]pyrimidine derivatives, are shown in Schemes 2-4. The starting materials, 5-amino-3-(4-methoxyphenylamino)-N-aryl-1H-pyrazole-4-car- boxamides 2a-c were prepared by reacting N-(aryl)-2-cyano-3- [(4-methoxyphenyl)amino]-3-(methylsulfanyl)acrylamides 1a-c with hydrazine hydrate in boiling absolute ethanol as described in the literature [17] (Scheme 1).
|
Download:
|
| Scheme1. Synthesis of the starting compounds 2a-c | |
|
Download:
|
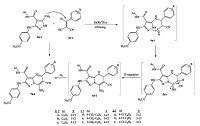
| Scheme2. Synthesis of 7-amino-6-cyano-5-aryl-2-(4-methoxyphenylamino)-N-aryl-pyrazolo[1, 5-a]pyrimidine-3-carboxamides 7a-i. | |
|
Download:
|
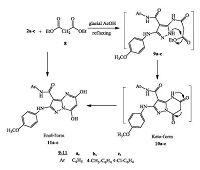
| Scheme3. Synthesis of N-aryl-5, 7-dihydroxy-2-(4-methoxyphenylamino)pyra- zolo[1, 5-a]pyrimidine-3-carboxamide 11a-c. | |
|
Download:
|
| Scheme4. Synthesis of N-aryl-5-(2-chlorobenzylideneamino)-3-(4-methoxyphe- nylamino)-1H-pyrazole-4-carboxamide (13a-c). | |
Base-catalyzed condensation of 5-amino-3-(4-methoxypheny- lamino)-N-aryl-1 -pyrazole-4-carboxamides 2a-c with 2-(o-, m-or p-chlorobenzylidene)malononitriles 3a-c in absolute ethanol afforded 7-amino-6-cyano-5-aryl-2-(4-methoxyphenylamino)-N- aryl-pyrazolo[1, 5-a]pyrimidine-3-carboxamides 7a-i (Scheme 2).
The structure of compounds 7a-i was confirmed and established by different spectral data (MS, IR, 1H NMR and 13C NMR) and elemental analysis. For example, the mass spectrum of compound 7c confirmed the molecular formula C27H20ClN7O2 (509.95) {MS (m/z, %): 511 (M++2, 2.20), 509 (M+, 6.97)}. The IR spectrum of 7c reveals the presence of absorption bands due to the NH, NH2, C ≡ N and C=O groups at 3434, 3290, 2217 and 1665 cm-1, respectively. Its 1H NMR (400 MHz, DMSO-d6, δ) spectrum showed protons of the OCH3 group as a singlet at 3.76. Protons of the N-phenyl ring (5H) appeared as a doublet (2H, JHH = 9.0 Hz) at 6.91, a triplet (1H, JHH = 7.4 Hz) at 7.10 and a triplet (2H, JHH = 7.6 Hz) at 7.37. Protons of the p-substituted benzene rings (AB-system) (8H) appeared as a four doublets at 7.60 (d, 2H,JHH=7.6Hz),7.72 (d, 2H,JHH= 8.6Hz), 7.85 (d, 2H, JHH= 9.0Hz) and at 8.02 (d, 2H, JHH= 8.6Hz). The spectrum also revealed three signals at 9.12, 9.26, 10.03 referred to the protons of NH2 (2H),NH (1H),NH (1H),respectively. The 13C NMR (100 MHz, DMSO-d6, δ) spectrum was characterized by four signals at 55.6, 116.4, 133.7 and 160.3 due to the -OCH3, -C≡N, C3a (pyrazolopyrimidine) and -C=O groups, respectively. Additionally, we investigated the structure of 7a-i from previous studies for similar analog by the single crystal X-ray structure analysis [18, 19].
The preparation of 5, 7-dihydroxy-2-(4-methoxyphenylamino)- N-aryl-pyrazolo[1, 5-a]pyrimidine-3-carboxamides 11a-c (enol- form) was performed via the reaction of compounds 2a-c with diethyl malonate 8 in refluxing glacial acetic acid with 70-80% yield (Scheme 3).
Structures of compounds 11a-c were confirmed by different spectral and elemental analyses. For example, the presence of OH, NH at 3358, 3298 cm-1 respectively, and C=O at 1652 cm-1 in the IR spectrum confirmed the structure of compound 11b. Its 1H NMR (400 MHz, DMSO-d6, δ ppm) spectrum showed two signals at 2.26 and 3.70 for CH3 and OCH3protons, respectively. The enol form of 11b was authenticated by its 1H NMR analysis, where, the H-6 proton of the pyrimidine nucleus appeared at 6.85, whereas, the CH2 protons in keto form (10b), were expected at ≈4.50 and not observed in the 1H NMR spectrum. The aromatic protons (AB-system) (8H) gave signals at 6.84 (d, 2H, JHH = 8.4Hz), 7.13 (d, 2H, JHH= 8.2 Hz), 7.28 (2H), 7.46 (d, 2H, JHH= 8.2Hz). Moreover, four D2O-exchangeable signals appeared at 8.28 (OH), 9.12 (NH), 10.34 (NH) and 12.51 (OH). Therefore, the reaction between 2a-c with 8 in acetic acid gives 11a-c in the enol-form and not 10a-c in the keto-form.
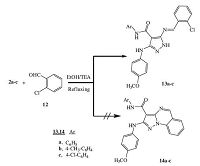
Next, when compounds 2a-c were allowed for condensation with 2-chlorobenzaldehyde, 12, in absolute ethanol by using triethylamine as a catalyst under reflux temperature, Schiff bases 13a-c were obtained and not the expected, N-aryl-pyrazolo[1, 5-a] quinazoline-3-carboxamides 14a-c (Scheme 4). From previous studies, Schiff bases show a variety of antifungal [20], antibacterial [21] and particularly anticancer activities [22].
The mass spectra confirmed structure of the Schiff bases 13a-c. For example, the mass spectrum of compound 13c revealed a molecular ion peak at m/z = 480 (M+, 27.75) corresponding to the molecular formula C24H19Cl2N5O2 (480.35); its IR spectrum confirmed presence of NH groups at 3277, 3196 cm-1and C=O at 1654cm-1. The 1H NMR (400 MHz, DMSO-d6, δ) spectrum was characterized by presence of two signals at 3.74 and 8.67 for OCH3 and -N=CH- (azomethine proton) protons, respectively. The aromatic protons (12H) appeared at 6.92 (d, 2H, JHH = 8.7Hz), 7.35-7.38 (m, 1H), 7.42 (d, 2H, JHH=8.8Hz), 7.62-7.65 (m, 1H), 7.67-7.71 (m, 5H), 8.32 (d, 1H, JHH =7.3 Hz). The appearance of three NH protons as three singlet signals at 9.39, 9.90 and 12.83 confirmed the structure of the produced Schiff bases 13a-c.
2.2. In vitro anti-tumor activityThe eighteen synthesized compounds, 5-amino-1H-pyrazoles 2a-c, pyrazolo[1, 5-a]pyrimidine derivatives 7a-i, 11a-c and Schiff bases 13a-c, were examined in vitro for their anti-tumor activities against three human carcinoma cell lines {colorectal carcinoma (HCT116), prostate adenocarcinoma (PC-3) and liver carcinoma (HepG-2)} using MTT assay [23, 24, 25].
The percentage of the intact cells was measured and compared to the control (Fig. 2). The activities of these compounds against three carcinoma cells were compared with that ofdoxorubicin. The results indicated that all tested compounds show dose-dependent anticancer activities against the three cell lines.

|
Download:
|
| Figure 2. Anticancer activities of the eighteen synthesized compounds against three human carcinoma cell lines (HCT-116, PC-3 and HepG-2) using MTT assay at 100 ppm. | |
From Fig. 2 we can deduce that, at 100 μg/mL, four compounds 7d, 7f, 7g and 11a showed good anticancer activities against HCT-116 carcinoma cells compared to that of doxorubicin (100 μmol/L). Seven compounds (2b, 7e, 7h, 7i, 11b, 11c and 13c) showed moderate activities, while, the rest of compounds showed weak activities against the same cell line. In addition, four compounds (2b, 7d, 7e and 11a) showed good antitumor activity; two compounds (7f, 7h) showed moderate activities and the rest of the compounds showed weak or no antitumor activities against PC-3 cancer cells. Furthermore, all the tested compounds showed weak activities against HepG-2 liver cancer cells.
The IC50(μg/mL) values were the concentration required for 50% inhibition of cell growth and were compiled in Table 1.
|
|
Table 1 The anticancer activity (IC50 (μg/mL) values) of the eighteen compounds using MTT assay against HepG-2, PC-3 and HCT-116 cancer cell lines. |
2.3. Structure activity relationship (SAR)
By comparing the anticancer activity of the eighteen synthesized compounds in this work to their structures, the following structure activity relationships (SAR) were deduced.
From the results of the screening of the 5-amino-1H-pyrazoles 2a-c against the HCT-116, PC-3 and HepG-2 cell lines, some derivatives bearing the 4-methylphenyl group were more active than those bearing the 4-chlorophenyl group and/or bearing only the phenyl group. Thus, on colon HCT-116 cell line, 2b(IC50 = 72.77 ± 5.1 μg/mL)>2c (IC50 = 98.54 ± 6.3 μg/mL)>2a (IC50 = 3 80.42 ± 10.5μg/mL). In addition, the screening of the 5-amino-1H- pyrazoles against the PC-3 (prostate) cell line showed that 2b (IC50 = 71.53 ± 4.9 μg/mL)> 2c (IC50 = 696.09 ± 3.8 μg/mL)>2a (IC50 3 1000 μg/mL). Moreover, the screening against the HepG2 (liver) cell lines showed that 2b (IC50=122.07 ± 9.8 μg/mL)> 2c (IC50=143.32 ± 11.5 μg/mL)> 2a (IC50 = 150.04 ± 10.5 μg/mL).
Furthermore, the same order of antitumor activity 4-CH3-C6H4 derivatives > 4-Cl-C6H4 derivatives > Ph derivatives was observed upon screening the pyrazolo[1, 5-a]pyrimidine derivatives 7a-i against the HCT-116 and PC-3 cell lines. Thus, 7d > 7g > 7a; 7e > 7h > 7b; 7f > 7i > 7c.
Moreover, the 5-amino-1H-pyrazoles 2a-c and pyrazolo[1, 5-a] pyrimidine derivatives 7a-i and 11a-c were more active than the 5-amino-1H-pyrazoles against the HCT-116 and PC-3 cell lines.
From the screening of the pyrazolo[1, 5-a]pyrimidine derivatives 7a-i against HCT-116 and PC-3 cancer cell lines, we noticed the effect of the position of chlorine atom in the aryl substituent on the antitumor activities. Thus, it has been found that some derivatives bearing chlorine atom at position-2 were more active than those bearing the same atom at position-3 or position-4 in the order that chlorine atom at position-2 > chlorine atom at position-3 >chlorine atom at position-4. Thus, on HCT-116 cancer cell lines, 7a (IC50 = 92.30 ± 5.3 μg/mL) > 7b (IC50 = 95.29 ± 4.9 μg/mL)> 7c (IC50 = 101.49 ± 10.1 μg/mL) and also, 7g (IC50 = 63.28 ± 5.9μg/mL)>7h (IC50 = 71.29 ± 9.2μg/mL)>7i (IC50 = 72.51 ±5.1 μg/mL). Moreover, the screening of 7a-i against the PC-3 cell lines, was found that 7a (IC50=109.87 ± 12.4 μg/mL)> 7b (IC50 = 152.31 ± 14.9μg/mL)>7c (IC50 = 286.37± 19.9μg/mL) and also, 7d (IC50 = 67.27 ± 3.8 μg/mL)> 7e (IC50 = 72.35 ± 2.9 μg/mL)> 7f (IC50 = 74.32 ± 5.3 μg/mL).
3. ConclusionIn conclusion, the present study fosters a simple and easily scalable approach for the preparation of a new series of pyrazolo [1, 5-a]pyrimidine derivatives 7a-i, 11a-c and their relevant Schiff bases 13a-c. All the newly synthesized compounds were screened for their in vitro antitumor activity against three human carcinoma cell lines, namely colorectal carcinoma (HCT116), prostate adenocarcinoma (PC-3) and liver carcinoma (HepG-2) using MTT cytotoxicity assay at 100 μg/mL. The results revealed that compounds 7d and 11a show good antitumor activity against both cell lines. Accordingly, this class of compounds could be considered as excellent templates for future optimization or modification to obtain potent antitumor agents. A study on the structure activity relationships (SAR) indicated the role was displayed by the position of the chlorine atom in the aryl substituent of the molecules of the tested compounds.
4. Experimental 4.1. ChemistrySynthesis of 7-amino-6-cyano-5-aryl-2-(4-methoxyphenyla- mino)-N-aryl-pyrazolo[1, 5-a]pyrimidine-3-carboxamides (7a-i): A mixture of compounds 2a-c (0.01 mol) with 2-(o-, m- or p-chlorobenzylidene)malononitrile 3a-c (0.01 mol) and a catalytic amount of triethylamine (four drops) in absolute ethanol (30 ml) was refluxed for 6 h. After cooling, the solvent was concentrated under reduced pressure and the solid obtained was collected and recrystallized from ethanol to give 7a-i.
7-Amino-5-(4-chlorophenyl)-6-cyano-2-(4-methoxyphenyla- mino)-N-phenylpyrazolo[1, 5-a]pyrimidine-3-carboxamide (7c): Orange crystals, m.p.>300℃, yield (78%). IR (KBr) vmax/cm-1 3434, 3290 (NH, NH2), 2217 (C≡N), 1665 (C=O). 1H NMR (400MHz, DMSO-d6): δ 3.76 (s, 3H, OCH3),6.91 (d, 2H, JHH= 9.0 Hz, ArH), 7.10 (t, 1H, JHH= 7.4 Hz, ArH), 7.37 (t, 2H, JHH= 7.6 Hz, ArH), 7.60 (d, 2H, JHH= 7.6 Hz, ArH), 7.72 (d, 2H, JHH= 8.6 Hz, ArH), 7.85 (d, 2H, JHH= 9.0Hz, ArH), 8.02 (d, 2H, JHH=8.6Hz, ArH), 9.12 (s, 2H, NH2), 9.26 (s, 1H, NH), 10.03 (s, 1H, NH). 13C NMR(100MHz, DMSO-d6): δ 55.6 (-OCH3), 75.5 (C6, pyrazolopyrimidine), 89.6 (C3, pyrazolo- pyrimidine), 114.6 (2C, Ar), 116.4 (C≡N), 119.4 (4C, Ar), 123.9 (C, Ar), 129.2, 129.6, 131.0 (6C, Ar), 133.7 (C3a, pyrazolopyrimidine), 135.9, 136.2, 138.7, 145.9, 149.7 (5C, Ar), 154.3 (C2, pyrazolopyr- imidine), 156.7 (C 5, pyrazolopyrimidine), 160.3 (C-O), 162.5 (C7, pyrazolopyrimidine). MS (m/z, %): 511 (M++2, 2.20), 509 (M+, 6.97). Anal. Calcd. (%) for C27H20ClN7O2 (509.95): C, 63.59; H, 3.95; N, 19.23. Found: C, 63.65; H, 3.90; N, 19.20%.
Synthesis of N-aryl-5, 7-dihydroxy-2-(4-methoxyphenylamino) pyrazolo[1, 5-a]pyrimidine-3-carboxamide (11a-c): A mixture of compounds 2a-c (0.01 mol) with diethyl malonate 7 (0.01 mol) in glacial acetic acid (20 mL) was refluxed for 6h, then poured onto crushed ice, and the separated solid was filtered, dried well, and recrystallized from ethanol to afford compounds 11a-c.
5, 7-Dihydroxy-2-(4-methoxyphenylamino)-N-(4-methyl- phenyl)pyrazolo[1, 5-a]pyrimidine-3-carboxamide (11b): White crystals, m.p. 200-202 ℃, yield (65%). IR (KBr) vmax/cm-1 3358 (OH), 3298 (NH), 1652 (C=O).1H NMR(400 MHz, DMSO-d6): δ 2.26 (s, 3H, CH3), 3.70 (s, 3H, OCH3), 6.85 (s, 1H, pyrimidine H-6), 6.84 (d, 2H, JHH= 8.4 Hz, ArH), 7.13 (d, 2H, JHH=8.2 Hz, ArH), 7.28 (2H, ArH), 7.46 (d, 2H, JHH=8.2 Hz, ArH), 8.28 (s, 1H, OH), 9.12 (s, 1H, NH), 10.34 (s, 1H, NH), 12.51 (s, 1H, OH). Anal. Calcd. (%) for C21H19N5O4 (405.41): C, 62.22; H, 4.72; N, 17.27. Found: C, 62.15; H, 4.75; N, 17.30%.
Synthesis of N-aryl-5-(2-chlorobenzylideneamino)-3-(4- methoxyphenylamino)-1H-pyrazole-4-carboxamide (13a-c): A mixture of compounds 2a-c (0.01 mol) with 2-chlorobenzaldehyde 12 (0.01 mol) in absolute ethanol (30 mL) and a catalytic amount of triethylamine (four drops) was refluxed for 6 h. The solvent was concentrated under reduced pressure and the solid obtained was collected and recrystallized from ethanol to give 13a-c.
5-(2-Chlorobenzylideneamino)-N-(4-chlorophenyl)-3-(4- methoxyphenylamino)-1H-pyrazole-4-carboxamide (13c): Yellow crystals, m.p. 222-223 ℃, yield (71%). IR (KBr) nmax/cm-1 3277, 3196 (NH), 1654 (C=O). 1H NMR(400MHz, DMSO-d6): d 3.74 (s, 3H, OCH3), 6.92 (d, 2H, JHH= 8.7Hz, ArH), 7.35-7.38 (m, 1H, ArH), 7.42 (d, 2H, JHH=8.8 Hz, ArH), 7.62- 7.65 (m, 1H, ArH), 7.67-7.71 (m, 5H, ArH), 8.32 (d, 1H, JHH= 7.3 Hz, ArH), 8.67 (s, 1H, -N=CH-), 9.39 (s, 1H, NH), 9.90 (s, 1H, NH), 12.83 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δ 55.7 (-OCH3), 99.9 (C4, pyrazole), 114.9, 121.2, 127.2, 128.4, 128.6, 129.0, 129.1, 129.3, 131.0, 132.2, 133.8, 134.7, 136.2, 137.8 (18C, Ar), 150.0 (C5, pyrazole), 151.1 (C3, pyrazole), 161.8 (-N=CH-), 163.0 (C=O). MS (m/z, %): 480 (M+, 27.75), 452 (BP, 100). Anal. Calcd. (%) for C24H19CbN5O2(480.35): C, 60.01; H, 3.99; N, 14.58. Found: C, 60.10; H, 3.95; N, 14.55%.
Physical and spectroscopic characterization data of all compounds were given in Supplementary data.
4.2. Biological evaluation 4.2.1. In vitro anticancer activityCell culture of HCT116 (human colorectal carcinoma), PC-3 (human prostate adenocarcinoma) and HepG-2 (human liver carcinoma) cell lines were purchased from the American Type Culture Collection (Rockville, MD) and maintained in RPMI- 1640 medium which was supplemented with 10% heat-inactivated FBS (fetal bovine serum), 100U/mL penicillin and 100U/mL streptomycin. The cells were grown at 37 ℃ in a humidified atmosphere of 5% CO2.
4.2.2. MTT cytotoxicity assayThe antitumor activity against HCT-116, PC-3 and HepG- 2 human cancer cell lines was estimated using the 3-[4, 5- dimethyl-2-thiazolyl]-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay, which is based on the cleavage of the tetrazolium salt by mitochondrial dehydrogenases in viable cells [23-25]. Cells weredispensed in a 96 well sterile microplate (5 × 104cells/well), and incubated at 37 ℃ with series of different concentrations, in DMSO, of each tested compound or Doxorubicin® (positive control) for 48 h in a serum free medium prior to the MTT assay. After incubation, media were carefully removed, 40 mL of MTT (2.5 μg/mL) were added to each well and then incubated for an additional 4 h. The purple formazan dye crystals were solubilized by the addition of 200 μL of DMSO. The absorbance was measured at 590 nm using a SpectraMax® Paradigm® Multi-Mode microplate reader. The relative cell viability was expressed as the mean percentage of viable cells compared to the untreated control cells.
4.2.3. Statistical analysisAll experiments were conducted in triplicate and repeated in three different days. All the values were represented as mean ± SD. IC50s were determined by probit analysis using SPSS software program (SPSS Inc., Chicago, IL).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.10.022.
| [1] | C. Avendaño, J.C. Menéndez, Medicinal Chemistry of Anticancer Drugs, Elsevier, Amsterdam, 2008. |
| [2] | S.A. Ahmed, A.M. Hussein, W.G.M. Hozayen, Synthesis of some pyrazolopyrimidines as purine analogues. J. Heterocycl. Chem. 44 (2007) 803–810. DOI:10.1002/jhet.v44:4 |
| [3] | H.A. Abdel-Aziz, T.S. Saleh, H.S.A. El-Zahabi, Facile synthesis and in-vitro antitumor activity of some pyrazolo[3,4-b]pyridines and pyrazolo[1,5-a] pyrimidines linked toa thiazolo[3,2-a]benzimidazole moiety. ArchPharm. 343 (2010) 24–30. |
| [4] | M.A. Gouda, M.A. Berghot, A.I. Shoeib, A.M. Khalil, Synthesis and antimicrobial of new anthraquinone derivatives incorporating pyrazole moiety. Eur. J. Med. Chem. 45 (2010) 1843–1848. DOI:10.1016/j.ejmech.2010.01.021 |
| [5] | A. Gopalsamy, H. Yang, J.W. Ellingboe, et al., Pyrazolo[1,5-a]pyrimidin-7-yl phenyl amides as novel anti-proliferative agents:parallel synthesis for lead optimization of amide region, Bioorg. Med. Chem. Lett. 15(2005) 1591-1594. |
| [6] | O.M. Ahmed, M.A. Mohamed, R.R. Ahmed, S.A. Ahmed, Synthesis and antitumor activities of some new pyridines and pyrazolo[1,5-a]pyrimidines. Eur. J. Med. Chem. 44 (2009) 3519–3523. DOI:10.1016/j.ejmech.2009.03.042 |
| [7] | A.S. Hassan, T.S. Hafez, S.A.M. Osman, M.M. Ali, Synthesis and in vitro cytotoxic activity of novel pyrazolo[1,5-a]pyrimidines and related Schiff bases. Turk. J. Chem. 39 (2015) 1102–1113. DOI:10.3906/kim-1504-12 |
| [8] | K. Paruch, M.P. Dwyer, C. Alvarez, Discovery of dinaciclib (SCH 727965):a potent and selective inhibitor of cyclin-dependent kinases. Med. Chem. Lett. (2010) 204–208. |
| [9] | K. Paruch, M.P. Dwyer, C. Alvarez, Pyrazolo[1,5-a]pyrimidines as orally available inhibitors of cyclin-dependent kinase 2. Bioorg Med. Chem. Lett. 17 (2007) 6220–6223. DOI:10.1016/j.bmcl.2007.09.017 |
| [10] | A.S. Hassan, T.S. Hafez, M.M. Ali, T.K. Khatab, Design, synthesis and cytotoxic activity of some new pyrazolines bearing benzofuran and pyrazole moieties. Res. J. Pharm. Biol. Chem. Sci. 7 (2016) 417–429. |
| [11] | A.S. Abd El-All, A.S. Hassan, S.A. Osman, Synthesis characterization and biological evaluation of new fused triazine derivatives based on 6-methyl-3-thioxo-1,2,4-triazin-5-one. Acta Pol. Pharm. 73 (2016) 79–92. |
| [12] | S.A. Osman, H.A. Mousa, H.A.A. Yosef, Synthesis, characterization and cytotoxicity of mixed ligand Mn(II) Co(II) and Ni(II) complexes. J. Serb. Chem. Soc. 79 (2014) 953–964. DOI:10.2298/JSC130813134O |
| [13] | T.S. Hafez, S.A. Osman, H.A.A. Yosef, Synthesis, structural elucidation, and in vitro antitumor activities of some pyrazolopyrimidines and Schiff bases derived from 5-amino-3-(arylamino)-1H-pyrazole-4-carboxamides. Sci. Pharm. 81 (2013) 339–357. DOI:10.3797/(ISSN)0036-8709 |
| [14] | S.A. Osman, H.A.A. Yosef, T.S. Hafez, Synthesis and antibacterial activity of some novel chalcones, pyrazoline and 3-cyanopyridine derivatives based on khellinone as well as Ni(II) Co(II) and Zn(II) complexes. Aust. J. Basic Appl. Sci. 6 (2012) 852–863. |
| [15] | G.H. Elgemeie, S.H. Elsayed, A.S. Hassan, Direct route to a new class of acrylamide thioglycosides and their conversions to pyrazole derivatives. Synth. Commun. 38 (2008) 2700–2706. DOI:10.1080/00397910802222605 |
| [16] | G.H. Elgemeie, S.H. Elsayed, A.S. Hassan, Design and synthesis of the first thiophene thioglycosides. Synth. Commun. 39 (2009) 1781–1792. DOI:10.1080/00397910802590928 |
| [17] | A.S. Hassan, T.S. Hafez, S.A. Osman, Synthesis, characterization, and cytotoxicity of some new 5-aminopyrazole and pyrazolo[1,5-a]pyrimidine derivatives. Sci. Pharm. 83 (2015) 27–39. DOI:10.3797/(ISSN)0036-8709 |
| [18] | H.F. Anwar, D.H. Fleita, H. Kolshorn, H. Meier, M.H. Elnagdi, 2H-Pyrazol-3-ylamines as precursors for the synthesis of polyfunctionally substituted pyrazolo[1,5-a]pyrimidines. Arkivoc xv (2006) 133–141. |
| [19] | M.D. Wendt, A. Kunzer, R.F. Henry, J. Crossb, T.G. Pagano, Regiochemistry of addition of aminoheterocycles to α-cyanocinnamonitriles:formation of azabridged bi- and tricycles. Tetrahedron Lett. 48 (2007) 6360–6363. DOI:10.1016/j.tetlet.2007.07.039 |
| [20] | N.B. Patel, H.R. Patel, F.M. Shaikh, D. Rajani, New 4-thiazolidinones from 5-ethyl pyridine-2-ethanol:their antibacterial, antifungal, and antitubercular activity. Med. Chem. Res. 23 (2014) 1360–1370. DOI:10.1007/s00044-013-0736-8 |
| [21] | K.H.M.E. Tehrani, M. Hashemi, M. Hassan, F. Kobarfard, S. Mohebbi, Synthesis and antibacterial activity of Schiff bases of 5-substituted isatins. Chin. Chem. Lett. 27 (2016) 221–225. DOI:10.1016/j.cclet.2015.10.027 |
| [22] | Neelima ${referAuthorVo.mingEn}, K. Poonia, S. Siddiqui, Md. Arshad, D. Kumar, In vitro anticancer activities of Schiff base and its lanthanum complex. Spectrochim. Acta A Mol. Biomol. Spectrosc. 155 (2016) 146–154. DOI:10.1016/j.saa.2015.10.015 |
| [23] | N.A. Hamdy, M.M. Anwar, K.M. Abu-Zied, H.M. Awad, Synthesis tumor inhibitory and antioxidant activity of new polyfunctionally 2-substituted 5,6,7,8-tetrahydronaphthalene derivatives containing pyridine, thioxopyridine and pyrazolopyridine moieties. Acta Pol. Pharm. 70 (2013) 987–1001. |
| [24] | H.A. Soliman, M.N.M. Yousif, M.M. Said, Synthesis of novel 1,6-naphthyridines, pyrano. Der Pharma Chem. 6 (2014) 394–410. |
| [25] | H.M. Awad, H.I. Abd-Alla, K.H. Mahmoud, S.A. El-Toumy, In vitro antinitrosative, antioxidant, and cytotoxicity activities of plant flavonoids:a comparative study. Med. Chem. Res. 23 (2014) 3298–3307. DOI:10.1007/s00044-014-0915-2 |
 2017, Vol. 28
2017, Vol. 28 



