Perylene diimides (PDIs) as a kind of the most investigated organic dyes have excellent optical and electronic properties applied in organic photovoltaic cells (OPVs), fluorescent solar collectors, organic field-effect transistors (OFETs), organic light emitting diodes (OLED) and etc.[1-4]. As one of the most intensively studied classes of organic fluorescent materials, PDIs possess high fluorescence quantum efficiency, especially nearunity fluorescence quantum yields in dilute solution. In addition, PDI derivatives, the typical polycyclic aromatic molecules, were prone to form aggregates due to the strong π-π interaction between the planer PDI rings in poor solvents or solid state [5]. The strong intermolecular interaction renders PDI derivatives to be excellent building blocks for self-organized molecular materials with highly ordered structure [6, 7]. However, the characteristic of the notorious aggregation-caused quenching (ACQ) has limited its potential applications in organic luminescent devices [8].
To solve this problem, the bulky and twisted moieties were introduced into the perylene core to develop solid light emitting materials [9]. Thus, some chemical modification to PDI by introducing twisted moieties into the bay positions have been carried out in order to improve the luminescent properties of PDI. As reported, an almost flat PDIs were synthesized and characterized by single crystal X-ray diffraction, which neither form π-stacks in concentrated solution nor in the solid state due to the bulky 2, 6-terphenyl units efficiently shield the PDI scaffold [10]. Therefore, modifications to the PDIs at bay position by twisted moieties maybe an effective method to inhibit the aggregation and improve the luminescent properties. A series of the oxygen bridged biphenyl were studied in our groups, and the dihedral angle between two phenyl rings was about40± 5° [11].The suppressing intermolecular aggregation was confirmed by the introduction of the oxygen bridged biphenyl in conjugated polymer [12-14].
In this report, one or two oxygen bridged twisted heptatomic biphenyl (THB) were attached to the bay positions of PDI through acetylene bond to develop a novel twisted PDI-based dyes by sonogashira coupling reaction. The photophysical and electrochemical properties of single, double oxygen bridged twisted heptatomic biphenyl substituted perylene diimide and its parent compound PDI were discussed to study the effect of structure on molecular aggregation.
2. Results and discussionUV-vis absorption and photoluminescence (PL) spectra of s-THBPDI, d-THBPDI and parent PDI were shown in Fig. 1a, and >their photophysical property data were summarized in Table 1. As shown in Fig. 1a, two pronounced peaks at 524nm and 488nm belong to the electronic 0-0 and 0-1 transition were found to PDI [15]. Compared to the PDI, the introduction ofTHB substituents in the bay positions led to a bathochromic shift of the absorption maximum to 547 nm for s-THBPDI and 572 nm for d-THBPDI, respectively. This can be explained by the fact that the increasing amount of substituents has enlarged the conjugation of the perylene diimide derivatives [16]. The PL spectra of all PDI dyes were also shown in Fig. 1a. The fluorescence spectrum of unsubstituented PDI depicted almost mirror image of its absorption [17]. However, the emission peaks of s-THBPDI and d-THBPDI were broader and displayed less vibronic structures. Moreover, the emission maxima of s-THBPDI and d-THBPDI appeared at 617 nm and 629nm, respectively, which exhibited a significantly bath- ochromic shift (approximately 40-50 nm) compared to that of PDI (577 nm).

|
Download:
|
| Figure 1. (a) Normalized UV-vis absorption (solid line) and photoluminescence (dotted line) spectra of PDI, s-THBPDI and d-THBPDI in dichloromethane; (b) The UV-vis absorption spectra of PDI, s-THBPDI and d-THBPDI in dichloromethane (solid line) and in casting films (dotted line). | |
|
|
Table 1 The photophysical and electrochemical properties data of PDI, s-THBPDI and d-THBPDI. |
UV-vis absorption spectra of PDIs are sensitive to the inter- chromophore distance and orientation and therefore have been widely used to study their π-π stacking. Thus, the aggregation level of the different PDI molecules can be estimated by comparison of the absorption spectra in their solutions and solid states (Fig. 1b). The results revealed that the absorption spectra in solid-state were significantly different from those in solution. Compared to their corresponding absorption in solution, the 0-0 and 0-1 transition of perylene diimide skeleton dramatically reversed and the fine vibration structure disappeared in the solid state, which attribute to strong intermolecular π-π stacking [18]. Furthermore, the intermolecular π-π stacking also led to a bathochromic shift of absorption spectra. Thus through contrast analysis, the absorption peak of 0-0 (and 0-1) transition of s-THBPDI and d-THBPDI in solid changed slightly compared to that in solutions (Table 1), which proved that THB substitutes have an inhibition effect on the π-π aggregation of the perylene diimide.
The concentration-dependent absorption and photoluminescence (PL) spectra of all PDI derivatives were investigated in order to deeply understand the dynamic process of π-π aggregation behaviors. According to the related literature [19], the ratio of the absorption intensity of 0-0 and 0-1 transitions (A0-0/A0-1) was directly correlated with the proportion of monomer against aggr egates in solution and dropped sharply upon perylene diimides aggregation. The relationships between the A0-0/A0-1 and concentrations for all PDI derivatives were shown in Fig. 2a. In general, the concentration that A0-0/A0-1 significantly decreased represents the critical concentration of molecular aggregation. Thus, the critical concentrations of molecular aggregation obtained were 4.5 × 10-5 mol/L, 1 × 10-4 mol/L, and 6.5 × 10-5 mol/L for PDI, s-THBPDI and d-THBPDI, respectively.

|
Download:
|
| Figure 2. (a) The relation curves of A0-0/A0-1 of PDI, s-THBPDI and d-THBPDI 'versusconcentrations; (b) the relationship between the Imax and their concentrations. | |
Furthermore, nuorescence quenching would appear along with the PDI molecules aggregated, so the concentration-dependent fluorescence spectra can also be used to study the aggregation behaviors. The fluorescence intensity of all these three compounds was increased and showed slight red-shift with the increase of the concentration until a certain concentration and then decreased (Fig. S5). The Imax-C curve for all PDI dye was consequently established and shown in Fig. 2b (Imax: the intensity of maximum emission peak; C: concentration, mol/L). The critical concentration is closely related with the aggregation ofPDI molecules. Obviously, as we can see, the critical of the s-THBPDI is the biggest among the three PDI dyes and the order was s-THBPDI, d-THBPDI and PDI.
The concentration-dependent absorption and photoluminescence (PL) spectra of these three PDI dyes were synergistically confirmed that the introduction of THB substituent definitely inhibited the π-π aggregation of PDI dyes. Furthermore, as we see, the aggregation concentration of s-THBPDI was higher than d-THBPDI described above could ascribe to the loss of the planarity of the perylene core due to the lower molecular symmetry by the bay substitution [20]. The optimized molecules structure of s-THBPDI and d-THBPDI were shown in Fig. S6.
To further investigate the luminescence properties of PDI, s-THBPDI and d-THBPDI deeply, the fluorescence lifetime measurement was performed (Fig. 3a). It can be seen that fluorescence decay profiles of PDI, s-THBPDI and d-THBPDI were in accordance with a mono-exponential decay, and their fluorescence lifetimes (t) were listed in Table 1. The fluorescence lifetimes of s-THBPDI and d-THBPDI were 7.81ns and 8.18 ns respectively, having an advantage over 6.12 ns of parent PDI.

|
Download:
|
| Figure 3. (a) PL decay times measured at 525 nm ofPDI, s-THBPDI and d-THBPDI in DMF; (b) Cyclic voltammetry curves of these molecules containing [nBu4N][PF6] (0.1 mol/L) with respect to Ag/AgCl in DMF | |
The electrochemical properties of all PDIs (s-THBPDI, d-THBPDI and PDI) in DMF at room temperature were investigated by cyclic voltammetry (CV). The CV curve was recorded versus the Ag/AgCl electrode as reference, using ferrocene as an internal standard (Fig. 3b). As expected, all PDIs exhibited two typical reversible reduction waves due to the first and second one-electron stepwise reductive process from the perylene core to the corresponding radical anions and dianions.
The onset reduction potential (Eonset) for d-THBPDI was0.009V. According to the empirical formulae [21] ELUMO = -([Eonsetred] + 4.8 eV) and EHomo = -(Egopt - ELumo). The calculated values for the HOMO and LUMO of s-THBPDI, d-THBPDI, and PDI were summarized in Table 1. The LUMO energy levels were -4.809eV, -4.645eV, and -4.574eV for d-THBPDI, s-THBPDI and PDI, respectively. The results indicated the introduction of the THB can decrease the LUMO energy levels of PDIs effectively. The lower LUMO energy levels for PDIs are more advantageous to the preparation of radical anion and dianion species under mild conditions [22], suggesting this materials may be candidates for gas sensor and the environment detection, etc.
3. ConclusionThe oxygen bridged twisted heptatomic biphenyl was successfully introduced in bay positions of PDI by Sonogashira coupling reaction. The introduction of the oxygen bridged twisted heptatomic biphenyl improved the aggregation concentrations of PDI derivatives. Moreover, it also decreased the reduction potential of PDIs and increase critical concentration of fluorescence quenching effectively.
4. Experimental 4.1. Reagents and measurementAll chemicals and solvents were purchased from commercial supplies and used without further purification unless otherwise specified. And the 1-bromo-3, 4, 9, 10-perylene tetracarboxylic dianhydride (s-BrPDI) and 1, 7-dibromo-3, 4, 9, 10-perylene tetra- carboxylic dianhydrid (d-BrPDI) were synthesized by our group.
1H NMR spectra was recorded on Bruker 400 MHz Spectrometer with CDCl3 and DMSO-d6 as the solvent and tetramethysilane (TMS) as an internal reference. Infrared measurement with the KBr pellet technique was performed within the 400-4000cm-1 on a Perkin Elemer SP100 Fourier transform infrared spectrometer. UV-vis spectra were measured using a Perkin Elemer Lambda 35. Fluorescence spectra (PL) were recorded on a Perkin Elmer RF- 5301PC spectra-photometer. Cyclic voltammetry measurement was performed in solution, under argon atmosphere with a computer controlled CHI600d electrochemical workstation in a three electrode single-compartment cell using platinum electrodes and Ag+/AgCl electrode as the reference electrode, with Fc/Fc* redox couple as internal standard, with a tetrabutyl-ammonium hexafluoro-phosphate (Bu4NPF6) solution (0.1 mol/L) in DMF at a scan rate of 0.1 V/s.
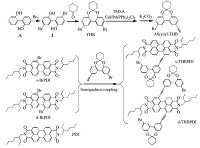
4.2. Synthesis and characterization5, 5'-Dibromine-2, 2'-dihydroxybiphenyl (1): 2, 2'-Dihydroxy- biphenyl (A) 1.86g (10mmol) and solvent CH2Cl2 (20 mL) were introduced into a three-necked round bottomed flask, and then bromine 1.1 mL (20 mmol) was dripwise with rapid stirring at room temperature. After the precipitation appeared, filtered to remove the solvent, white powder was received as the target product (3.10g, 90%).
6, 6'-Cyclohexy-2, 10-dibromine oxygen bridged twisty hepta- tomic biphenyl (THB) (2): As reported [11], THB was prepared from compound (1) (3.44g, 10mmol) with excess of cyclohexanone in anhydrous benzene in the presence of P2O5, and the reaction mixture was refluxed stirred for 8 h. The mixture was filtered and washed thoroughly with water to extract the P2O5, and removed the solvent under reduced pressure, the resulting product mixture was subjected to silica gel column chromatography using 10% ethyl acetate in petroleum ether as eluent to give product (2) THB as a colorless crystalline (2.15g, 30%). FT-IR (KBr, cm-1): 2930, 2858, 1475 (-CH2-), 1253 (-O-), 1875-1550, 875-825 (Ar-H), 550 (-Br). 1H NMR (400 MHz, DMSO-d6): δ 7.85 (s, 2H, Ar-H), 7.60 (d, H, J =8 Hz, Ar-H), 7.16 (d, 2H, J =8 Hz, Ar-H), 1.83 (s, 4H, CH2), 1.66 (s, 4H, CH2), 1.50(s, 2H, CH2).Anal.calcd.for C18H16Br2O2:C, 50.97;H, 3.80; Found C, 50.51; H, 3.77.
6, 6'-Cyclohexy-2-bromine-10-alkynyl oxygen bridged twisty heptatomic biphenyl (alkynyl-THB): As reported [23], in a threenecked round bottomed flask, compound (2) THB (0.74 g, 4 mmol), CuI (38mg, 0.2mmol), Pd(PPh3)2Cl2 (84mg, 0.12mmol) and solvent TEA were added, then (trimethylsilyl)acetylene (TMSA) (1 mL, 8 mmol) was added dropwise. The reaction was conducted at 80℃ under argon atmosphere for 24h. Then the residue was stirred in co-solvent MeOH/THF (1:2) with K2CO3 (mol, 6 eq.) at room temperature for 2h, and purified by silica gel column chromatography with 5% ethyl acetate in petroleum ether to give THB as a colorless crystalline (0.74g, 50%). FT-IR(KBr, cm-1): 3335 (CrC-H), 2985, 2910, 1450(-CH2-), 2160(-C≡C-), 1268 (-O-), 1800-1660, 890(Ar-H), 605(-Br).1HNMR(400MHz, DMSO-d6): δ 7.84(s, 1H, Ar-H), 7.75(s, 1H, Ar-H), 7.58(d, 1H, J =8Hz, Ar-H), 7.52 (d, 1H, J =8 Hz, Ar-H), 7.19 (d, 1H, Ar-H), 7.15 (d, 1H, Ar-H), 4.22 (s, 1H, C≡CH), 1.83(s, 4H, CH2), 1.66(s, 4H, CH2), 1.50(s, 2H, CH2). Anal. calcd. for C20H17BrO2: C, 65.05; H, 4.64; Found C, 65.57; H, 4.55.
Single oxygen bridged twisted heptatomic biphenyl perylene diimides (s-THBPDI): As reported [24-26],the s-BrPDI (0.63g, 1 mmol), alkynyl-THB (0.37 g, 1 mmol), CuI (9 mg, 0.05 mmol), and Pd(PPh3)2 Cl2 (21 mg, 0.03 mmol) were added in 15 mL solvents of TEA/THF (1:1). The reaction mixture was stirred and refluxed for 24 h under argon atmosphere. The residue was purified by silica gel column chromatography using petroleum ether and dichloro- methane (4:1) as the eluent to give s-THBPDI as a dark red solid (0.37g, 40%). FT-IR (KBr, cm-1): 3010, 1387 (-CH3), 2980, 2900, 1400 (-CH2-), 2244(-C≡C-), 1752, 1710(C=O), 1645 (-CO-N-), 1269(-O-).1HNMR(400MHz, CDCl3, ):δ10.13(d, 1H, J =8Hz, Per- H), 8.72 (s, 1H, Per-H), 8.62 (d, 1H, J = 4Hz, Per-H), 8 .60 (d, 1H, J = 4Hz, Per-H), 8.57 (d, 1H, J =8 Hz, Per-H), 8.51 (d, 2H, J =8 Hz, Per- H), 7.78 (s, 1H, Ar-H), 7.74 (s, 1H, Ar-H), 7.70 (d, 1H, J = 4 Hz, Ar-H), 7.63 (d, 1H, J = 8Hz, Ar-H), 7.51 (d, 1H, J =8Hz, Ar-H), 7.06 (d, 1H, J = 8Hz, Ar-H), 4.10-4.15 (m, 4H, N-CH2), 1.94-1.98 (m, 6H, -CH-, cyclohexyl-CH2), 1.77-1.79 (t, 4H, -CH2-CH2-CH2-), 1.70-1.74 (t, 2H, -CH2-CH2-CH2-), 1.31-1.42 (m, 16H, alkyl-CH2), 0.94-0.98 (t, 6H, -CH2-CH3), 0.88-0.91 (t, 6H, -CH2-CH3). Anal. calcd. for C60H57BrN2O6: C, 73.38; H, 5.85; N, 2.85; Found C, 73.95; H, 5.97; N, 2.64.
Double oxygen bridged twisted heptatomic biphenyl perylene diimides (d-THBPDI): The synthesis procedures of d-THBPDI were identical to that of s-THBPDI, only s-BrPDI was replaced with d-BrPDI (1 mmol), and petroleum ether and dichloromethane (3:2) was selected as the eluent to give pure d-THBPDI as a dark red solid (0.52g, 40%). FT-IR (KBr, cm-1): 3000, 1378 (-CH3), 2978, 2905, 1395 (-CH2-), 2235 (-C≡C-), 1748, 1705 (C=O), 1647 (-CO-N-), 1259 (-O-).1H NMR(400MHz, CDCl3): δ 9.6 (d, 2H, J =16Hz, Per- H), 8.75 (s, 1H, Per-H), 8.70 (s, 1H, Per-H), 8.66 (d, 1H, J = 4Hz, Per- h), 8.59 (d, 1H, J =12Hz, Per-H), 7.71 (d, 4H, J = 8Hz, Ar-H), 7.57 (d, 2H, J =8Hz, Ar-H), 7.48 (d, 2H, J =8Hz, Ar-H), 7.19 (d, 2H, J = 8Hz, Ar-H), 7.03 (d, 2H, J =8Hz, Ar-H), 4.07-4.18 (m, 4H, N-CH2), 1.88-1.96 (m, 10H, -CH-, cyclohexyl-CH]), 1.68-1.77 (t, 12H, -CH2-CH2-CH2-), 1.33-1.42 (m, 16H, alkyl-CH2), 0.94-0.97 (t, 6H, -CH2-CH3), 0.88-0.91 (t, 6H, -CH2-CH3). Anal. Calcd. for C80H72Br2N2O8: C, 71.21; H, 5.38; N, 2.08; Found C, 71.33; H, 6.01; N, 1.86 (The synthesis routes were shown in Scheme 1).
|
Download:
|
| Scheme1. Synthesis routes of s-THBPDI, d-THBPDI and the structure of the parent compound PDI. | |
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos: 51173155, 51472214) and the Colleges and Universities Science and Technology Research Project ofHebei Province (No. QN20131070).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.Org/10.1016/j.cclet.2016.10.011.
| [1] | E. Kozma, M. Catellani, Perylene diimides based materials for organic solar cells. Dyes Pigm. 98 (2013) 160–179. DOI:10.1016/j.dyepig.2013.01.020 |
| [2] | T. Ishi-I, K.I. Murakami, Y. Imai, S. Mataka, Light-harvesting and energytransfer system based on self-assembling perylene diimide-appended hexaazatriphenylene. Org. Lett. 7 (2005) 3175–3178. DOI:10.1021/ol050919t |
| [3] | R. Schmidt, J.H. Oh, Y.S. Sun, High-performance air-stable n-channel organic thin film transistors based on halogenated perylene bisimide semiconductors. J. Am. Chem. Soc. 131 (2009) 6215–6228. DOI:10.1021/ja901077a |
| [4] | G. Li, Y.B. Zhao, J.B. Li, Synthesis characterization, physical properties, and OLED application of single BN-fused perylene diimide. J. Org. Chem. 80 (2015) 196–203. DOI:10.1021/jo502296z |
| [5] | L. Yang, Y.Y. Yu, J. Zhang, Time-dependent aggregation-induced enhanced emission, absorption spectral broadening, and aggregation morphology of a novel perylene derivative with a large D-π-A structure. Chem. Asian J. 10 (2015) 1215–1224. DOI:10.1002/asia.v10.5 |
| [6] | F. Würthner, Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. (2004) 1564–1579. |
| [7] | M.J. Ahrens, L.E. Sinks, B. Rybtchinski, Self-assembly of supramolecular light-harvesting arrays from covalent multi-chromophore perylene-3,4:9,10-bis (dicarboximide) building blocks. J. Am. Chem. Soc. 126 (2004) 8284–8294. DOI:10.1021/ja039820c |
| [8] | Q.L. Zhao, S. Zhang, Y. Liu, Tetraphenylethenyl-modified perylene bisimide:aggregation-induced red emission, electrochemical properties and ordered microstructures. J. Mater. Chem. 22 (2012) 7387–7394. DOI:10.1039/c2jm16613e |
| [9] | Z.J. Zhao, P. Lu, J.W.Y. Lam, Molecular anchors in the solid state:restriction of intramolecular rotation boosts emission efficiency of luminogen aggregates to unity. Chem. Sci. 2 (2011) 672–675. DOI:10.1039/C0SC00521E |
| [10] | M.J. Lin, Á.J. Jiménez, C. Burschka, F. Würthner, Bay-substituted perylene bisimide dye with an undistorted planar scaffold and outstanding solid state fluorescence properties. Chem. Commun. 48 (2012) 12050–12052. DOI:10.1039/c2cc36719j |
| [11] | H.Q. Zhang, B. Yang, G.D. Yang, Y.G. Ma, Investigation on conformation of a series of functional bridged biphenyl containing seven-member heterocycle by X-ray single crystal diffraction. Acta Phys. Chim. Sin. 24 (2008) 1879–1883. |
| [12] | P. Lu, H.Q. Zhang, F.Z. Shen, A wide-bandgap semiconducting polymer for ultraviolet and blue light emitting diodes. Macromol. Chem. Phys. 204 (2003) 2274–2280. DOI:10.1002/(ISSN)1521-3935 |
| [13] | P. Lu, H.Q. Zhang, Y. Zheng, et al., New ultraviolet emissive wide-bandgap semiconductive polymers, Synth. Met. 135-136(2003) 205-206. |
| [14] | F. He, H.Q. Zhang, L. He, et al., Twisted PPV copolymers with efficient blue light emitting, Synth. Met. 135-136(2003) 209-210. |
| [15] | Z.J. Chen, U. Baumeister, C. Tschierske, F. Würthner, Effect of core twisting on self-assembly and optical properties of perylene bisimide dyes in solution and columnar liquid crystalline phases. Chemistry 13 (2007) 450–465. DOI:10.1002/(ISSN)1521-3765 |
| [16] | Y.W. Huang, Y. Yan, B.M. Smarsly, Z.X. Wei, C.F.J. Faul, Helical supramolecular aggregates, mesoscopic organisation and nanofibers of a perylenebisimide-chiral surfactant complex via ionic self-assembly. J. Mater. Chem. 19 (2009) 2356–2362. DOI:10.1039/b817838k |
| [17] | G. Boobalan, P.M. Imran, S. Nagarajan, Synthesis of highly fluorescent and water soluble perylene bisimide. Chin. Chem. Lett. 23 (2012) 149–153. DOI:10.1016/j.cclet.2011.10.017 |
| [18] | J.Q. Feng, B.L. Liang, D.L. Wang, Synthesis and aggregation behavior of perylenetetracarboxylic diimide trimers with different substituents at bay positions. Langmuir 24 (2008) 11209–11215. DOI:10.1021/la801463u |
| [19] | K. Sugiyasu, N. Fujita, S. Shinkai, Visible-light-harvesting organogel composed of cholesterol-based perylene derivatives. Angew. Chem. Int. Ed. 116 (2004) 1249–1253. DOI:10.1002/(ISSN)1521-3757 |
| [20] | K.J. Sonogashira, Development of Pd-Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. J. Organomet. Chem. 653 (2002) 46–49. DOI:10.1016/S0022-328X(02)01158-0 |
| [21] | R. Mishra, J.M. Lim, M. Son, Tuning the electronic nature of Mono-Bay alkynyl-phenyl-substituted perylene bisimides:synthesis, structure, and photophysical properties. Chemistry 20 (2014) 5776–5786. DOI:10.1002/chem.201400099 |
| [22] | Z.S. An, S.A. Odom, R.F. Kelley, Synthesis and photophysical properties of donor-and acceptor-substituted 1,7-bis(arylalkynyl)perylene-3,4:9,10-bis (dicarboximide)s. J. Phys. Chem. A 113 (2009) 5585–5593. DOI:10.1021/jp900152r |
| [23] | J. Mizuguchi, K. Tojo, Electronic structure of perylene pigments as viewed from the crystal structure and excitonic interactions. J. Phys. Chem. B 106 (2002) 767–772. |
| [24] | F. Würthner, M. Stolte, Naphthalene and perylene diimides for organic transistors. Chem. Commun. 47 (2011) 5109–5115. DOI:10.1039/c1cc10321k |
| [25] | R.O. Marcon, S. Brochsztain, Aggregation of 3,4,9,10-perylenediimide radical anions and dianions generated by reduction with dithionite in aqueous solutions. J. Phys. Chem. A 113 (2009) 1747–1752. DOI:10.1021/jp808383e |
| [26] | H. Zhao, Y.Y. Zhang, H. Xu, Synthesis and properties of perylene diimide dyes bearing unsymmetrical and symmetrical phenoxy substituents at bay positions. Tetrahedron 71 (2015) 7752–7757. DOI:10.1016/j.tet.2015.07.032 |
 2017, Vol. 28
2017, Vol. 28 



