b Department of Entomology, College of Plant Protection, China Agricultural University, Beijing 100193, China
Aphids are important agricultural pests throughout the world, often causing major economic losses. Their abilities of fast breeding and easily developing resistance to insecticides make their population control challenging . Recently, the eco-toxicity of some neonicotinoid insecticides to bees has led to call for restrictions on their use in agriculture [2]. Therefore, it becomes necessary to find eco-friendly chemicals with new strategy. The major component of aphid alarm pheromone, (E) -β-farnesene (Eβf, Fig. 1), is released when aphids are attacked by natural enemies and is also repellent to other aphids, causing avoidance behaviour when detected [3-6]. Moreover, its insecticidal activity at high doses has also been demonstrated [7]. Parasitoid and predators, on the other hand, eavesdrop on aphid communication and utilize Eβf as a kairomone, which attracts aphid predators and enhances foraging behaviour of parasitoids [8, 9]. Therefore, it is a potentially valuable tool in the development of new aphid control strategies. However, field application of this pheromone presents some disadvantages for its relative volatility and instability in the environment caused by easy oxidation of its conjugated double bonds [10]. Therefore, it is necessary to find new Eβf analogues that are more stable and efficient.
As an important class of nitrogen-containing compounds, 1, 2, 3- thiadiazole with various interesting properties is becoming a rapidly growing and independent branch of the heterocyclic chemistry [11]. It is widely used in chemicals discovery, and has increasingly aroused attention because of its versatile biological activities, such as insecticidal, systemic acquired resistance, fungicidal, herbicidal and antiviral activities [12-19]. Recently, a large number of 1, 2, 3-thiadiazole derivatives were found to exhibit insecticidal activities. For example, Fan found that a series of 1, 2, 3- thiadiazole derivatives containing diacylhydrazine moiety possessed superior insecticidal activities against Plutella xylostella L. and Culex pipiens pallens [20]. More significantly, the structure of 1, 2, 3-thiadiazole can be easily decomposed into lower molecular weight compounds by releasing N2, which favours the use of its derivatives as eco-friendly pesticides with low toxicity and suitable duration of efficiency to the target biology and half-life in the agroecosystem [11].
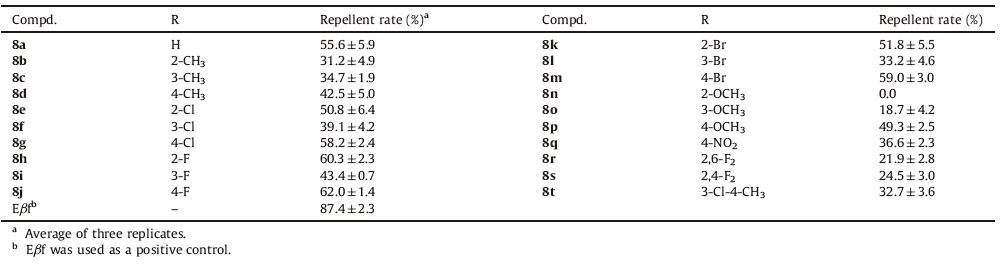
In our previous studies, some Eβf analogues with good stability and biological activity were obtained by introducing a variety of aromatic ring skeleton to replace the conjugated double bonds in Eβf [1, 21, 22]. These studies inspired our current hypothesis that the introduction of 1, 2, 3-thiadizole moiety into the lead compound Eβf may favour the biological activity of Eβf. Here we designed and synthesized a series of Eβf analogues containing 1, 2, 3-thiadiazole (Fig. 1) thanks to its superior properties as described above. Accordingly, the designed compounds contain the geranyl group, mimicking the terpene structure of Eβf, linked to 1, 2, 3-thiadizole ring. Especially, the conjugated double bonds of Eβf are part of the aromatic ring in the analogues. The good stability and biological activities of these Eβf analogues were expected. The stability of representative compounds was studied by HPLC and 1H NMR technologies. Their aphicidal and repellent activities against Myzus persicae (Sulzer) were evaluated. It is expected that this study may be valuable for the discovery of eco-friendly aphid control agents.
|
Download:
|
| Figure 1. Design strategy of the target compounds. | |
2. Results and discussion 2.1. Chemistry
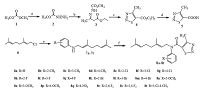
The synthetic route for the title compounds 8a-8t was illustrated in Scheme 1. First, the key intermediate 4-methyl- 1, 2, 3-thiadiazole-5-carboxylic acid 5 was synthesized from dimethyl carbonate through four steps: Hydrazidation, condensation, cyclization, and saponification with the total yield 68.2%. Second, the synthetic method of compounds 7a-7t have been reported in Ref. [22], when geranyl chloride as alkylating agents reacted with aniline only in the present of K2CO3, the reaction proceeded slowly and dialkylation by-products are observed. In order to improve the selectivity and reaction yield, we choose one optimized reaction condition: 3 equiv. of aniline and a catalytic amount of KI (0.1 equiv.) in acetonitrile, additional 1 equiv. K2CO3 and 3 equiv. N, N-dimethyldodecylamine-N-oxide (DDAO), at room temperature for 4 h. And we successfully obtained 7b-7t in this condition through the reaction of geranyl chloride with different substituted aniline in the yields of 39%-83%. Finally, the target compounds were readily obtained in 34%-92% yields through the condensation reaction between compound 5 and compounds 7a- 7t at room temperature in the presence of the condensation reagent N, N'-dicyclohexyl-carbodiimide (DCC) and the promoter 4-dimethy-lamino-pryidine (DMAP).
The structures of the synthesized compounds (8a-8t) were established on the basis of their spectroscopic data of NMR, IR and HRMS (Supporting information). As indicated by 1H NMR, all aryl protons showed multiplets at δ 6.78 to δ 8.20, and the positions of the two double bonds' protons were showed multiplet at δ 4.91 to d 5.02 and triplet at about δ 5.26 respectively. Signals corresponding to the -CH3 of the 1, 2, 3-thiadiazole ring were observed at about d 2.82 and signals for the C-CH2-CH2-C protons were observed at d 1.92 to δ 2.05. Three -CH3 absorption peaks showed singlet at about δ 1.48, δ 1.59 and δ 1.68, respectively. Interestingly, the signal of CH2-N protons is change along with the substituent in different position of phenyl. Normally, this absorption peak is a doublet at about δ 4.46 with coupling constants 7.20 Hz. However, when the substituent is at the ortho of phenyl ring, the signal will split two quartets in average (Supporting information). We considered that this phenomenon is from the existing steric hindrance of R at the ortho position. Therefore, the conformational switching of corresponding compound is slowly at room temperature (low energy), and this led to the compound not existed with only one preferential conformation. In order to verify this deduction, we further chose compound 8n (R = 2-OCH3) as example to study by dynamic 1H NMR spectroscopy. As a result, the 'abnormal split' of the signal of CH2-N protons was vanish and replaced with a singlet at d 4.37 in the 100 ℃ 1H NMR spectrum (Supporting information). This result is identical with our guess, in which the conformational switching of compound 8n is quickly at high temperature (high energy).
|
Download:
|
| Scheme1. Synthetic route of the designed compounds. Reagents and conditions: (a) hydrazine hydrate, ethanol, r.t. 24 h; (b) ethyl acetoacetate, ethanol, r.t. 10 h; (c) SOCl2, CH2Cl2, 0 ℃-r.t. 24 h; (d) CH3OH/NaOH, HCl/H2O, r.t. 12 h; (e) substituted anilines, K2CO3, KI, DDAO, CH3CN, r.t. 4 h; (f) 4-methy-1, 2, 3-thiadizole-5-carboxylic acid 5, DCC, DMAP, CH2Cl2, r.t. 6-10 h. | |
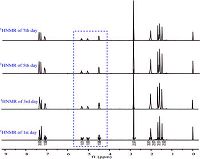
These compounds were found to be stable at room temperature. In particular, we checked the contents change of eight compounds by HPLC technique, and these data were shown in Table 1. The Eβf is easy oxidation because of its conjugated double bonds, and it degrades 77% and 94% after exposure to air for 24 h and 45 h at room temperature, respectively [23]. We have found the accordant result in our previous study that Eβf was decomposed absolutely after leaving it at room temperature and in contact with air for periods up to 48 h [21]. As indicated in Table 1, in such conditions the degradation of the candidate compounds could not be detected. Moreover, we took 1H NMR technique to study the protons change of compound 8a, and Fig. 2 illustrated the 1H NMR spectrums of compound 8a after exposure to air for 1 day, 3 days, 5 days and 7 days. As a result, there was neither proton signals disappeared nor new proton signals appeared. On the basis of the above results, the stability of the title compounds is much better than that of the lead compound Eβf.
|
|
Table 1 Stability data of representative compounds. |
|
Download:
|
| Figure 2. The 1H NMR spectrums of compound 8a. | |
2.2. Biological activity
The repellent effects of analogues on M. persicae were evaluated and listed in Table 2. The results indicated that except for 8n, compounds 8a-8t displayed certain repellent activities against M. persicae at 5 mg dose. Some of them displayed promising repellent effect with values of 18.7%-62.0%. Particularly, compounds 8h and 8j showed 60.3% and 62.0% repellent rates, respectively. Repellent activities of target compounds were affected by both variation of the substitute's type and position on the phenyl. When the substituent R was at para position of phenyl, compounds with weak electron-withdrawing groups (such as 4-F, 4-Br) were more effective than those with electron-donating groups (such as 4-CH3, 4-OCH3). Additionally, compounds with di-substituents on the phenyl showed lower effectiveness than those with monosubstituent. For example, the repellent rate of compound 8h (with a 2-F substituent) was much higher than those of 8r (with a 2, 6-F2 substituent) and 8s (with a 2, 4-F2 substituent). Furthermore, the position of the substituent R on the phenyl interestingly affected the repellent activity. Amongst the analogues containing a halogen substituent, the order of the repellent activities of compounds 8k (2-Br), 8l (3-Br), and 8m (4-Br) could be placed as following: 8m > 8k > 8l. Also, compounds 8e, 8f, 8g, and 8h, 8i, 8j, had the similar results. However, the analogues with electrondonating groups on the benzene ring exhibited another rule, for instance, the repellent activities of compounds 8n (2-OCH3), 8o (3- OCH3), and 8p (4-OCH3) could be found as following: 8p > 8o > 8n.
|
|
Table 2 The repellent activities of title compounds 8a-8t (5 μg/test, Myzus persicae) |
The aphicidal activity of the lead compound, the target compounds, and pymetrozine against M. persicae was shown in Table 3. The preliminary bioassay results (at a concentration of 200 μg/mL, 48 h) indicated that all the target compounds exhibited aphicidal activity. The aphicidal activities of analogues 8d, 8e, 8f, 8h, 8j, 8l, 8o, 8p, 8r, 8s, and 8t are comparable to or even better than that of the lead compound Eβf. Unluckily, the aphicidal activities of all the compounds are weaker than that of pymetrozine. On the basis of the primary experimental results, analogues showing a mortality rate higher than 70% were chosen to determine the LC50 values. As shown in Table 4, compounds 8l, 8s, and 8t exhibited high aphicidal activity against M. persicae with LC50 values of 33.4, 50.2 and 61.8 μg/mL, respectively. However, the aphicidal activities of analogues 8l, 8s, and 8t are lower than pymetrozine with a LC50 of 7.1 μg/mL.
|
|
Table 3 The aphicidal activity against Myzus persicae of title compounds 8a-8t (200μg/mL, 48h). |
|
|
Table 4 The LC50 of compounds 8l, 8r and 8t. |
3. Conclusion
In summary, a series of novel (E) -β-farnesene analogues containing 1, 2, 3-thiadiazole were designed and synthesized by replacing unstable conjugated double bonds of Eβf with1, 2, 3- thiadiazole ring according to the principle of linking bioactive substructures. The stability of representative compounds is much better than the lead Eβf based on the results made by HPLC and 1H NMR techniques. Behaviour experiment results indicated that compounds 8h and 8j displayed 60.3% and 62.0% repellent rate, respectively. Interestingly, compounds with weak electron-withdrawing groups (such as 4-F, 4-Br) were more effective than those with electron-donating groups (such as 4-CH3, 4-OCH3) when the substituent is at para position of phenyl. The aphicidal bioassay results showed that most analogues exhibited considerable aphicidal activity against M. persicae, especially analogues 8l, 8s and 8t exhibited high aphicidal activity against M. persicae with LC50 values of 33.4, 50.2 and 61.8 μg/mL, respectively, which exhibited improved aphicidal activity compared with the lead Eβf. This work could afford some valuable information on the discovery of eco-friendly aphid control agent.
4. Experimental 4.1. SynthesisMelting points of some compounds were determined on an X-4 binocular microscope (Fukai Instrument Co., Beijing, China), with an uncorrected thermometer. 1H NMR spectra and 13C NMR spectra were recorded on Bruker AM-300 (300 MHz, 75 MHz respectively) spectrometer with CDCl3 as the solvent and TMS as the internal standard. High resolution mass spectrometry (HRMS) data were obtained on an FTICR-MS Varian 7.0 T FTICR-MS instrument. Purity was analysed by high performance liquid chromatography (HPLC) on a LC-1AT HPLC instrument (Shimadzu). Allthe reagentswere obtainedcommerciallyandused after further purification. Column chromatography purification was carried out by using silica gel (Merck 60, 200-300 mesh).
The general synthetic scheme for representative compounds 8a-8t is shown in Scheme 1.Keyintermediate 5 was prepared from dimethyl carbonate according to Ref. [12]. Compounds 7a-7t were obtained through N-alkylation reaction between geranyl chloride and different substituted aniline according to the reported method [22, 24-26]. The general procedures of 5 and 7a-7t are described in the Supporting information.
The target compounds 8a-8t were prepared using condensation reaction between compounds 5 and 7a-7t. The general procedure was described as below. To a solution of 5 (0.87 g, 6 mmol), DMAP (0.15 g, 1.2 mmol) and dichloromethane (20 mL), DCC (1.36 g, 6.6 mmol) was added in batches. And then solution of compounds 7a-7t (6.0 mmol) in dichloromethane was dropped slowly, the resulted mixture was stirred for 6-10 h. After the reaction completed, filtered, the filtrate was washed with water. The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo. Then the residue was purified by hromatography on silica-gel column (petroleum ether:ethyl acetate = 15:1, v/v) to obtain the corresponding analogues 8a-8t.
4.2. Stability testThe representative analogues 8a (H), 8b (2-CH3), 8g (4-Cl), 8h (2-F), 8l (3-Br), 8n (2-OCH3), 8q (4-NO2) and 8s (2, 4-F2) were dissolved in methanol (chromatographically pure), respectively. After exposure to air for 48 h at room temperature, their changes of content were analysed by high performance liquid chromatography (HPLC) on a LC-1AT HPLC instrument (Shimadzu). Chromatographic experiments were performed on C18 reversed-phase column (4.5 mm × 250 mm, 5 μm), the mobile phase was methanol and water (85:15); the detection wavelength was 254 nm, column temperature was 25 ℃, the flow rate was 0.7 mL/min and the injection volume was 5 μL.
Meanwhile, the compound 8a was selected to study its stability by 1H NMR technology. Dissolved in CDCl3, 8a was tested 4 times at corresponding time successively after exposure to air for 1 day, 3 days, 5 days, 7 days at room temperature, the changes of proton were analysed by 1H NMR spectrograms.
4.3. BioassaysThe behavioural activity and aphicidal assay against M. persicae was evaluated according to the reference methods [27-31]. In the repellent assay, in each test 5 μg compound (2.5 μL of hexane solution of each compound with a concentration of 2000 μg/mL) was placed in the glass stimulus chamber of the "treatment" arm. As a control, 2.5 μL of hexane was placed in the glass stimulus chamber of the "control" arm of the olfactometer. The detailed procedures were listed in the Supporting information.
AcknowledgmentsWe thank the financial support from the National Natural Science Foundation of China (No. 21132003).
| [1] | Y.F. Sun, H.L. Qiao, Y. Ling, New analogues of (E)-β-farnesene with insecticidal activity and binding affinity to aphid odorant-binding proteins. J. Agric. Food Chem. 59 (2011) 2456–2461. DOI:10.1021/jf104712c |
| [2] | European Food Safety Authority, Statement on the findings in recent studies investigating sub-lethal effects in bees of some neonicotinoids in consideration of the uses currently authorised in Europe. EFSA J. 10 (2012) 2752. DOI:10.2903/j.efsa.2012.2752 |
| [3] | C.J. Kislow, L.J. Edwards, Repellent odour in aphids. Nature. 235 (1972) 108–109. DOI:10.1038/235108a0 |
| [4] | R.W. Gibson, J.A. Pickett, Wild potato repels aphids by release of aphid alarm pheromone. Nature. 302 (1983) 608–609. DOI:10.1038/302608a0 |
| [5] | W.S. Bowers, L.R. Nault, R.E. Webb, S.R. Dutky, Aphid alarm pheromone:isolation, identification, synthesis. Science. 177 (1972) 1121–1122. DOI:10.1126/science.177.4054.1121 |
| [6] | T.J.A. Bruce, M.A. Birkett, J. Blande, Response of economically important aphids to components of Hemizygia petiolata essential oil. Pest Manag. Sci. 61 (2005) 1115–1121. DOI:10.1002/(ISSN)1526-4998 |
| [7] | A.M. Van Oosten, J. Gut, P. Harrewijn, P.G.M. Piron, Role of farnesene isomers and other terpenoids in the development of different morphs and forms of the aphids Aphis fabae and Myzus persicae. Acta. Phytopathol. Entomol. Hung. 25 (1990) 331–342. |
| [8] | S. Vandermoten, F. Francis, E. Haubruge, W.S. Leal, Conserved odorant-binding proteins from aphids and eavesdropping predators. PLoS ONE. 6 (2011) e23608. DOI:10.1371/journal.pone.0023608 |
| [9] | G. Kunert, J. Trautsch, W.W. Weisser, Density dependence of the alarm pheromone effect in pea aphids, Acyrthosiphon pisum (Sternorrhyncha:Aphididae). Eur. J. Entomol. 104 (2007) 47–50. DOI:10.14411/eje.2007.007 |
| [10] | M.H. Beale, M.A. Birkett, T.J.A. Bruce, Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior. Proc. Natl. Acad. Sci. U.S.A. 103 (2006) 10509–10513. DOI:10.1073/pnas.0603998103 |
| [11] | V.A. Bakulev, W. Dehaen, The Chemistry of 1,2,3-Thiadiazoles, Wiley, New York, 2004. |
| [12] | Z.J. Fan, Z.G. Shi, H.K. Zhang, Synthesis and biological activity evaluation of 1,2,3-thiadiazole derivatives as potential elicitors with highly systemic acquired resistance. J. Agric. Food Chem. 57 (2009) 4279–4286. DOI:10.1021/jf8031364 |
| [13] | W.T. Mao, H. Zhao, Z.J. Fan, Synthesis and bioactivity of N-tert-butyl-N'-acyl-5-methyl-1,2,3-thiadiazole-4-carbohydrazides. Chin. Chem. Lett. 23 (2012) 1233–1236. DOI:10.1016/j.cclet.2012.09.024 |
| [14] | Z.J. Fan, Z.K. Yang, H.K. Zhang, Synthesis, crystal structure, and biological activity of 4-methyl-1,2,3-thiadiazole-containing 1,2,4-triazolo[3,4-b] [1,3,4] thiadiazoles. J. Agric. Food Chem. 58 (2010) 2630–2636. DOI:10.1021/jf9029628 |
| [15] | F.Y. Li, X.F. Guo, Z.J. Fan, Synthesis and biological activities of novel 2-amino-1,3-thiazole-4-carboxylic acid derivatives. Chin. Chem. Lett. 26 (2015) 1315–1318. DOI:10.1016/j.cclet.2015.05.040 |
| [16] | Y.P. Luo, Q. Gong, Q. Chen, G.F. Yang, Synthesis and herbicidal activities of tetrazolinone derivatives containing oxime ether. Chin. J. Org. Chem. 28 (2008) 1561–1565. |
| [17] | W.M. Xu, S.Z. Li, M. He, Synthesis and bioactivities of novel thioether/sulfone derivatives containing 1,2,3-thiadiazole and 1,3,4-oxadiazole/thiadiazole moiety. Bioorg. Med. Chem. Lett. 23 (2013) 5821–5824. DOI:10.1016/j.bmcl.2013.08.107 |
| [18] | S.X. Wang, Z. Fang, Z.J. Fan, Synthesis of tetrazole containing 1,2,3-thiadiazole derivatives via U-4CR and their anti-TMV activity. Chin. Chem. Lett. 24 (2013) 889–892. DOI:10.1016/j.cclet.2013.05.026 |
| [19] | Y.D. Li, W.T. Mao, Z.J. Fan, Synthesis and biological evaluation of novel 1,2,4-triazole containing 1,2,3-thiadiazole derivatives. Chin. Chem. Lett. 24 (2013) 1134–1136. DOI:10.1016/j.cclet.2013.06.024 |
| [20] | H. Wang, Z.K. Yang, Z.J. Fan, Synthesis and insecticidal activity of N-tertbutyl-N,N'-diacylhydrazines containing 1,2,3-thiadiazoles. J. Agric. Food Chem. 59 (2011) 628–634. DOI:10.1021/jf104004q |
| [21] | Y.G. Qin, Y.Y. Qu, J.P. Zhang, Synthesis and biological activity of different heterocyclic substituted (E)-β-farnesene analogues. Chin. J. Org. Chem. 35 (2015) 455–461. DOI:10.6023/cjoc201408002 |
| [22] | J.P. Zhang, Y.G. Qin, Y. Ling, Design, synthesis and repellent activity of ((E)-β-farnesene analogues containing benzene ring with different substitutions. Chin. J. Org. Chem. 36 (2016) 1883–1889. DOI:10.6023/cjoc201603021 |
| [23] | G.W. Dawson, D.C. Griffiths, J.A. Pickett, M.C. Smith, C.M. Woodcock, Natural inhibition of the aphid alarm pheromone. Entomol. Exp. Appl. 36 (1984) 197–199. DOI:10.1111/eea.1984.36.issue-2 |
| [24] | K. Tani, T. Yamagata, S. Akutagawa, Metal-assisted terpenoid synthesis. 7. Highly enantioselective isomerization of prochiral allylamines catalyzed by chiral diphosphine rhodium(I) complexes. Preparation of optically active enamines. J. Am. Chem. Soc. 106 (1984) 5208–5217. DOI:10.1021/ja00330a029 |
| [25] | J.L. Romera, J.M. Cid, A.A. Trabanco, Potassium iodide catalysed monoalkylation of anilines under microwave irradiation. Tetrahedron Lett. 45 (2004) 8797–8800. DOI:10.1016/j.tetlet.2004.10.002 |
| [26] | C. Siswanto, J.F. Rathman, Selective N-alkylation of aniline by micellar catalysis. J. Colloid Interface Sci. 196 (1997) 99–102. DOI:10.1006/jcis.1997.5171 |
| [27] | M. Hori, Repellency of rosemary oil against Myzus persicae in a laboratory and in a screen house. J. Chem. Ecol. 24 (1998) 1425–1432. DOI:10.1023/A:1020947414051 |
| [28] | M. Hori, Onion aphid (Neotoxoptera formosana) attractants, in the headspace of Allium fistulosum and A. tuberosum leaves. J. Appl. Entomol. 131 (2007) 8–12. DOI:10.1111/jen.2007.131.issue-1 |
| [29] | J.R. Busvine, Recommended Methods for Measurement of Pest Resistance to Pesticides, FAO Plant Protection Papers No. 21, Food and Agricultural Organization, Rome, Italy, 1980. |
| [30] | C.L. Zhang, Y.Y. Qu, X.Q. Wu, Eco-friendly insecticide discovery via peptidomimetics:design, synthesis, and aphicidal activity of novel insect kinin analogues. J. Agric. Food Chem. 63 (2015) 4527–4532. DOI:10.1021/acs.jafc.5b01225 |
| [31] | W.S. Abbott, A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18 (1925) 265–267. |
 2017, Vol. 28
2017, Vol. 28