The comprehensive properties of poly (butylene succinate) (PBS) are comparable to the conventional polypropylene and polyethylene, and it is considered to be a promising bio-based polymers [1, 2]. However, its melt strength/viscosity and crystallization rate are low, which lead to a poor blow-molding processability. A cross-linking of PBS should be an effective way to address those issues [3-7]. The traditional cross-linking modification is achieved by post-processing. Not only extra equipment is needed, but also some gels are formed during modification, which will influence the reprocessability and biodegradability of PBS. If we make PBS slightly cross-linking at the polycondensation stage instead of post-processing, the crosslinking degree is so low as not to affect PBS further thermal processing.
Diacetylene group is prone to cross-linking reaction under UVirradiation or at high temperature [8-10]. In this study, a functional monomer containing a diacetylene group, dimethyl 4, 40- (buta-1, 3- diyne-1, 4-diyl) dibenzoate (DA), was designed and synthesized. It was found that DA can partly cross-linked at the melt polymerization temperature (Fig. S1 in Supporting information), and therefore a small amount of DA is copolymerized with 1, 4-butanediol, and dimethyl succinate to prepare PBS copolyesters. It is expected that the slightly-cross-linking reaction caused by DA will increase the melt strength/viscosity and the crystallization rate of PBS.
2. Results and discussion 2.1. Rheological behaviour of PBDASxThe dynamic rheological behaviours of neat PBS and PBDASx were studied systematically. Fig. 1a shows the complex viscosity- frequency curves. For neat PBS, it presented low complex viscosity and acted as a Newtonian behaviour. For PBDASx, it still showed Newtonian behaviour like neat PBS, illustrating slightly crosslinking did not change its flowing type. But it can be seen obviously that the complex viscosity of PBDASx increased significantly compared to PBS. This phenomenon can be due to the formation of slightly cross-linking network and an increase in molecular weight caused by the cross-linking of DA.

|
Download:
|
| Figure 1. Dynamic rheological (a) complex viscosities vs. frequency, (b) storage modulus G' vs. frequency, (c) loss modulus G" vs. frequency and (d) storage modulus G' vs. loss modulus G" for neat PBS and PBDASx. | |
From the storage modulus G'-frequency and loss modulus G"- frequency curves (shown in Fig. 1b and c), we can see that G' of PBDASx was higher than that of PBS, suggesting that the PBDASx have an improved elastic response. Meanwhile, PBDASx also kept higher G" than neat PBS, demonstrating that copolyesters have an enhanced molecular interaction and reduced chain mobility [11]. This phenomenon may owe to the formation of cross-linking network at the polycondensation step, which can be demonstrated in Fig. 1d. The curves of PBDASx shown in Fig. 1d were almost superimposed and deviated with that of neat PBS, suggesting that some microstructure change occurred in PBDASx [11-13]. From the above rheological data we can make a conclusion that a small amount of DA monomers cross-linked at the polycondensation step, and some cross-linking networks formed at the same time.
The capillary rheological behaviours of the neat PBS and PBDAS0.3 are shown in Fig. 2. We can see that PBDAS0.3 showed higher shear viscosity at the whole shear region, and even at the testing temperature up to 140 ℃, PBDAS0.3 still remained higher shear viscosity than PBS. This demonstrated that with the introduction of DA content, the melt viscosity of PBDASx was improved due to the formation of cross-linking network, which will benefit the blow molding processing in the future.

|
Download:
|
| Figure 2. Capillary rheological shear viscosities vs. shear rate (a) at 130 ℃ and (b) at 140 ℃ for neat PBS and PBDAS0.3. | |
2.2. Crystallization behaviour of PBDASx
The crystallization behaviour of PBDASx was evaluated by DSC (Fig. S3a in Supporting information). The detailed crystallization data are summarized in Table 1. It can be observed clearly that the crystallization temperatures (Tc) of PBDASx were much higher than that of neat PBS, even at different cooling rates (Fig. S3b in Supporting information). To make clear how diacetylene group affects the crystallization process of PBDASx, isothermal crystallization processes were investigated at 94 ℃ by DSC. Avrami equation was used to analyse the isothermal crystallization kinetics [14]:
| $1-Xt=\text{exp}\left( -k{{t}^{\text{n}}} \right)$ | (1) |
|
|
Table 1 Crystallization data of PBS and PBDASx. |
The equation can be rewritten as:
| $\text{log}\left[ -\text{ln}\left( 1-Xt \right) \right]\text{=log}k+n\text{log}t$ | (2) |
A plot of log[-ln (1 - Xt) ] versus log t (shown in Fig. S3c in Supporting information) gives a straight line from which both the Avrami exponent and the rate constant could be determined. n is the Avrami exponent, which denotes the nature of the nucleation and growth process. By calculation, the n values of PBS and PBDASx were 2.6, 2.2, 2.1 and 2.4, suggesting that the crystallization of the neat PBS corresponded to a three dimensional growth with heterogeneous nucleation, while the segments of PBDASx tended to be gathered on a two dimensional axial axe because of thedecreased segment integrity and mobility caused by the cross-linking, the copolyesters corresponded to a two dimensional growth with heterogeneous nucleation.Thehalf-time of crystallization (t1/2) values of PBS and PBDASx were 14.7, 1.2, 1.4, and 2.6 min, respectively, illustrating PBDASx crystallized faster than PBS.
It is well known that the crystallization process of the melting polymer can be divided into two steps, nucleation and crystal growth. We can reasonably suppose that the cross-linking and rigid DA unit structure may prevent the segment motion to a certain extent, and, therefore, the crystal growth because they reduce the segment regularity of PBS chain. However, the crystallization rate and temperature were not decreased, but increased considerably. Therefore, the increase in crystallization rate can only be ascribed to the improvement in nucleation efficiency by the introduction of cross-linkable DA unit structure.
To estimate the nucleating efficiency (NE) of PBDASx, the following method put forward by Fillion et al. is used [15, 16] (Fig. S4 in Supporting information) :
| $NE=\frac{{{T}_{cNA}}-{{T}_{c}}}{{{T}_{c}}\text{max}-{{T}_{c}}}\times 100$ | (3) |
The detailed NE values of PBDASx are given in Table 1. It was found that cross-link points can be regarded as crystal nucleus in the nucleation step [7, 17], therefore, it is beneficial to high nucleating efficiency (NE), which will lead to a higher crystallization temperature and faster crystallization. At the same time, we can see that the Tc of PBDASx decreased a little with the increase of DA content, illustrated that more cross-linking networks would inhibit the crystal growth. However, due to the very low crosslinking content, PBDASx still kept higher crystallization temperature than the neat PBS.
Meanwhile, compared to PBS, the crystalline morphology showed that the crystals for PBDASx were tiny and imperfect (Fig. S5 in Supporting information). WAXD was used to investigate the crystalline structure of PBS and PBDASx (Fig. S3d in Supporting information). It was found that although cross-linking enhanced the crystallization rate, it had no influence on the crystalline structure of PBS.
2.3. Tensile properties of PBDASxThe mechanical properties play an important role in the application of materials, and the tensile properties of PBDASx are provided in Table 2. We can see that the tensile strength of PBDAS0.3 was close to neat PBS while the elongation at break decreased slightly. The Young's modulus increased with the increase of DA content. This was ascribed to the cross-linking network and the increased rigidity of copolyesters, which will reduce the segment mobility. As a result, the elongation at break decreased, and the tensile strength and Young's modulus increased. Although the introduction of DA content can improve the melt viscosity and crystallization properties of PBDASx, the segment regularity of copolyesters was reduced with the increase of DA content. To take all this into account, PBDAS0.3 was considered to possess optimum comprehensive properties.
|
|
Table 2 Mechanical properties of neat PBS and PBDASx. |
3. Conclusions
In summary, a comonomercontaining a diacetylenegroup (DA), was synthesized and introduced into PBS main chains successfully via melt polymerization to prepare a series of slightly cross-linked PBS copolyesters (PBDASx). PBDASx had high molecular weights and showed good solubility in chloroform. The cross-link points could be regarded as crystal nucleus favouring crystallization, and PBDASx copolyesters had higher Tc and faster crystallization rate than neat PBS. PBDASx had higher melt viscosity than neat PBS due to the formation of cross-linking network caused by introducing DA into its polymer chains. PBDAS0.3 exhibited better comprehensive properties than neat PBS, which will widen applications of PBS.
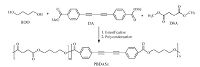
4. Experimental 4.1. Materials1, 4-Butanediol (BDO, AR grade), and zinc acetate (Zn (OAc)2, AR grade) were purchased from Kelong Chemical Corporation (Chengdu, China). Dimethyl succinate (DSA, AR grade) was purchased from Alfa Aesar Chemical Corporation (Tianjin, China). Tetrabutyl titanate (TBT), used as a catalyst, was also provided by Kelong Chemical Corporation, and dissolved in anhydrous toluene to prepare 0.2 g/mL solution. Methyl 4-iodobenzoate (AR grade), ethynyltrimethylsilane (AR grade), copper (I) iodide (CuI, AR grade) and bis (triphenylphosphine) palladium (II) chloride (Pd (PPh3)2Cl2, AR grade) were purchased from Yinuokai Chemical Corporation (Beijing, China). Copper (I) chloride (CuCl, AR grade), dimethyl sulfoxide (DMSO, AR grade), potassium carbonate (K2CO3, AR grade), triethylamine (Et3N), tetrahydrofuran (THF), hydrochloric acid (HCl), methanol and ethanol were purchased from Kelong Chemical Corporation. DA monomer (described in Supporting information) is synthesized according to the following synthetic procedures (Scheme 1).

|
Download:
|
| Scheme1. Synthetic routes of DA. | |
4.2. Synthesis and characterizations of PBS copolyesters containing DA (PBDASx)
The copolyesters (PBDASx) containing DA were synthesized by a two-step melt polymerization (esterification and polycondensation, shown in Scheme 2). In the abbreviations, x refers to the molar percentage (mol%) of DA units relative to the total diesters. In the first esterification step, diesters (the compositions of DSA and DA mixtures are shown in Table 3) and BDO with the molar ratio of 1:1.06, as well as the transesterification catalyst Zn (OAc)2 (0.2 wt%) were added into a three-necked round-bottom flask equipped with mechanical stirrer, water separator, and nitrogen inlet pipe. The reactor was heated to 175 ℃ and maintained for 4 h, and then the catalyst TBT (0.1 wt%) was added to the reaction system. The polycondensation was carried out at 230 ℃ for 6 h under the vacuum of 20-70 Pa. All the characterizations were described in Supporting information. 1H NMR spectra of PBDASx (shown in Fig. S2) confirm that DA has been successfully introduced into PBS main chains. PBDASx show good solubility in chloroform and their intrinsic viscosities are in the range of 0.81-0.97 dL/g (Table 3). The molecular weights can be determined by GPC, and the data are also shown in Table 3. We can see that the Mw values of PBDASx are greater than that of PBS, which all are higher than 23 ×104 g/mol, and their PDI values are also increased when DA is introduced.
|
Download:
|
| Scheme2. Synthetic procedure of PBDASx copolyesters. | |
|
|
Table 3 Composition and molecular weight data of PBDASx copolyesters. |
Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (Nos. 21634006 and 51121001), and the Program for Changjiang Scholars and Innovative Research Teams in University of China (No. IRT1026).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.10.014.
| [1] | J. Xu, B. Guo, Poly(butylene succinate) and its copolymers:research, development and industrialization. Biotechnol. J. 5 (2010) 1149–1163. DOI:10.1002/biot.v5.11 |
| [2] | R.T. Duan, X. Dong, D.F. Li, X.L. Wang, Y.Z. Wang, Preparation and properties of bio-based PBS multiblock copolyesters containing isosoorbide units. Acta Polym. Sin. 1 (2016) 70–77. |
| [3] | D. Kim, W. Kim, D. Lee, Modification of poly(butylene succinate) with peroxide crosslinking physical and thermal properties, and biodegradation. J. Appl. Polym. Sci. 81 (2001) 1115–1124. DOI:10.1002/(ISSN)1097-4628 |
| [4] | D. The, F. Yoshii, N. Nagasawa, Synthesis of poly(butylene succinate)/glass fiber composite by irradiation and its biodegradability. J. Appl. Polym. Sci. 91 (2004) 2122–2127. DOI:10.1002/(ISSN)1097-4628 |
| [5] | N. Teramoto, M. Ozeki, I. Fujiwara, M. Shibata, Crosslinking and biodegradation of poly(butylene succinate) prepolymers containing itaconic or maleic acid units in the main chain. J. Appl. Polym. Sci. 95 (2005) 1473–1480. DOI:10.1002/(ISSN)1097-4628 |
| [6] | X. Huang, C. Li, W. Zhu, Ultraviolet-induced crosslinking of poly(butylene succinate) and its thermal property dynamic mechanical property, and biodegradability. Polym. Adv. Technol. 22 (2011) 648–656. DOI:10.1002/pat.v22.5 |
| [7] | P. Ma, Z. Ma, W. Dong, Y. Zhang, P.J. Lemstra, Structure/property relationships of partially crosslinked poly(butylene succinate). Macromol. Mater. Eng. 298 (2013) 910–918. DOI:10.1002/mame.v298.8 |
| [8] | J.M. Kim, J.S. Lee, H. Choi, D. Sohn, D.J. Ahn, Rational design and in-situ FTIR analyses of colorimetrically reversibe polydiacetylene supramolecules. Macromolecules. 38 (2005) 9366–9376. DOI:10.1021/ma051551i |
| [9] | D. Likhatchev, L. Alexandrova, R. Salcedo, T. Ogawa, Topochemical polymerization of butadiynylene dibenzamides. Polym. Bull. 34 (1995) 149–154. DOI:10.1007/BF00316389 |
| [10] | B. Yoon, H. Shin, E.M. Kang, Inkjet-compatible single-component polydiacetylene precursors for thermochromic paper sensors. ACS Appl. Mater. Interfaces. 5 (2013) 4527–4535. DOI:10.1021/am303300g |
| [11] | R.T. Zeng, W. Hu, M. Wang, S.D. Zhang, J.B. Zeng, Morphology, rheological and crystallization behavior in non-covalently functionalized carbon nanotube reinforced poly(butylene succinate) nanocomposites with low percolation threshold. Polym. Test. 50 (2016) 182–190. DOI:10.1016/j.polymertesting.2016.01.003 |
| [12] | M.H. Al-Saleh, U. Sundararaj, Processing-microstructure-property relationship in conductive polymer nanocomposites. Polymer. 51 (2010) 2740–2747. DOI:10.1016/j.polymer.2010.03.022 |
| [13] | S.J. Chin, S. Vempati, P. Dawson, Electrical conduction and rheological behaviour of composites of poly(ε-caprolactone) and MWCNTs. Polymer. 58 (2015) 209–221. DOI:10.1016/j.polymer.2014.12.034 |
| [14] | M. Avrami, Kinetics of phase change. I General theory. J. Chem. Phys. 7 (1939) 1103–1112. DOI:10.1063/1.1750380 |
| [15] | B. Fillon, B. Lotz, A. Thierry, J.C. Wittmann, Self-nucleation and enhanced nucleation of polymers. definition of a convenient calorimetric efficiency scale and evaluation of nucleating additives in isotactic polypropylene (a phase). J. Polym. Sci. Polym. Phys. 31 (1993) 1395–1405. DOI:10.1002/polb.1993.090311014 |
| [16] | B. Fillon, J.C. Wittmann, B. Lotz, A. Thierry, Self-nucleation and recrystallization of isotactic polypropylene (a phase) investigated by differential scanning calorimetry. J. Polym. Sci. Polym. Phys. 31 (1993) 1383–1393. |
| [17] | J.B. Zeng, F. Wu, C.L. Huang, Y.S. He, Y.Z. Wang, Urethane ionic groups induced rapid crystallization of biodegradable poly(ethylene succinate). ACS Macro Lett. 1 (2012) 965–968. DOI:10.1021/mz300243t |
 2017, Vol. 28
2017, Vol. 28 





