b University of the Chinese Academy of Sciences, Beijing 100049, China
Organic conjugated polymers have been widely used for organic solar cells and field effect transistors because of their outstanding electronic properties and solution processability [1-7]. Plenty of researches demonstrate that the device performance is closely related to molecular structure, molecular packing and film morphology [8-12]. Changing the side chain structure can contribute to adjust the interactions between molecules, increasing the solubility, adjusting the molecular arrangement and optimizing the film morphology. The influence mechanism is embodied in the following aspects.
Increasing the length of side chains can promote aggregation, weaken rod-rod interactions and decrease crystallization velocity [13-16]. Besides, longer side chains can also increase the cohesive energy and reduce the solubility, which can influence the processability and film morphology of polymer films [17]. When the length of side chains is relatively shorter, the molecular arrangement of polymer chains is significantly dependent on the odd-even characteristics. Molecules with side chains of evennumbered carbons exhibited greater packing density [18]. The cistrans isomerism of side chains can also affect the film morphology. trans-Alkenes exhibit zigzag structures that are beneficial for close packing, cis-alkenes are curved and thus possess a less regular shape that is disadvantageous to thin film ordering [19]. Fei et al. found that the regiochemistry of the solubilizing side chains on the backbone had a significant impact on the optoelectronic properties of the polymers and their propensity to aggregate in solution [20]. These differences were rationalized on the basis of differences in backbone torsion, which could influence intermolecular π-π interaction. Kim et al. found that the existence of bulky side chains linked to a tetrahedral carbon could prevent the polymer from massive aggregation and inhibit side-chain interdigitation, which endowed liquid-like mobility to polymers so that they could align along the shear direction [21].
In this work, we chose a series of isoindigo-based conjugated polymer (IIDDT, IIDDT-C3 and IIDDT-C4) with different length of side chains and bifurcation positions to investigate the relationship between the degree of alignment and the length of side chains and bifurcation positions. We found that the weak interaction of side-chain was beneficial for the molecules being rearranged in parallel during the contact line receding and the strong π-π stacking could accelerate the interchain assembly of the parallel molecules through π-π interaction to form parallel fibers.
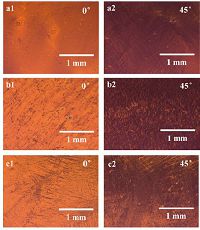
2. Results and discussionThe isoindigo-based conjugated polymer (Fig. 1) films were prepared by drop-casting at high temperature under solvent vapor conditions. We characterized the macro anisotropy of the IIDDT, IIDDT-C3 and IIDDT-C4 films by optic microscopy at 100× magnification under crossed polarizers (Fig. 2). The optic macro scope image of IIDDT film obtained at 0° with respect to crossed polarizer and analyzer showed similar brightness in contrast to the images obtained at 45°, which indicated the weak alignment of IIDDT film [22, 23]. As the side chains of isoindigo-based conjugated polymers became longer and the bifurcation position of side chains was away from the backbone, the contrast of brightness between images obtained at 0° and 45° became more obvious. The most evident contrast of brightness was attained in the IIDDT-C4 film which was with the longest side chains and the farthest bifurcation position. This suggested the highest degree of alignment in the IIDDT-C4 film.

|
Download:
|
| Figure 1. The molecular structure of IIDDT, IIDDT-C3 and IIDDT-C4. | |
|
Download:
|
| Figure 2. Optic microscope images under crossed polarizers of (a1, a2) IIDDT (b1, b2) IIDDT-C3 and (c1, c2) IIDDT-C4 films prepared by drop-casting at 70 ℃ under the condition of SVE = 0.3 mL. | |
To further study the alignment of IIDDT, IIDDT-C3 and IIDDTC4 films quantitatively, we characterized the dichroic ratio (Fig. 3). The dichroic ratio of IIDDT, IIDDT-C3 and IIDDT-C4 were 2.37, 3.42 and 5.23, respectively. This demonstrated that with the increasing of the side chain length and bifurcation away from the backbone, the alignment degree of isoindigo-based conjugated polymer films was improved, which was in accordance with the conclusion obtained in Fig. 2.

|
Download:
|
| Figure 3. UV-vis absorption spectra of (a) IIDDT (b) IIDDT-C3 (c) IIDDT-C4 films with radial direction parallel and perpendicular to the polarized direction. (d) The variation of dichroic ratio of IIDDT, IIDDT-C3, IIDDT-C4 films. The films were prepared by drop-casting at 70 ℃ under the condition of SVE = 0.3 mL. | |
Then we characterized the micro morphology of IIDDT, IIDDTC3 and IIDDT-C4 films through TEM. In Fig. 4, it was found that with the increasing of the side chain length and bifurcation away from the backbone, the fiber structure was formed in all the isoindigobased conjugated polymer films. While the IIDDT-C4 film exhibited the highest degree of alignment since the diffraction ring was the most sharply. To study the molecular arrangement in detail, we calculated the lattice parameter. It was found that the intermolecular π-π stacking distance of IIDDT, IIDDT-C3 and IIDDTC4 were 3.67 Å, 3.63 Å and 3.61 Å, respectively. The π-π stacking became closer as the bifurcation position was away from the backbone. This was because of the smaller steric hindrance between adjacent molecules when the bifurcation position was away from the backbone and the π-π interaction was improved. According to the Marcus theory, charge transfer mobility had a close relationship with reorganization energy and transfer integral. Through decreasing the π-π stacking direction, we could increase transfer integral, which was beneficial for the improvement of charge transfer mobility [24].

|
Download:
|
| Figure 4. The TEM images of (a) IIDDT, (b) IIDDT-C3, (c) IIDDT-C4 films prepared by drop-casting at 70 ℃ under the condition of SVE= 0.3 mL. The red arrow in electron diffraction patterns represents the π-π stacking directions. | |
It was found that with the increasing of the side chain length and bifurcation away from the backbone, the intensity of diffraction peak was improved, which indicated the enhancement of crystallization, as shown in Fig. 5. The peak position of IIDDT, IIDDT-C3 and IIDDT-C4 were 4.40°, 3.59° and 3.50°, respectively. It was calculated that the peaks in XRD profiles with a d-spacing of 20.06 Å, 24.58 Å and 25.21 Å for IIDDT, IIDDT-C3 and IIDDT-C4, respectively, was correspond to the alkyl-stacking (10 0). The three kinds of molecules adopted an edge-on orientation. Through the TEM and XRD images, it was found that the backbone of IIDDT, IIDDT-C3 and IIDDT-C4 molecules all arranged parallel with the growth direction of fibers.

|
Download:
|
| Figure 5. The XRD profile of IIDDT, IIDDT-C3 and IIDDT-C4 films prepared by dropcasting at 70 ℃ under the condition of SVE = 0.3 mL. | |
According to the viewpoint of Kim et al. [21], the alignment degree of polymers had a relationship with the molecular structure. The excellent coplanarity and mobility were beneficial for alignment. In our work, we found that with the increasing of the side chain length and bifurcation away from the backbone, the better alignment and crystallization was obtained in the isoindigo based conjugated polymer films.
We thought that when the side chain was longer, the distance between adjacent molecular backbones was farther in the direction of a-axis. The interaction between them was weak in the direction of a-axis. The mobility of molecules in solution was better. As the solvent was evaporated, a flow field would form along the contact line receding direction. The molecular backbone which had better mobility in solution could rearrange to parallel more quickly along the direction of the flow field. Besides, it was found from the TEM and XRD image that the backbone of isoindigo-based conjugated polymers were paralleled with the growth direction of fibers. At the same time, the molecules would have stronger π-π interaction and better planarity when the bifurcation was away from the backbone because of its smaller steric hindrance [25, 26]. As a result, during the solvent evaporating, it was more easily for the parallel molecules with strong π-π interaction to form aligned fibers.
Long-range alignment polymer films could not only reduce the number of grain boundaries efficiently, but also induce better coplanarity of tie molecules in amorphous region. The conformation of tie molecules and arrangement of crystals could be optimized simultaneously. As a result, more continuous pathways necessary for efficient charge transport could be obtained, which was beneficial to the improvement of charge carrier mobility.
3. ConclusionIn this work, we chose a series of isoindigo-based conjugated polymers (IIDDT, IIDDT-C3 and IIDDT-C4) with different length of side chains and bifurcation positions to investigate the relationship between alignment degree and the length of side chains and bifurcation positions. We found that the better alignment and crystallization was obtained in the isoindigo-based conjugated polymer films with the increasing of the side chain length and bifurcation away from the backbone. The weak interaction in long side-chain was beneficial for the molecules being rearranged in parallel during the contact line receding and the strong π-π interaction when the bifurcation was away from the backbone could accelerate the interchain assembly of the parallel molecules through π-π interaction to form aligned fibers.
4. Experimental 4.1. MaterialsThe copolymer of isoindigo and bithiophene with different length of side chains and bifurcation positions (IIDDT, IIDDTC3 and IIDDT-C4) were purchased from 1-Material Inc. Fig. 1 shows the molecular structures of IIDDT, IIDDT-C3 and IIDDT-C4. The molecular weight (Mn) and polydispersity index (PDI) were 28 kDa and 3.58, 26 kDa and 2.66, 32 kDa and 2.92 respectively for IIDDT, IIDDT-C3 and IIDDT-C4. Chlorobenzene (CB) was purchased from Sinopharm Chemical Reagent Co., Ltd. The glass slides as substrates were cleaned in piranha solution (70/30 v/v of concentrated H2SO4 and 30% H2O2) at 90 ℃ for 30 min and then treated with deionized water by ultrasound. Finally the glass slides were blown dry by nitrogen.
4.2. Sample preparationIIDDT, IIDDT-C3, IIDDT-C4 were all dissolved by CB at a concentration of 0.5 mg/mL. The solution was heated at 70 ℃ to be fully dissolved. All film samples were prepared by drop-casting the solutions at high temperature under the condition of solvent vapor enhancement (SVE) with 50 mL in each glass slide. We placed the glass slides into the weighing bottles with 0.3 mL CB. Then the weighing bottles were sealed at 70 ℃ until the solvent was dried.
4.3. CharacterizationThe macroscopic morphology of IIDDT-C3 films were characterized by Karl-Zeiss optic microscopy under crossed polarizers. Transmission electron microscopy (TEM) images and selected area electron diffraction patterns (SAED) were obtained with a JEOL JEM-1011 transmission electron microscope (Japan). The accelerating voltage was 100 kV. Dichroic ratio was characterized by an AvaSpect-3648 optical fiber spectrometer (Netherlands) equipped with a polarizer. The anisotropy was defined as the dichroic ratio between max absorption parallel and perpendicular to polarization direction. GIXRD pattern was obtained on Bruker D8 Discover Reflector (Cu Ka, λ= 1.54056 Å) under 40 kV and 40 mA tube current.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21334006, 51577138, 21474113), and the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB12020300).
| [1] | H. Sirringhaus, 25th anniversary article:organic field-effect transistors:the path beyond amorphous silicon. Adv. Mater. 26 (2014) 1319–1335. DOI:10.1002/adma.201304346 |
| [2] | Y. Olivier, D. Niedzialek, V. Lemaur, 25th anniversary article:highmobility hole and electron transport conjugated polymers:how structure defines function. Adv. Mater. 26 (2014) 2119–2136. DOI:10.1002/adma.v26.14 |
| [3] | Y. Li, M.G. Li, Y.J. Su, Liquid crystal character controlled by complementary discotic molecules mixtures:columnar stacking type and mesophase temperature range. Chin. Chem. Lett. 27 (2016) 475–480. DOI:10.1016/j.cclet.2015.12.024 |
| [4] | X. Gao, J.G. Liu, Y. Sun, R.B. Xing, Y.C. Han, Effects of aggregation of poly(3-hexylthiophene) in solution on uniaxial alignment of nanofibers during zone casting. Chin. Chem. Lett. 24 (2013) 23–27. DOI:10.1016/j.cclet.2013.01.014 |
| [5] | X. Gao, Y.C. Han, P3HT stripe structure with oriented nanofibrils enabled by controlled inclining evaporation. Chin. J. Polym. Sci. 31 (2013) 610–619. DOI:10.1007/s10118-013-1259-y |
| [6] | X. Gao, R.B. Xing, J.G. Liu, Y.C. Han, Uniaxial alignment of poly(3-hexylthiophene) nanofibers by zone-casting approach. Chin. J. Polym. Sci. 31 (2013) 748–759. DOI:10.1007/s10118-013-1284-x |
| [7] | Y. Sun, J.G. Liu, Y. Ding, Y.C. Han, Controlling the surface composition of PCBM in P3HT/PCBM blend films by using mixed solvents with different evaporation rates. Chin. J. Polym. Sci. 31 (2013) 1029–1037. DOI:10.1007/s10118-013-1295-7 |
| [8] | P.M. Beaujuge, J.M.J. Fréchet, Molecular design and ordering effects in π-functional materials for transistor and solar cell applications. J. Am. Chem. Soc. 133 (2011) 20009–20029. DOI:10.1021/ja2073643 |
| [9] | R.M. Pinto, E.M.S. Maçôas, A.I. Neves, Effect of molecular stacking on exciton diffusion in crystalline organic semiconductors. J. Am. Chem. Soc. 137 (2015) 7104–7110. DOI:10.1021/ja512886h |
| [10] | A.J. Pearson, T. Wang, A.D.F. Dunbar, Morphology development in amorphous polymer:fullerene photovoltaic blend films during solution casting. Adv. Funct. Mater. 24 (2014) 659–667. DOI:10.1002/adfm.201301922 |
| [11] | H.L. Dong, S.D. Jiang, L. Jiang, Nanowire crystals of a rigid rod conjugated polymer. J. Am. Chem. Soc. 131 (2009) 17315–17320. DOI:10.1021/ja907015p |
| [12] | H.L. Dong, H.X. Li, E.J. Wang, Ordering rigid rod conjugated polymer molecules for high performance photoswitchers. Langmuir. 24 (2008) 13241–13244. DOI:10.1021/la8026094 |
| [13] | C.K. Mai, R.A. Schlitz, G.M. Su, Side-chain effects on the conductivity, morphology, and thermoelectric properties of self-doped narrow-band-gap conjugated polyelectrolytes. J. Am. Chem. Soc. 136 (2014) 13478–13481. DOI:10.1021/ja504284r |
| [14] | A.T. Yiu, P.M. Beaujuge, O.P. Lee, Side-chain tunability of furan-containing low-band-gap polymers provides control of structural order in efficient solar cells. J. Am. Chem. Soc. 134 (2012) 2180–2185. DOI:10.1021/ja2089662 |
| [15] | M.J. Eslamibidgoli, J.B. Lagowski, The effect of side-chain length on the solidstate structure and optoelectronic properties of fluorene-alt-benzothiadiazole based conjugated polymers-a DFT study. J. Phys. Chem. A. 116 (2012) 10597–10606. DOI:10.1021/jp304974p |
| [16] | J.H. Xia, Y. Jiang, S.M. Gong, Z. Sun, Y.H. Wang, Effects of side chains with similar lengths and different structures of polyimides on liquid crystal alignment behavior. Chin. J. Polym. Sci. 32 (2014) 1610–1619. DOI:10.1007/s10118-014-1550-6 |
| [17] | S. Inoue, H. Minemawari, J.Y. Tsutsumi, Effects of substituted alkyl chain length on solution-processable layered organic semiconductor crystals. Chem. Mater. 27 (2015) 3809–3812. DOI:10.1021/acs.chemmater.5b00810 |
| [18] | J.Y. Back, H. Yu, I. Song, Investigation of structure-property relationships in diketopyrrolopyrrole-based polymer semiconductors via side-chain engineering. Chem. Mater. 27 (2015) 1732–1739. DOI:10.1021/cm504545e |
| [19] | F. Hinkel, T. Marszalek, W. Zajaczkowski, Tuning packing and solubility of donor (D)-acceptor (A) polymers by cis-trans isomerization within alkenyl side chains. Chem. Mater. 26 (2014) 4844–4848. DOI:10.1021/cm5021355 |
| [20] | Z.P. Fei, P. Pattanasattayavong, Y. Han, Influence of side-chain regiochemistry on the transistor performance of high-mobility, all-donor polymers. J. Am. Chem. Soc. 136 (2014) 15154–15157. DOI:10.1021/ja508798s |
| [21] | B.-G. Kim, E.J. Jeong, J.W. Chung, A moleculardesignprinciple of lyotropic liquid-crystalline conjugated polymers with directed alignment capability for plastic electronics. Nat. Mater. 13 (2013) 659–664. |
| [22] | H. Yao, J.T. Liu, L.Q. Zhang, S.K. Yan, Phase structureand crystallizationbehavior of polyethylene in its blends with cis-1,4-butadiene rubber. Chin. J. Polym. Sci. 33 (2015) 386–394. DOI:10.1007/s10118-015-1592-4 |
| [23] | L.G. Chai, H.X. Zhou, X.L. Sun, Structure of friction-transferred highly oriented poly(3-hexylthiophene) thin films. Chin. J. Polym. Sci. 34 (2016) 513–522. DOI:10.1007/s10118-016-1770-z |
| [24] | J.L. Bredas, J.P. Calbert, D.A. da Silva Filho, J. Cornil, Organic semiconductors:a theoretical characterization of the basic parameters governing charge transport. Proc. Natl. Acad. Sci. U. S. A. 99 (2002) 5804–5809. DOI:10.1073/pnas.092143399 |
| [25] | J.G. Mei, D.H. Kim, A.L. Ayzner, M.F. Toney, Z. Bao, Siloxane-terminated solubilizing side chains:bringing conjugated polymer backbones closer and boosting hole mobilities in thin-film transistors. J. Am. Chem. Soc. 133 (2011) 20130–20133. DOI:10.1021/ja209328m |
| [26] | T. Lei, J.H. Dou, J. Pei, Influence of alkyl chain branching positions on the hole mobilities of polymer thin-film transistors. Adv. Mater. 24 (2012) 6457–6461. DOI:10.1002/adma.v24.48 |
 2017, Vol. 28
2017, Vol. 28 


