b College of Chemistry & Chemical Engineering, Yangzhou University, Yangzhou 225002, China
As one of the most important classes of spirooxidole, spiro[indoline-3, 2'-pyrrolidine] skeleton can be found in many alkaloids such as formasanine, isorhynchophylline, spirotryprostatin A, strychnofoline, etc. [1, 2]. Many of its derivatives were described with different biological activities including antitumor, antimicrobial, antipyretics and antagonists [3-7]. As a consequence, there is a continual interest in organic and pharmaceutical chemistry to develop elegant synthetic methodologies for diverse spiro[indoline-3, 2'-pyrrolidine] derivatives [8-13]. A literature survey indicated that the most common synthetic method for the spiro[indoline-3, 2'-pyrrolidine] is 1, 3-dipolar cycloaddition reaction, which could allow rapidly access versatile spiro[indoline-3, 3'-pyrrolizine] scaffolds with most changeful regio-, diastereo-, and enantioselectivity [14-22]. In this respect, 1, 3-dipolar cycloaddition reaction of azomethine ylides generated in situ from the reactions of isatin with a-amino acid such as sarcosine, proline with alkene dienophiles, and 1, 3-dipolar cycloaddition reaction of various 1, 3-dipoles with 3-methyleneoxindoles are two main practical procedures for the construction of spiro[indoline-3, 3'-pyrrolizine] skeleton [23-32]. The electron-deficient alkynes have been used as effective dienophiles in 1, 3-dipolar cycloaddition for synthesis of versatile pyrrolidines or pyrrolizidines. However, there are very few reports about employing electron-deficient alkynes as dienophiles in 1, 3-dipolar cycloaddition reaction for construction of spiro[indoline-3, 2'-pyrrolidines] [33-35]. Recently, we found that three-component reaction of L-proline, isatin and dialkyl acetylenedicarboxylate not only gave the expected spiro[indoline-3, 3'-pyrrolizines] through 1, 3-dipolar cycloaddition, but also afforded unexpected spiro[indoline-3, 7'-pyrrolo[1, 2-a]azepines] via sequential annulation process [36]. In order to further illustrate the synthetic values of this practical protocol, herein we report the BF3OEt2 catalyzed domino 1, 3-dipolar cycloaddition reaction of benzylamines, isatins and dimethyl acetylenedicarboxylate for the diastereoselective synthesis of functionalized spiro[indoline-3, 2'-pyrroles].
2. ExperimentalGeneral procedure for the procedure for synthesis of dihydrospiro[indoline-3, 2'-pyrroles] (1a-1l): In a 50 mL of round flask, a mixture of benzylamine (1.0 mmol), substituted isatin (1.0 mmol) and dry methylene dichloride (20.0 mL) was stirred at room temperature. To this solution was added dropwise boron trifluoride etherate solution (0.5 mmol). Then, the mixture was refluxed about 12 h. TLC monitor indicated that the condensation reaction has been finished. Dimethyl acetylenedicarboxylate (2.6 mmol) was added and the solution was refluxed overnight. After removing the solvent by rotatory evaporation at reduced pressure, the residue was subjected to column chromatography with light petroleum and ethyl acetate (v/v=2:1) as elute to give the pure product for analysis.
Dimethyl 1-benzyl-1'-(1, 4-dimethoxy-1, 4-dioxobut-2-en-2-yl)-2-oxo-5'-phenyl-1', 5'-dihydrospiro[indoline-3, 2'-pyrrole]-3', 4'-dicarboxylate (1a): White solid, 65%, mp 20'-202 ℃; 1H NMR (400 MHz, CDCl3): δ 7.87 (s, 2H, ArH), 7.52 (m, 3H, ArH), 7.39-7.34 (m, 7H, ArH), 7.07 (s, 1H, ArH), 6.81 (s, 1H, ArH), 6.18 (s, 1H, CH), 5.03-5.02 (m, 2H, CH2), 4.36 (s, 1H, CH), 3.59 (s, 3H, OCH3), 3.40 (d, 6H, J=6.8 Hz, OCH3), 3.13 (s, 3H, OCH3); 13C NMR (100 MHz, CDCl3): δ 172.3, 166.5, 164.2, 162.3, 160.6, 131.1, 129.0, 128.8, 128.4, 128.1, 127.8, 124.8, 124.1, 123.7, 109.7, 92.4, 76.3, 72.0, 52.5 (2 C), 52.4, 50.9, 45.4; IR (KBr, cm-1): υ 3007, 2951, 1730, 1590, 1435, 1393, 1346, 1286, 1231, 1156, 1114, 1070, 973, 925, 841, 805, 756, 702; HRMS (ESI) Calcd. for C34H30N2NaO9 ([M + Na]+): 633.1844. Found: 633.1833.
Dimethyl 1-benzyl-1'-(1, 4-dimethoxy-1, 4-dioxobut-2-en-2-yl)-5-methyl-2-oxo-5'-phenyl-1', 5'-dihydrospiro[indoline-3, 2'-pyrrole]-3', 4'-dicarboxylate (1c): White solid, 75%, mp 18'-182 ℃; 1H NMR (400 MHz, CDCl3): δ 7.88 (d, 2H, J=7.2 Hz, ArH), 7.51 (d, 2H, J=7.8 Hz, ArH), 7.41 (d, 2H, J=7.2 Hz, ArH), 7.37-7.29 (m, 4H, ArH), 7.06 (d, 2H, J=10.8 Hz, ArH), 6.70 (d, 1H, J=7.8 Hz, ArH), 6.20 (s, 1H, CH), 5.02 (s, 2H, CH2), 4.35 (s, 1H, CH), 3.60 (s, 3H, OCH3), 3.43 (s, 3H, OCH3), 3.40 (s, 3H, OCH3), 3.15 (s, 3H, OCH3), 2.29 (s, 3H, CH3); 13C NMR (100 M Hz, CDCl3): δ 172.1, 166.6, 164.4, 162.3, 160.6, 146.9, 142.1, 140.7, 136.1, 135.5, 133.5, 132.3, 131.4, 130.0, 128.8, 128.4, 128.1, 127.8, 125.4, 123.9, 109.5, 92.1, 76.4, 71.9, 52.5 (2 C), 52.4, 50.8, 45.3, 21.0; IR (KBr, cm-1): υ 3470, 3377, 3292, 2946, 1739, 1691, 1579, 1497, 1438, 1391, 1332, 1286, 1235, 1166, 1111, 1029, 968, 884, 813, 740, 701;HRMS (ESI) Calcd. for C35H32N2NaO9 ([M + Na]+): 647.2000. Found: 647.1993.
Dimethyl 1-butyl-5-chloro-1'-(1, 4-dimethoxy-1, 4-dioxobut-2-en-2-yl)-2-oxo-5'-phenyl-1', 5'-dihydrospiro[indoline-3, 2'-pyrrole]-3', 4'-dicarboxylate (1f): White solid, 68%, mp 176-178 ℃; 1H NMR (400 MHz, CDCl3): δ 7.79 (d, 2H, J=7.2 Hz, ArH), 7.39-7.30 (m, 4H, ArH), 7.23 (s, 1H, ArH), 6.84 (d, 1H, J=8.0 Hz, ArH), 6.15 (s, 1H, CH), 4.28 (s, 1H, CH), 3.80 (t, 2H, J=7.2 Hz, CH2), 3.58 (s, 3H, OCH3), 3.53 (s, 3H, OCH3), 3.41 (s, 3H, OCH3), 3.16 (s, 3H, OCH3), 1.78-1.72 (m, 2H, CH2), 1.51-1.45 (m, 2H, CH2), 0.99 (t, 3H, J=7.2Hz, CH3); 13C NMR (100 MHz, CDCl3): δ 171.4, 166.4, 164.1, 162.2, 160.3, 146.6, 143.1, 142.1, 135.7, 131.0, 129.1, 128.9, 128.8, 128.4, 125.9, 125.1, 109.8, 92.4, 75.9, 72.1, 52.6 (2 C), 52.4, 50.9, 41.1, 29.2, 20.5, 13.8; IR (KBr, cm-1): υ 2974, 1738, 1695, 1582, 1484, 1435, 1389, 1337, 1289, 1225, 1162, 1110, 1022, 969, 882, 816, 746, 701; HRMS (ESI) Calcd. for C31H31ClN2NaO9 ([M + Na]+): 633.1610. Found: 633.1604.
Dimethyl 1-benzyl-5'-(2-chlorophenyl)-1'-(1, 4-dimethoxy-1, 4-dioxobut-2-en-2-yl)-2-oxo-1', 5'-dihydrospiro[indoline-3, 2'-pyrrole]-3', 4'-dicarboxylate (1 h): White solid, 62%, mp 20'-202 ℃; 1H NMR (400 MHz, CDCl3): δ 8.69 (d, 1H, J=7.6 Hz, ArH), 7.51 (d, 1H, J=7.6 Hz, ArH), 7.44 (t, 1H, J=7.6 Hz, ArH), 7.35 (t, 3H, J=7.2 Hz, ArH), 7.31-7.26 (m, 4H, ArH), 7.07 (t, 1H, J=7.6 Hz, ArH), 6.80 (d, 1H, J=7.6 Hz, ArH), 6.71 (s, 1H, CH), 5.10 (d, 1H, J=16.0 Hz, CH), 4.92 (d, 1H, J=16.0 Hz, CH), 4.46 (s, 1H, CH), 3.67 (s, 3H, OCH3), 3.42 (s, 3H, OCH3), 3.38 (s, 3H, OCH3), 3.21 (s, 3H, OCH3); 13C NMR (100 MHz, CDCl3): δ 172.8, 166.4, 163.8, 162.3, 160.3, 146.7, 143.4, 142.7, 135.3, 133.4, 133.0, 131.8, 131.1, 130.6, 130.2, 129.0, 128.8, 128.5, 128.1, 127.8, 124.9, 124.6, 123.7, 109.6, 93.3, 75.8, 67.3, 58.4, 52.7 (2 C), 52.5, 50.9, 45.4, 18.4; IR (KBr, cm-1): υ 3060, 3017, 2950, 2844, 1733, 1662, 1593, 1479, 1436, 1395, 1343, 1289, 1228, 1158, 1114, 1044, 970, 925, 806, 7=; HRMS (ESI) Calcd. for C34H29ClN2NaO9 ([M + Na]+): 667.1454. Found: 667.1452.
Dimethyl 1-benzyl-5-chloro-5'-(2-chlorophenyl)-1'-(1, 4-dimethoxy-1, 4-dioxobut-2-en-2-yl)-2-oxo-1', 5'-dihydrospiro[indoline-3, 2'-pyrrole]-3', 4'-dicarboxylate (1k): White solid, =%, mp 21'-212 ℃; 1HNMR (400MHz, CDCl3): δ 8.64 (d, 1H, J=7.6 Hz, ArH), 7.49-7.42 (m, 3H, ArH), 7.37-7.27(m, 5H, ArH), 7.04 (d, 1H, J=4.2Hz, ArH), 6.98-6.94 (m, 1H, ArH), 6.72-6.69 (m, 2H, CH), 5.05 (d, 1H, J=16.0 Hz, CH), 4.93 (d, 1H, J=16.0 Hz, CH), 4.45 (s, 1H, CH), 3.68 (s, 3H, OCH3), 3.44 (d, 6H, J=2.4Hz, OCH3), 3.36 (s, 3H, OCH3); 13C NMR (100 MHz, CDCl3): δ 172.6, 166.3, 163.7, 162.2, 160.8, 160.1, 158.4, 146.5, 143.4, 139.5 (d, J=2.1 Hz), 135.0, 133.4, 133.3, 132.9, 130.9, 130.5, 130.3, 129.1, 128.9 (d, J=6.3 Hz), 128.5, 128.0, 127.9 (d, J=5.0 Hz), 126.2, 117.6, 112.7, 110.4 (d, J=7.6 Hz), 93.7, 75.8, 67.4, 52.8(2 C), 52.6, 51.0, 45.6, 18.4; IR (KBr, cm-1): y2954, 1735, 1589, 1491, 1442, 1392, 1336, 1286, 1229, 1164, 1113, 1040, 970, 810, 766; HRMS (ESI) Calcd. for C34H28ClFN2NaO9 ([M + Na]+): 685.1360. Found: 685.1351.
Dimethyl 1-butyl-5-chloro-5'-(2-chlorophenyl)-1'-(1, 4-dimethoxy-1, 4-dioxobut-2-en-2-yl)-2-oxo-1', 5'-dihydrospiro[indoline-3, 2'-pyrrole]-3', 4'-dicarboxylate (1l): White solid, 66%, mp 22'-222 ℃; 1H NMR (400 MHz, CDCl3): δ 8.6'-8.58 (m, 1H, ArH), 7.44-7.40 (m, 1H, ArH), 7.36-7.33 (m, 2H, ArH), 7.29-7.26 (m, 2H, ArH), 6.84 (d, 1H, J=16.0 Hz, ArH), 6.72 (s, 1H, CH), 4.4 (s, 1H, CH), 3.86-3.73 (m, 2H, CH2), 3.68 (s, 3H, OCH3), 3.54 (s, 3H, OCH3), 3.44 (s, 3H, OCH3), 3.28 (s, 3H, OCH3), 1.79-1.72 (m, 2H, CH2), 1.52-1.46 (m, 2H, CH2), 1.00 (t, 3H, J=7.6 Hz, CH3); 13C NMR (100 MHz, CDCl3): δ 169.3, 163.7, 161.1, 159.6, 157.4, 143.9, 140.9, 139.7, 130.7, 130.3, 128.5, 128.1, 128.0, 127.7, 126.5, 126.3, 125.9, 123.8, 122.5, 107.1, 90.7, 72.9, 64.9, 50.2, 50.1(2 C), 48.4, 38.5, 26.6, 18.0, 11.2; IR (KBr, cm-1): υ 3471, 3016, 2954, 2870, 1730, 1661, 1591, 1484, 1438, 1395, 1343, 1298, 1226, 1159, 1113, 1045, 968, 915, 809, 760; HRMS (ESI) Calcd. for C31H30Cl2N2NaO9 ([M + Na]+): 667.1221. Found: 667.1212.
3. Results and discussionInitially, an equivalent amount of benzylamine, N-benzylisatin and dimethyl acetylenedicarboxylate in ethanol was refluxed according to our previous established reaction condition for the three-component reaction of L-proline, isatin and dialkyl acetylenedicarboxylate [30]. TLC monitor indicated that a complicate mixture was formed, which was not worthy for separation. Then, the three-component reaction was carried out in aprotic solvent such as toluene, chloroform and methylene dichloride. The results were still very disappointed and no main product was obtained from these reactions. Thus, our attention turned to one-pot reaction procedure and many reaction conditions were carefully examined. At last, we were pleased to find that a domino reaction procedure could reveal satisfactory results for us. That is, a mixture of benzylamine and isatin were refluxed in dry methylene dichloride in the presence of boron trifluoride etherate for about 12 h to give the desired 3-benzyliminooxindole. Then, dimethyl acetylenedicarboxylate was added and the mixture was refluxed for another 12 h. After workup, the polysubstituted spiro[indoline-3, 2'-pyrroles] 1a was successfully obtained in 65% yield. The spectroscopic data also indicated that a scaffold of dimethyl maleate connected to the nitrogen atom of spiropyrrole ring, which is obviously coming from the addition of the initially formed spiro[indoline-3, 2'-pyrrole] to the remaining dimethyl acetylenedicarboxylate. Under this one-pot domino reaction conditions, the reactions containing various substituted isatins and o-chlorobenzylamine afforded the expected spiro[indoline-3, 2'-pyrroles] 1b-1l in moderate to good yields. The results are summarized in Table 1.
|
|
Table 1 Synthesis of dihydrospiro[indoline-3, 2'-pyrroles] 1a-1l.a |
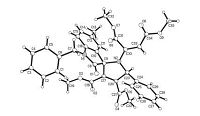
The structures of the functionalized spiro[indoline-3, 2'-pyrroles] 1a-1l were fully characterized with IR, HRMS, 1H NMR and 13C NMR spectroscopy (1H NMR and 13C NMR spectra are available in Supporting information). 1H NMR spectra clearly reveals one set of characteristic absorptions for the functional groups in the molecule, which strongly indicated only one kind of diastereoisomer exist in the obtained samples of the products. As for example, the spiro compound 1c displays one singlet at 6.20 ppm for one proton at C=C double bond, a singlet at 5.02 ppm for methylene unit of benzyl group and a singlet at 4.35 ppm for one proton at spiropyrrole ring. This result also indicated that this cycloaddition reaction has very high diastereoselectivty. In order to determine the relative configuration of spiro[indoline-3, 2'-pyrroles], the single crystal structure of one representative product 1c (CCDC 1478667) was determined by X-ray diffraction method (Fig. 1). It can be seen that the 5'-phenyl group and the phenyl group of oxindole moiety exist at the trans-configuration in the newly formed dihydropyrrole ring. Additionally, the two methoxycarbonyl groups exist at the cis-configuration on the N-connected alkene chain. On the basis of NMR spectra and single crystal structure, we concluded that the obtained spiro[indoline-3, 2'-pyrroles] all have this kind of relative configuration.
|
Download:
|
| Figure 1. Single crystal structure of spiro compound 1c. | |
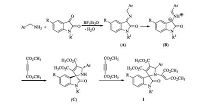
For explaining the reaction mechanism, a plausible domino reaction mechanism is concisely outlined in the Scheme 1 based on the previous reported 1, 3-dipolar cycloaddition reaction. Under the catalysis of Lewis acid BF3OEt2, benzylamine condensed with isatin to give N-benzyliminoisatin (A), which transferred to the active 1, 3-dipole intermediate (B). Then, the concerted 1, 3-dipolar cycloaddition of 1, 3-dipole (B) with dimethyl acetylenedicarboxylate to give the dihydrospiro[indoline-3, 2'-pyrrole] (C). Finally, the nucleophilic addition of amino group of intermediate (C) to another molecular dimethyl acetylenedicarboxylate to give the obtained functionalized dihydrospiro[indoline-3, 2'-pyrrole] 1. In this reaction mechanism, the trans-configuration of the product is mainly controlled by the concerted 1, 3-dipolar cycloaddition step and the steric effect of the substituents.
|
Download:
|
| Scheme 1. The proposed formation mechanism for dihydrospiro[indoline-3, 20-pyrrole]. | |
4. Conclusion
In summary, we have successfully provided an efficient protocol for the diastereoselective synthesis of functionalized spiro[indoline-3, 2'-pyrroles] from the Lewis acid catalyzed onepot reaction of benzylamine, isatin and dimethyl acetylenedicarboxylate. The reaction has the advantages of the using readily variable substrates, milder reaction condition, easiness of handling, high yields and with high diastereoselectivity. This reaction not only broadens the synthetic potentials of 1, 3-dipolar cycloaddition of azomethine ylide with electron-deficient alkynes, but also offers a rich repertoire of one-pot reactions for the efficient construction of nitrogen-containing spiro heterocycles. This reaction might be found potential applications for the synthesis of the complex spiro heterocycles in synthetic and medicinal chemistry.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21172189, 21572196) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. We also thank the Analysis and Test Center of Yangzhou University providing instruments for analysis.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.07.016.
| [1] | N.R. Ball-Jones, J.J. Badillo, A.K. Franz, Strategies for the enantioselective synthesis of spirooxindoles. Org. Biomol. Chem. 10 (2012) 5165–5181. DOI:10.1039/c2ob25184a |
| [2] | G.S. Singh, Z.Y. Desta, Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev. 112 (2012) 6104–6155. DOI:10.1021/cr300135y |
| [3] | R. Kumar, S. Perumal, P. Senthilkumar, P. Yogeeswari, D. Sriram, A facile synthesis and antimycobacterial evaluation of novel spiro-pyrido-pyrrolizines and pyrrolidines. Eur. J. Med. Chem. 44 (2009) 3821–3829. DOI:10.1016/j.ejmech.2009.05.010 |
| [4] | S.M. Rajesh, S. Perumal, J.C. Menendez, P. Yogeeswari, D. Sriram, Antimycobacterial activity of spirooxindole-pyrrolidine, pyrrolizine and pyrrolothiazole hybrids obtained by a three-component regio-and stereoselective 1, 3-dipolar cycloaddition. Med. Chem. Comm. 2 (2011) 626–630. DOI:10.1039/c0md00239a |
| [5] | A. Kaur, B. Singh, B. Vyas, O. Silakari, Synthesis and biological activity of 4-aryl-3-benzoyl-5-phenylspiro[pyrrolidine-2.3'-indolin]-2'-one derivatives as novel potent inhibitors of advanced glycation end product. Eur. J. Med. Chem. 79 (2014) 282–289. DOI:10.1016/j.ejmech.2014.04.022 |
| [6] | I.D. Premachandra, K.V. Scott, C.T. Shen, Potent synergy between spirocyclic pyrrolidinoindolinones and fluconazole against Candida albicans. Chem. Med. Chem. 10 (2015) 1672–1686. DOI:10.1002/cmdc.201500271 |
| [7] | N.Y. Chen, L.P. Ren, M.M. Zou, Design, synthesis and insecticidal activity of spiro heterocycle containing neonicotinoid analogs. Chin. Chem. Lett. 25 (2014) 197–200. DOI:10.1016/j.cclet.2013.12.004 |
| [8] | M. Santos, Recent advances in the synthesis of biologically active spirooxindoles. Tetrahedron 70 (2014) 9735–9757. DOI:10.1016/j.tet.2014.08.005 |
| [9] | C. Wang, Y.H. Jiang, C.G. Yan, [indoline-3, 4'-pyrano[2, 3-c]pyrazole] and spiro[acenaphthyl-3, 4'-pyrano[2, 3-c]pyrazoles] via fourcomponent reaction. Chin. Chem. Lett. 26 (2015) 889–893. DOI:10.1016/j.cclet.2015.05.018 |
| [10] | D. Zhang, D.M. Zhang, G.Y. Xu, J.T. Sun, Copper-catalyzed 1, 3-dipolar cycloaddition of methyleneindolinones and N, N'-cyclic azomethine imines. Chin. Chem. Lett. 26 (2015) 301–303. DOI:10.1016/j.cclet.2014.11.015 |
| [11] | S.A. Padvi, Y.A. Tayade, Y.B. Wagh, D.S. Dalal, [bmim]OH:An efficient catalyst for the synthesis of mono and bis spirooxindole derivatives in ethanol at room temperature. Chin. Chem. Lett. 27 (2016) 714–720. DOI:10.1016/j.cclet.2016.01.016 |
| [12] | Y.B. Wagh, Y.A. Tayade, S.A. Padvi, B.S. Patil, N.B. Patil, D.S. Dalal, A cesium fluoride promoted efficient and rapid multicomponent synthesis of functionalized 2-amino-3-cyano-4H-pyran and spirooxindole derivative. Chin. Chem. Lett. 26 (2015) 1273–1277. DOI:10.1016/j.cclet.2015.06.014 |
| [13] | R.G. Shi, C.G. Yan, Three-component reaction for synthesis of functionalized spiro[indoline-3, 4'-pyrano[3, 2-h]quinolines]. Chin. Chem. Lett. 27 (2016) 575–578. DOI:10.1016/j.cclet.2016.02.016 |
| [14] | D. Fokas, W.J. Ryan, D.S. Casebier, D.I. Coffen, Solution-phase synthesis of a spiro[pyrrolidine-2, 3'-oxindole] library via a three component 1, 3-dipolar cycloaddition reaction. Tetrahedron Lett. 39 (1998) 2235–2238. DOI:10.1016/S0040-4039(98)00234-2 |
| [15] | S. Rehn, J. Bergman, B. Stensland, The three-component reaction between isatin, α-amino acids, and dipolarophiles, Eur. J. Org. Chem. (2004) 413-418. |
| [16] | R.R. Kumar, S. Perumal, Sacrificial azomethine ylide cycloaddition controlled chemoselective nitrile oxide cycloaddition to 1-methyl-3, 5-bis[(E)-arylmethylidene]tetrahydro-4(1H)-pyridinones:formation of mono-spiro-isoxazolines. Tetrahedron 63 (2007) 12220–12231. DOI:10.1016/j.tet.2007.09.033 |
| [17] | J. Liu, H.B. Sun, X.J. Liu, Direct construction of novel exo'-selective spiropyrrolidine bisoxindoles via a three-component 1, 3-dipolar cycloaddition reaction. Tetrahedron Lett. 53 (2012) 2336–2340. DOI:10.1016/j.tetlet.2012.02.099 |
| [18] | L. Tian, X.Q. Hu, Y.H. Li, P.F. Xu, Organocatalytic asymmetric multicomponent cascade reaction via 1, 3-proton shift and[3+2] cycloaddition:an efficient strategy for the synthesis of oxindole derivatives. Chem. Commun. 49 (2013) 7213–7215. DOI:10.1039/c3cc43755h |
| [19] | J.A. Xiao, H.G. Zhang, S. Liang, synthesis of pyrrolo (spiro-[2.3']-oxindole)-spiro-[4.3"]-oxindole via 1, 3-dipolar cycloaddition of azomethine ylides with 3-acetonylideneoxindole. J. Org. Chem. 78 (2013) 11577–11583. DOI:10.1021/jo4017259 |
| [20] | C.Q. Peng, J.W. Ren, J.A. Xiao, Additive-assisted regioselective 1, 3-dipolar cycloaddition of azomethine ylides with benzylideneacetone. Beilstein J. Org. Chem. 10 (2014) 352–360. DOI:10.3762/bjoc.10.33 |
| [21] | M. Sankaran, C. Uvarani, K. Chandraprakash, A regioselective multicomponent protocol for the synthesis of novel bioactive 4-hydroxyquinolin-2(1H)-one grafted monospiropyrrolidine and thiapyrrolizidine hybrids. Mole. Divers. 18 (2014) 269–283. DOI:10.1007/s11030-013-9498-y |
| [22] | H.B. Sun, X.Y. Wang, C. Yi, Efficient construction of highly functionalized endo-selective spiro[pyrrolidin-2, 3'-oxindoles] via a regioselective 1, 3-dipolar cycloaddition reaction between 3-amino oxindoles as azomethine ylide precursors and nitroalkenes. Tetrahedron Lett. 55 (2014) 5434–5438. DOI:10.1016/j.tetlet.2014.08.030 |
| [23] | J.A. Xiao, Q. Liu, J.W. Ren, et al., Highly enantioselective construction of polycyclic spiro-oxindoles by organocatalytic 1, 3-dipolar cycloaddition of 2-cyclohexenone catalyzed by proline-sulfonamide, Eur. J. Org. Chem. (2014) 5700-5704. |
| [24] | X.P. Yin, X.P. Zeng, Y.L. Liu, Asymmetric Triple Relay Catalysis:Enantioselective Synthesis of Spirocyclic Indolines through a One-Pot Process Featuring an Asymmetric 6π Electrocyclization. Angew. Chem. Int. Ed. 53 (2014) 13740–13745. DOI:10.1002/anie.201407677 |
| [25] | S. Haddad, S. Boudriga, T.N. Akhaja, Strategic approach to the synthesis of functionalized spirooxindole pyrrolidine derivatives:in vitro antibacterial, antifungal, antimalarial and antitubercular studies. New J. Chem. 39 (2015) 520–528. DOI:10.1039/C4NJ01008F |
| [26] | W. Dai, X.L. Jiang, Q. Wu, F. Shi, S.J. Tu, Diastereo-and Enantioselective Construction of 3, 3'-Pyrrolidinyldispirooxindole Framework via Catalytic Asymmetric 1, 3-Dipolar Cycloadditions. J. Org. Chem. 80 (2015) 5737–5744. DOI:10.1021/acs.joc.5b00708 |
| [27] | M.X. Ma, Y.Y. Zhu, Q.T. Sun, The asymmetric synthesis of CF3-containing spiro[pyrrolidin-3, 2'-oxindole] through the organocatalytic 1, 3-dipolar cycloaddition reaction. Chem. Commun. 51 (2015) 8789–8792. DOI:10.1039/C4CC10216A |
| [28] | X. Zhu, S. Chiba, Construction of 1-pyrroline skeletons by Lewis acid-mediated conjugate addition of vinyl azides. Chem. Commun. 52 (2016) 2473–2476. DOI:10.1039/C5CC10299E |
| [29] | G.D. Zhu, B.M. Wang, X.Z. Bao, Catalytic asymmetric construction of spiro[pyrrolidine-2, 3'-oxindole] scaffolds through chiral phosphoric acid-catalyzed 1, 3-dipolar cycloaddition involving 3-amino oxindoles. Chem. Commun. 51 (2015) 15510–15513. DOI:10.1039/C5CC05798A |
| [30] | H. Dong, S.C. Song, J.J. Li, The discovery of oxazolones-grafted spirooxindoles via three-component diversity oriented synthesis and their preliminary biological evaluation. Bioorg. Med. Chem. Lett. 25 (2015) 3585–3591. DOI:10.1016/j.bmcl.2015.06.076 |
| [31] | Q.T. Sun, X.Y. Li, J.H. Su, The Squaramide-Catalyzed 1, 3-Dipolar Cycloaddition of Nitroalkenes with N-2, 2, 2-Trifluoroethylisatin Ketimines:An Approach for the Synthesis of 5'-Trifluoromethyl-spiro[pyrrolidin-3, 2'-oxindoles]. Adv. Synth. Catal. 357 (2015) 3187–3196. DOI:10.1002/adsc.201500416 |
| [32] | R.S. Kumar, A.I. Almansour, N. Arumugam, ionic liquid-promoted synthesis and cholinesterase inhibitory activity of highly functionalized spiropyrrolidines. Australian J. Chem. 68 (2015) 863–871. DOI:10.1071/CH14370 |
| [33] | W. Tan, X.T. Zhu, S. Zhang, Diversity-oriented synthesis of spiro-oxindolebased 2, 5-dihydropyrroles via three-component cycloadditions and evaluation on their cytotoxicity. RSC Adv. 3 (2013) 10875–10886. DOI:10.1039/c3ra40874d |
| [34] | S.N. Singh, S. Regati, A.K. Paul, Cu-mediated 1, 3-dipolar cycloaddition of azomethine ylides with dipolarophiles:a faster access to spirooxindoles of potential pharmacological interest. Tetrahedron Lett. 54 (2013) 5448–5452. DOI:10.1016/j.tetlet.2013.07.126 |
| [35] | G.H. Shi, X.W. He, Y.J. Shang, M.H. Xie, Combinatorial synthesis of spiro[indoline-3, 2'-pyrrole] derivatives via a three-component reaction under catalyst-free conditions. RSC Adv. 6 (2016) 10412–10418. DOI:10.1039/C5RA23860A |
| [36] | F. Yang, J. Sun, H. Gao, C.G. Yan, Unprecedented formation of spiro[1, 2-a]azepine] from multicomponent reaction of L-proline, isatin and but-2-ynedioate. RSC Adv. 5 (2015) 32786–32794. DOI:10.1039/C5RA04102C |
 2017, Vol. 28
2017, Vol. 28