b College of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou 450001, China;
c School of Information Engineering, Zhengzhou University, Zhengzhou 450001, China
Mobile phones, laptop computers and digital cameras have become indispensible portable electronic products. In addition to these devices, hybrid electric vehicles (HEVs) and electric vehicles (EVs) are green technologies that depend on rechargeable energy storage devices. Lithium ion batteries (LIBs) are one of the most sophisticated energy storage devices, which exhibit obvious advantages over other secondary batteries, including lead-acid batteries and nickel-metal hydride batteries, with regards to energy density, cycling performance and environmental friendliness.
In order to more efficiently power the above electrochemical devices, especially EVs and HEVs, LIBs have to be designed with further improved performance, especially in their energy density and rate capability [1-3]. The performance of rechargeable LIBs is related to the intrinsic properties of the electrode materials which store and release electric energy by insertion and extraction of Liions and electrons. The commonly used graphite anode materials limit the performance of lithium-ion batteries due to their low storage capacity and poor rate performance [4]. To solve this problem, great efforts have been made to develop alternative anode materials with elevated capacity and rate performance [1-6].
SnS2 has been studied as an alternative candidate for commercial graphite anode materials because of its higher capacity (645 vs. 372 mAh/g) [7-9]. SnS2 has a layered CdI2 crystalline-like structure composed of tin atoms sandwiched between two layers of hexagonal sulfur atoms, and the neighboring sulfur layers are connected with weak van der Waals forces. Thus, the Li ion can access the Sn atoms in the SnS2 structure, and the resulting volume change can be alleviated to a certain degree. Therefore, compared to other Li-alloying anodes, SnS2 has better cycling performance, but it is still far from practical application. In addition, because of the poor intrinsic electronic and ionic conductivity of SnS2, modification is necessary to improve its rate performance. The poor electronic conductivity can be solved by combination with other conductive materials [5]. Graphene is particularly attractive because of its unique physicochemical properties including flexibility, strength, and high conductivity [10-12]. Therefore it is suggested as a promising matrix to be composited with other high-capacity electrode materials. Compared with electronic conductivity, ionic conductivity is of equal importance to rate performance. According to the theoretical diffusion time of ions in electrode materials (t=L2/D, L is the diffusion length while D is the diffusion coefficient) [8], decreasing the particle size of the electrode materials should be an effective way to improve ionic conductivity, because for certain electrode materials, the diffusion coefficient is a constant. Thus, the design of such a nano-architecture, in which small SnS2 particles are uniformly anchored on conductive graphene nanosheets would be an ideal anode material for LIBs with excellent cycling and rate performance.
According to the above analysis, in this work, we developed a facile solvothermal method to synthesize SnS2 nanoparticles/ graphene (SnS2 NP/GNs) nanocomposite, in which SnS2 nanoparticles (SnS2 NP) are well distributed on graphene nanosheets. The as-designed nano-architecture exhibits improved Li storage performance, especially in rate performance.
2. Experimental 2.1. Preparation of materialsGraphene oxide (GO) was prepared according to the previous literature [13]. In a typical procedure of SnS2 NP/GN synthesis, 0.2 g of the as-prepared GO was added into 80 mL ethylene glycol (EG) and subjected to ultrasonic treatment for about 2 h until completely dispersed. Next, 2 mmol (~231 μL) SnCl4 solution was injected into the above prepared GO solution with constant stirring. Then, 4.2 mmol sulfourea were added to the mixed solution. After another 20 min of ultrasonic treatment, the obtained solution was immediately transferred to a 100 mL Teflon autoclave. The solvothermal process was carried out at 160 ℃ for 20 h with a heating rate of~2 ℃/min. After cooling to r.t. naturally, the black product was collected by centrifugation, washed thoroughly with water and alcohol several times, and then freeze dried at -80 ℃ for 30 h.
2.2. CharacterizationsThe morphologies of samples were obtained using a scanning electron microscope (SEM, Hitachi S-7500, 5.0 kV). TEM and high resolution TEM (HRTEM) investigation accompanied by SAED (selected area electron diffraction) was carried out by a JEOL JEM-2100F microscope at an acceleration voltage of 20 kV. The specific surface area of the SnS2 NP/GNs nanocomposite was measured at 77 K by the Brunauer-Emmett-Teller (BET) nitrogen adsorption-desorption (NOVA 2200e, Quanthachrome, USA.) method; pore volume was calculated by the Barrett-Joyner-Halenda (BJH) method. Powder X-ray diffraction (XRD) patterns of the samples were carried out on a Bruker D8 X-ray diffraction meter with CuKa radiation (1.5416 Å). TGA was performed on a Pyris Diamond TG/DTA (PerkinElemer Inc., USA) with a heating rate of 5 ℃/min from 50 to 880 ℃ under oxygen atmosphere. Raman spectra of GO before and after reduction were obtained from a RM-1000 Renishaw confocal Raman micro-spectroscope in the range of 500-2000 cm-1.
2.3. Electrochemical performanceThe electrochemical performance of samples as anode materials for Li ion batteries were investigated with Li foil as counter and reference electrode. The mixture of 80 wt% SnS2 NP/GNs active material, 10 wt% carbon black as conductive agent, and 10 wt% polyvinylidene fluoride (PVDF) was prepared as the working electrode; Cu foil was used as substrate. The disk electrodes were first dried overnight at 60 ℃ under vacuum and then compressed at 1.0 × 106 Pa. In an Ar-filled glove box, the as-prepared electrodes were assembled into 2016 type coin cells, using polypropylene (PP) micro-porous film as the separator, a solution of 1 mol/L LiPF6 in ethylene carbonate (EC)/dimethyl carbonate (DMC) (1:1, v/v) as the electrolyte, and metallic lithium foil as the counter electrode. The electrochemical tests were performed on Land BT2013A battery testing systems (Wuhan Rambts Co. Ltd., China). The charging and discharging tests were performed galvanostatically at various currents and at a constant temperature of 25 ℃ in the voltage ranging from 0 to 1.5 V. Cyclic voltammetric (CV) was performed on an IM6 electrochemical workstation (Zahner elektrik) at an scan rate of 0.1 mV/s. The electrochemical impedance measurements were also performed at an AC voltage of 5 mV amplitude in the 100 kHz to 0.01 Hz at 10 cycles (0 V).
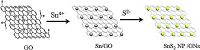
3. Results and discussionThe proposed formation process of the SnS2 NP/GNs nanocomposite is schematically illustrated in Fig. 1. In the ultrasonic process, SnCl4 could be easily dissolved in the EG solution. Sn4+ would be selectively bonded with the oxygenated groups by electrostatic forces, because GO nanosheets contain epoxyl and hydroxyl groups on the basal planes and carboxylic acid groups on the edges [14]. In the solvothermal process, sulfourea could also be dissolved in EG, and then S2- was released. Under the high temperature and pressure condition, S2- reacts with Sn4+, which was bonded with GO, forming numerous SnS2 nuclei (or so-called nanoparticles), thus producing the close combination of SnS2 nuclei and GO nanosheets. Simultaneously, GO was reduced to graphene during the solvothermal process, because EG also plays a role as a reducing agent. Thus, SnS2 NP/GNs nanocomposite was prepared. The proposed chemical reactions of the formation of SnS2 was suggested as follows:
| ${{\text{(C}{{\text{H}}_{\text{2}}}\text{OH)}}_{\text{2}}}\text{ }\!\!~\!\!\text{ + S}{{\text{n}}^{\text{4+}}}\text{ }\!\!~\!\!\text{ }\leftrightarrow \text{Sn(C}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{4}}}\text{ }\!\!~\!\!\text{ + 4}{{\text{H}}^{\text{+}}}$ | (1) |
| $\text{N}{{\text{H}}_{\text{2}}}\text{CSN}{{\text{H}}_{\text{2}}}\text{ }\!\!~\!\!\text{ + 2(C}{{\text{H}}_{\text{2}}}\text{OH}{{\text{)}}_{\text{2}}}\text{ }\!\!~\!\!\text{ + 2}{{\text{H}}^{\text{+}}}\text{ }\!\!~\!\!\text{ }\leftrightarrow \text{2N}{{\text{H}}_{\text{3}}}\text{ }\!\!~\!\!\text{ + 2C}{{\text{H}}_{\text{2}}}\text{OC}{{\text{H}}_{\text{2}}}\text{ }\!\!~\!\!\text{ + C}{{\text{O}}_{\text{2}}}\text{ }\!\!~\!\!\text{ + }{{\text{H}}_{\text{2}}}\text{S}$ | (2) |
| $\text{Sn(C}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{4}}}\text{ }\!\!~\!\!\text{ + }{{\text{H}}_{\text{2}}}\text{S }\to \text{ Sn}{{\text{S}}_{\text{2}}}\downarrow \text{ + (C}{{\text{H}}_{\text{2}}}\text{OH}{{\text{)}}_{\text{2}}}\text{ }\!\!~\!\!\text{ }$ | (3) |
|
Download:
|
| Figure 1. Schematic illustration of the formation process of SnS2 NP/GNs nanocomposite. | |
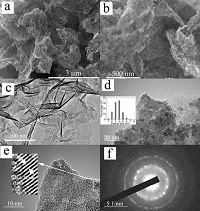
From Fig. 2a, it can be observed that SnS2 nanoparticles are densely anchored on the graphene nanosheets. The graphene nanosheets are separated by SnS2 nanoparticles without obvious restacking. From Fig. 2b, the sheet thickness of the nanocomposite is estimated to be 10-20 nm and the diameter of the SnS2 nanoparticles is several nanometers.
|
Download:
|
| Figure 2. (a and b) SEM image of the SnS2 NP/GNs nanocomposite, (a) low and (b) high magnified images. (c-f) TEM and HR-TEM images of the SnS2 NP/GNs nanocomposite, (c) low magnification image, (d) high magnification image, inset of (d) is the SnS2 NP size distribution estimated from the image, (e) HR-TEM image of the SnS2 NP/GNs nanocomposite and the inset image is the detail view of the lattice fringes of SnS2 NP, (f) the selected electron diffraction of the SnS2 NP/GNs nanocomposite. | |
We employ TEM and HR-TEM to further investigate the quality of SnS2 NP and graphene. Fig. 2c-f shows the TEM images of the asprepared SnS2 NP/GNs sample at different magnifications. At low magnification as shown in Fig. 2c, the presence of few-layer graphene sheets without obvious re-stacking could be clearly seen. SnS2 nanoparticles anchored onto the graphene nanosheets. Compared with the SEM image, fewer SnS2 NPs appear in the TEM image, which may caused by the high black-white contrast of the TEM image, so some of the small SnS2 NP ( < 2 nm) are difficult to resolve. The higher magnification image (Fig. 2d) clearly reveals the presence of ultrafine SnS2 nanoparticles distributed on the graphene nanosheets surface distinctly. The diameter of the SnS2 estimated from the TEM images is 2-5 nm in diameter. The TEM images in Fig. 2c and d appear to be inconsistent with the SEM images in Fig. 2a and b, as it seems there are more SnS2 NPs in Fig. 2a and b sample. In fact, two factors may result in this illusion, the one is the image contrast of TEM, and the other is the ultrasonic treatment procedure when preparing for TEM test, which may cause the loss of SnS2 NPs. Inset of Fig. 2d shows the size distribution of SnS2 nanoparticles according to TEM images. It shows that the average particle size is about 4 nm. The ultra small and uniform SnS2 particle size together with the separated graphene nanosheets ensure large specific surface area of the as-prepared nanocomposite, and BET test shows that the nanocomposite has a high specific surface area up to 129.6 m2/g and a pore volume of 0.17 cc/g (Fig. S1 in Supporting information). The high specific surface area and pore volume of the material facilitate electrolyte penetration, which benefits the fast transport of electrons and Li ions. The HR-TEM image (Fig. 2e) shows the clear lattice fringes with d values of 3.2 Å corresponding to (10 0) planes of SnS2 (JCPDS#23-677). The distinct ring pattern of the SAED, as displayed in Fig. 2f, reveals the polycrystalline nature of the SnS2 NP.
Next, we use wide-angle powder XRD diffraction to analyze the crystal structure of the as-prepared SnS2 NP/GNs nanocomposite (Fig. S2 in Supporting information). The diffraction peaks at 15°, 28°, 32°, 50°, and 52° can be indexed to (0 01), (10 0), (101), (110), and (111) planes of the 2T-type hexagonal SnS2 (JCPDS Card File No. 23-677), respectively. The relatively weak diffraction peak of the nanocomposite is believed to be caused by the small particle size of SnS2. However, at such a high content (32.1 wt% determined by TGA, Fig. S3 in Supporting information), the graphene diffraction peak (0 0 2 plane, generally at 24°-28°) cannot be detected in the nanocomposite, implying that the restacking of graphene nanosheets may be restricted by the uniformly loaded SnS2 nanoparticles [15], which is consistent with the SEM and TEM analysis. The reduction of GO does occur, as evidenced from the presence of the (0 0 2) characteristic peak of graphene for bare graphene prepared by a similar route (Fig. 4, black pattern).
Raman spectra was employed to supply further evidence of the reduction of GO. From Fig. 5 it can be found that there is an increased D/G intensity ratio in graphene in comparison with pure GO [16], which implies the GO were reduced during the solvothermal process.
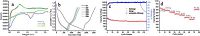
The electrochemical performance of the SnS2 NP/GNs nanocomposite anodes were evaluated using 2016 type coin cells. First, cyclic voltammetric (CV) experiments were performed in a voltage range of 0.01-2 V with a scan rate of 0.1 mV/s. As shown in Fig. 3a, a broad reduction peak is located at 1.5-1.0 V in the first potential sweeping process, which can be ascribed to the decomposition of SnS2 into metallic Sn and Li2S and the formation of solid electrolyte interface (SEI) film [17]. The 0.3-0 V reduction peak was related to the subsequent formation of a series of LiSn alloys [18]. During the subsequent cycle, a broad oxidation peak at 0.3-0.7 V corresponded to the de-intercalation of Li from the LiSn alloys [17, 18]. From Fig. 3a we can observe that the CV curves of the 10th cycle and the 30th cycle almost overlap, suggesting good capacity retention in the SnS2 NP/GNs nanocomposite. The charge/ discharge profiles of the SnS2 NP/GNs nanocomposite electrode at a current density of 0.1 A/g are shown in Fig. 3b. The initial discharge and charge capacities of the SnS2 NP/GNs nanocomposite are 1830 and 820 mAh/g, respectively, corresponding to a Coulumbic efficiency of about 49%. The large initial irreversible capacity is mainly related to the side reactions that occur at the voltage around 1.5V to form the SEI film, and to the irreversible reaction of Li ions and sulfur to form Li2S. Annealing the electrode materials at high temperature can improve initial Coulombic efficiency by reducing side reactions [19]. Another possible way is to widen the voltage window, because when the voltage was increased to 2.0V, the Li2S would decompose and the irreversible reaction between Li and S would become partially reversible [20].
The cycling performance of SnS2 NP/GNs nanocomposite anode was demonstrated in Fig. 3c. In comparison, the pure graphene and commercial SnS2 electrodes were also prepared and tested; their corresponding SEM images and cycling performance are shown in Figs. S4 and S5 in Supporting information. Although the cycling performance is quite good (with a capacity retention of 91% for 100 cycles), the pure graphene anode exhibits a low initial charge capacity of 191mAh/g. The low capacity can be attributed to the sluggish lithium ion diffusion related to the well-ordered structure of the graphene anode during the charge (Li extraction) process [21]. The commercial SnS2 anode exhibits a higher initial charge capacity 532mAh/g, however, it shows poor cycling performance, after 100 cycles, a capacity retention of only 53% can be reached. The poor cycling performance of the commercial SnS2 anode is related to the large volume expansion that occurs during the Li insertion and extraction process, leading to structural collapse. As shown in Fig. 3c, compared with the pure graphene and the commercial SnS2, on the one hand, the SnS2 NP/GNs anode exhibits a capacity retention of 81% after 100 cycles (77% after 150 cycles), which is a bit lowerthan the puregrapheneanode but muchhigher than the commercial SnS2 anode. On the other hand, the SnS2 NP/ GNs anode shows much higher initial charge capacity than both the graphene (820mAh/g vs.191mAh/g) and commercial SnS2 anodes (820mAh/g vs.525mAh/g). In addition to these, the SnS2 NP/GNs anode shows a Coulombic efficiency close to 100% except the first several cycles, revealing the excellent reversibility of the material.
The high rate capability of the SnS2 NP/GNs anode was tested as shown in Fig. 3d. The sample was cycled at different current densities ranging from 0.1A/g to 20A/g and then back to the initial current rate of 0.1A/g. From Fig. 3d, we can observe that the average (10 cycles) reversible specific capacities of the electrode cycling at progressively increasing current densities are 731mAh/ g, 674mAh/g, 621mAh/g, 561mAh/g, 525mAh/g, 443mAh/g, and 378mAh/g, respectively. It is noted that, even at the high current rate of 20A/g, the nanocomposite electrode can still deliver a capacity of 378mAh/g, even higher than the theoretical capacity of commercial graphite (372mAh/g). The rate capability of our SnS2 NP/GNs nanocomposite electrode is equivalent or better compared with the recently reported results [17-20, 22-26]. When the current density switches back from 20A/g to 0.1mA/g again, the reversible capacity recovers to 715mAh/g. This result indicates that our SnS2 NP/GNs nanocomposite electrode is tolerant to a variable charge-discharge current, which is a desirable characteristic required for high power application.
|
Download:
|
| Figure 3. Electrochemical tests of the SnS2 NP/GNs nanocomposite, (a) cyclic voltammetric curves at the scan rate of 0.1mV/S, (b) charge-discharge voltage profiles, (c) cycling performance and the corresponding columbic efficiency, (d) rate capability at various cycling rates ranging from 0.1A/g to 10A/g. | |
To explain the excellent high rate performance of the SnS2 NP/ GNs nanocomposite electrode, we draw a sketch map of the electrons and Li ions transport pathways in the nanocomposite material (Fig. S6 in Supporting information). As shown in Fig. S6, due to the excellent electron conductivity of the graphene nanosheets, we are clear that once the electrons reach the graphene nanosheets they can go everywhere. Because the SnS2 nanoparticles are firmly anchored on the graphene nanosheets, duringthe charge-dischargeprocess, electrons can easilyreachthe active position and participate in the electrochemical reaction. For the Li ions in the electrolyte, they can directly participate in the electrochemical reaction because the SnS2 nanoparticles are anchored in the surface of the graphene nanosheets. The asdesigned electrode material with the unique structure can greatly facilitate the transportation of electrons and Li ions. In addition, the ultra small particle size of SnS2 also reduced the inner diffusion pathway of Li ions.
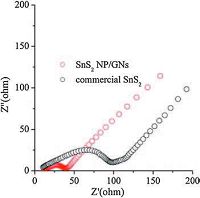
Fig. 4 shows AC impedance spectra of the SnS2 NP/GNs and the commercial SnS2 electrodes. Generally, the semicircle of the AC impedance spectra is assigned to the charge-transfer impedance on electrode, and the inclined line in the low-frequency range corresponds to the lithium-diffusion process within electrodes [27].From Fig. 4, it is obvious that the SnS2NP/GNssemicircleismuch smaller than that of commercial SnS2, which indicates the former electrode possesses lower charge-transfer resistances. The lower resistance of SnS2 NP/GNs electrode can be attributed to the good conductivity of graphene [28, 29] and the small particle size of SnS2.
|
Download:
|
| Figure 4. AC impedance spectra of the sample electrodes measured at the open potential of 0V (after the 10th cycle). | |
TEM image of the SnS2 NP/GNs nanocomposite after cycling is provided (Fig. S7 in Supporting information). Before the TEM test, the electrode material was washed in EC/DMC (1:1, v/v) solution with the absence of LiPF6, in order to remove the impurities from [(Fig._4) TD↔FIG the electrolyte. From Fig. S7, neither aggregated nor detached SnS2 particles can be observed. The ability to keep intact structure during cycling is extremely important to the electrochemical performance of the material. Specifically, for the SnS2 NP/GNs nanocomposite, it is speculated that the separated graphene nanosheets, the uniform distribution of the SnS2 NP, and the intrinsic crystalline structure of SnS2, are the three key factors that contributed to the material's structural stability.
4. ConclusionIn summary, SnS2 NP/GNs nanocomposite consisting of SnS2 nanoparticles and graphene nanosheets in the form where SnS2 nanoparticles were uniformly anchored on the graphene nanosheets, have been investigated as potential anode material for LIB. Electrochemical test results demonstrated an excellent electrochemical performance for the SnS2 NP/GNs nanocomposite anode in terms of the high charge/discharge capacities, good cycling performance, and good rate capability at various current densities.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 21475085) and the key scientific research project of high schools in Henan Province (Nos. 16A430025 & 17A480009).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.09.003.
| [1] | P.F. Hu, H. Wang, Y. Yang, Renewable-biomolecule-based full lithium-ion batteries. Adv. Mater. 28 (2016) 3486–3492. DOI:10.1002/adma.201505917 |
| [2] | H. Wang, H.B. Feng, J.H. Li, Graphene and graphene-like layered transition metal dichalcogenides in energy conversion and storage. Small 10 (2014) 2165–2181. DOI:10.1002/smll.201303711 |
| [3] | C.X. Zhai, N. Du, H. Zhang, J.X. Yu, D.R. Yang, Multiwalled carbon nanotubes anchored with SnS2 nanosheets as high-performance anode materials of lithium-ion batteries. ACS Appl. Mater. Interfaces 3 (2011) 4067–4074. DOI:10.1021/am200933m |
| [4] | W. Wei, Z.H. Wang, Z. Liu, Metal oxide hollow nanostructures:fabrication and Li storage performance. J. Power Sources 238 (2013) 376–387. DOI:10.1016/j.jpowsour.2013.03.173 |
| [5] | D.Z. Chen, W. Wei, R.N. Wang, J.C. Zhu, L. Guo, α-Fe2O3 nanoparticles anchored on graphene with 3D quasi-laminated architecture:in situ wet chemistry synthesis and enhanced electrochemical performance for lithium ion batteries. New J. Chem. 36 (2012) 1589–1595. DOI:10.1039/c2nj40151g |
| [6] | J.G. Ren, Q.H. Wu, H. Tang, Germanium-graphene composite anode for high-energy lithium batteries with long cycle life. J. Mater. Chem. A 1 (2013) 1821–1826. DOI:10.1039/C2TA01286C |
| [7] | S.Y. Liu, X. Lu, J. Xie, Preferential c-axis orientation of ultrathin SnS2 nanoplates on graphene as high-performance anode for li-ion batteries. ACS Appl. Mater. Interfaces 5 (2013) 1588–1595. DOI:10.1021/am302124f |
| [8] | T.F. Zhou, W.K. Pang, C.F. Zhang, Enhanced sodium-ion battery performance by structural phase transition from two-dimensional hexagonal-SnS2 to orthorhombic-SnS. ACS Nano 8 (2014) 8323–8333. DOI:10.1021/nn503582c |
| [9] | S. Liu, X.M. Yin, L.B. Chen, Q.H. Li, T.H. Wang, Synthesis of self-assembled 3D flowerlike SnS2 nanostructures with enhanced lithium ion storage property. Solid State Sci. 12 (2010) 712–718. DOI:10.1016/j.solidstatesciences.2010.02.033 |
| [10] | A.K. Geim, K.S. Novoselov, The rise of graphene. Nat. Mater. 6 (2007) 183–191. DOI:10.1038/nmat1849 |
| [11] | K.S. Novoselov, A.K. Geim, S.V. Morozov, Electric field effect in atomically thin carbon films. Science 306 (2004) 666–669. DOI:10.1126/science.1102896 |
| [12] | A.K. Geim, Graphene:status and prospects. Science 324 (2009) 1530–1534. DOI:10.1126/science.1158877 |
| [13] | N.I. Kovtyukhova, P.J. Ollivier, B.R. Martin, Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 11 (1999) 771–778. DOI:10.1021/cm981085u |
| [14] | J.F. Liang, W. Wei, D. Zhong, One-step in situ synthesis of SnO2/graphene nanocomposites and its application as an anode material for li-ion batteries. ACS Appl. Mater. Interfaces 4 (2012) 454–459. DOI:10.1021/am201541s |
| [15] | M. Zhang, D.N. Lei, Z.F. Du, Fast synthesis of SnO2/graphene composites by reducing graphene oxide with stannous ions. J. Mater. Chem. 21 (2011) 1673–1676. DOI:10.1039/C0JM03410J |
| [16] | S. Stankovich, D.A. Dikin, R.D. Piner, Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45 (2007) 1558–1565. DOI:10.1016/j.carbon.2007.02.034 |
| [17] | N. Du, X.L. Wu, C.X. Zhai, H. Zhang, D.R. Yang, Large-scale synthesis and application of SnS2-graphene nanocomposites as anode materials for lithiumion batteries with enhanced cyclic performance and reversible capacity. J. Alloys Compd. 580 (2013) 457–464. DOI:10.1016/j.jallcom.2013.06.079 |
| [18] | H.X. Zhong, G.Z. Yang, H.W. Song, Vertically aligned graphene-like SnS2 ultrathin nanosheet arrays:excellent energy storage, catalysis, photoconduction, and field-emitting performances. J. Phys.Chem. C 116 (2012) 9319–9326. DOI:10.1021/jp301024d |
| [19] | Y. Wang, F.B. Su, J.Y. Lee, X.S. Zhao, Crystalline carbon hollow spheres, crystalline carbon-SnO2 hollow spheres, and crystalline SnO2 hollow spheres:synthesis and performance in reversible li-ion storage, Chem. Mater.18(2006) 1347-1353. |
| [20] | M. Sathish, S. Mitani, T. Tomai, I. Honma, Ultrathin SnS2 nanoparticles on graphene nanosheets:synthesis, characterization, and li-ion storage applications. J. Phys. Chem. C 116 (2012) 12475–12481. DOI:10.1021/jp303121n |
| [21] | A. Abouimrane, O.C. Compton, K. Amine, S.T. Nguyen, Non-annealed graphene paper as a binder-free anode for lithium-ion batteries. J. Phys. Chem. C 114 (2010) 12800–12804. DOI:10.1021/jp103704y |
| [22] | G. Wang, J. Peng, L.L. Zhang, Two-dimensional SnS2@PANI nanoplates with high capacity and excellent stability for lithium-ion batteries. J. Mater. Chem. A 3 (2015) 3659–3666. DOI:10.1039/C4TA06384H |
| [23] | B. Luo, Y. Fang, B. Wang, Two dimensional graphene-SnS2 hybrids with superior rate capability for lithium ion storage. Energy Environ. Sci. 5 (2012) 5226–5230. DOI:10.1039/C1EE02800F |
| [24] | S.C. Yan, K.Y. Li, Z.X. Lin, Fabrication of a reversible SnS2/RGO nanocomposite for high performance lithium storage. RSC Adv. 6 (2016) 32414–32421. DOI:10.1039/C6RA03124B |
| [25] | L. Mei, C. Xu, T. Yang, Superior electrochemical performance of ultrasmall SnS2 nanocrystals decorated on flexible RGO in lithium-ion batteries. J. Mater. Chem. A 1 (2013) 8658–8664. DOI:10.1039/c3ta11269a |
| [26] | D.B. Kong, H.Y. He, Q. Song, A novel SnS2@graphene nanocable network for high-performance lithium storage. RSC Adv. 4 (2014) 23372–23376. DOI:10.1039/c4ra03052d |
| [27] | L.H. Tang, Y. Wang, Y.M. Li, et al., Preparation structure, and electrochemical properties of reduced graphene sheet films, Adv. Funct.Mater.19(2009)2782-2789. |
| [28] | Y.M. Wu, M.J. Liu, H.B. Feng, J.H. Li, Carbon coated MnO@Mn3N2 core-shell composites for high performance lithium ion battery anodes. Nanoscale 6 (2014) 14697–14701. DOI:10.1039/C4NR05043F |
| [29] | Y.M. Li, X.J. Lv, J. Lu, J.H. Li, Preparation of SnO2-nanocrystal/graphenenanosheets composites and their lithium storage ability. J. Phys. Chem. C 114 (2010) 21770–21774. DOI:10.1021/jp1050047 |
 2017, Vol. 28
2017, Vol. 28 


