b Department of Food Science and Human Nutrition, University of Florida, Bldg 475 Newell Drive, FL 32611, USA;
c Zhejiang Provincial Key Laboratory of Fiber Materials and Manufacturing Technology, Hangzhou 310018, China;
d Department of Materials Engineering, Zhejiang Sci-Tech University, Hangzhou 310018, China
Copper is a nonferrous metal largely applied in industry for the production of various conduction parts of cables, electrical units, and jewelry. It serves as a component of different alloys, and is also used as catalytic agents. Meanwhile, copper is known as a trace element necessary for life; however, the excessive intake of heavy metals causes significant harm to human health. Since heavy metal pollution has made a great impact to environment in recent years, development of novel methods to remove and reuse copper from waste disposal is vital to the environment for alleviating issues of copper pollution [1-8]. Recently, adsorption has been considered one of the most popular and effective methods for the removal of pollutants at different industrial facilities and from different natural environments. Because adsorption is an interfacial phenomenon, it has certain qualities, such as fast kinetics, flexibility in design, and mild regeneration condition [9].
Polyacrylonitrile (PAN) is an ideal polymeric matrix with a series of merits such as mechanical stability, solvent resistance, and abrasion resistance [10]. Active nitrile groups (C≡N) in PAN can easily be converted into a number of new functional groups via special reactions. Deng et al. [11-13] used aminated polyacrylonitrile fibers to remove copper, lead, and chromium ions from aqueous solutions; however, the adsorption capacity was not high, and selectivity was not mentioned. Our initial report showed that the cysteine functionalized polyacrylonitrile resin can be used for selective adsorption of Cu (II) from solutions with a good adsorption capacity.
Response surfacemethodology (RSM) is a collection of statistical and mathematical techniques which has been successfully used for developing, improving, and optimizing processes [14].
In this work, we designed a novel chelating resin (PAN-CS) by grafting cysteine on the surface of PAN beads, which has promising potential for the application of the separation and preconcentration of Cu (II) from multicomponent solutions. The synthesis conditions such as reaction temperature, molar ratio of reagents, and reaction time have been optimized with the use of RSM. The synthetic resin was characterized by FT-IR and elemental analysis (EA). The resin was designed to fit in with environmental protection goals.
2. Experimental 2.1. MaterialsMesoporous-type cross-linked polyacrylonitrile beads (PAN), cross-linked with 7% divinylbenzene (DVB), nitrogen content 22.18%, functional groups content 15.83 CN mmol/g, specific surface area 27.8 m2/g, pore size 25.1 nm, were purchased from Chen Guang Chemical Industrial Institute of China. Cysteine was purchased from Aladdin Industrial Corporation, China. Aqueous solutions of ions at various concentrations were prepared from NiSO4-6H2O, HgCl2, CuCl2-2H2O, Zn (NO3)2-6H2O, Cd(NO3)2-4H2O, and Pb (NO3)2, and were used as sources for Ni (II), Hg (II), Cu (II), Zn (II), Cd(II), and Pb (II), respectively. All other reagents and solvents were of analytical reagent grade and were used without further purification.
2.2. ApparatusIR spectra for the samples were obtained from a Nicolet 380 Fourier transform infrared (FT-IR) spectrometer. The concentrations of metal ions were measured by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES). C, N, and S elements were analyzed by a Vario EL III Elemental Analyzer. The specific surface area and the mean pore size of the resins were determined on an Autosorb-1 automatic surface area and pore size analyzer. A Mettler Toledo delta 320 pH meter was used for pH measurements.
2.3. Preparation of PAN-CSThe preparation procedure is simple and described as follows: PAN beads and N, N-dimethylformamide (DMF) were added into a three-neck round-bottom flask, swelling overnight. Then, CS and a small amount of metallic sodium used as catalyst were added to the flask. The mixture reacted with 100 rpm stirring speed under a nitrogen atmosphere. The solid product was carefully washed thoroughly with DMF and deionized and then washed with acetone and ether. After that, the obtained resin was dried in vacuum at 50 ℃. The conversion of the functional group of the synthetic resin can be calculated from the nitrogen content by the following equations:
| ${{F}_{c}}~=~\frac{{{N}_{c}}}{14\times {{n}_{c}}~}~\times 1000$ |
| $X\text{ }=~\frac{{{F}_{n}}\times 1000}{1000\times {{F}_{0}}-\Delta m\times {{F}_{n}}\times {{F}_{0}}~}\text{ }\times 100\%$ |
where F0 (mmol of Cl/g) and Fc are the contents of the functional group of polystyrene and the synthesized resin, respectively, X is the functional group conversion (%), mis the incremental synthesis reaction resin (g/mol), nc is the number of nitrogen atoms of ligand molecules, and Nc is the nitrogen content of the synthesized resin (%).
2.4. Resin adsorption and desorption experimentsBatch experiments were carried out to investigate the Cu (II) adsorption property on the prepared PAN-CS resin by placing 15.0 mg resin in a series of flasks containing 30 mL of the studied metal ions at the desired initial concentration and pH. Then, the contents of the flasks were shaken in a flask-shaker at specific temperatures for a given time with a speed of 100 rpm. The residual concentration of the studied metal ions in the solution was determined by ICP-OES. The adsorption capacity (Q, mg/g) and distribution coefficient (D, mL/g) were calculated with the following expression:
| $\mathsf{Q =}\frac{\mathsf{~}{{\mathsf{C}}_{\mathsf{0}}}\mathsf{-}{{\mathsf{C}}_{\mathsf{e}}}}{\mathsf{W}}\mathsf{V}$ |
| $\mathsf{D =}\frac{\mathsf{~}{{\mathsf{C}}_{\mathsf{0}}}\mathsf{-}{{\mathsf{C}}_{\mathsf{e}}}}{\mathsf{W}{{\mathsf{C}}_{\mathsf{e}}}}\mathsf{~V}$ |
where C0 is the initial concentration of Cu (II) (mg/mL), Ce is the residual concentration of Cu (II) in solution (mg/mL), V is the solution volume (mL), andWis the resin dry weight (g). Desorption experiments were carried out following the completion of the adsorption experiments. After adsorption experiments, the resins were separated from the aqueous solution by filtration, washed with deionized water, and shaken with different eluent solutions of various concentrations at 298 K for 24 h. After that time the concentration of Cu (II) was similarly analyzed as described above. After each adsorption-desorption cycle, the resin beads were washed and reconditioned for adsorption in the succeeding cycle. The desorption ratio (E) was calculated as follows:
| $E\left( \% \right)\text{ }=~\frac{{{C}_{d}}{{V}_{d}}}{({{C}_{0}}-{{C}_{e}})V}~\text{ }\times 100\%$ |
where Cd is the concentration of the solutes in the desorption solutions, Vd is the volume of the desorption solution, and C0, Ce and V are the same as defined above.
3. Results and discussion 3.1. Analysis of variance (ANOVA) and development of regression model equationExperimental data obtained using the Design Expert 8.0.4 software for multiple regression equation, and gained objective function quadratic regression equation on the conversion rate of the functional group of PAN-CS:
| $\begin{align} & Y\text{ }=\text{ 43}\text{.28 + 1}\text{.43A-1}\text{.44B + 2}\text{.27C-} \\ & \text{1}\text{.45}{{\text{A}}^{\text{2}}}\text{-0}\text{.49}{{\text{B}}^{\text{2}}}\text{-2}\text{.29}{{\text{C}}^{\text{2}}}\text{-1}\text{.38AB + 0:18AC-0}\text{.54BC} \\ \end{align}$ |
The results for analysis of variance for quadratic regression are given in Table 1. From the analysis results in Table 1, the overall model is highly significant ("Pr > F" value < 0.0001), no significant lack of fit items ("Pr > F" value=0.7348 > 0.05), which is desirable. The model can be used to predict the conversion rate of the functional group of PAN-CS for different conditions. The determination coefficient R2=0.9840, and the coefficient of variation (C.V%) 1.27% is within an acceptable range, which indicates that the experiment as described is operable. The adjusted R-square Radj2=0:9635, demonstrates 96.35% of the variability of the data can be explained by the model. Adeq Precision is to detect a noise signal ratio and it is operable once the ratio is over 4. The experimental Adeq Precision is 19.072, which is moderate, indicating that this model can accurately predict the experimental results. It is seen from the F value of each single factor that the molar ratio has the most significant impact on the conversion rate. Compared with the single factor experimental results, which found that the molar ratio within a certain range has the greatest impact on the conversion rate; the effects of time and temperature were not significant ("Pr > F" value > 0.05).
|
|
Table 1 Analysis of variance of RSM. |
3.2. Optimization of synthesis conditions
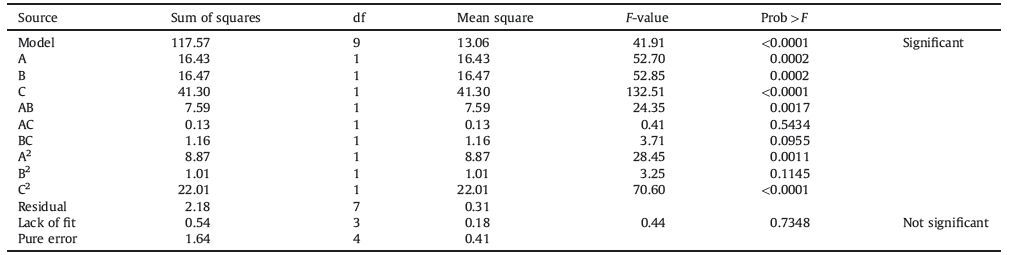
Three-dimensional plots were drawn to investigate the relationship between the responses and the reaction parameters. By Design Expert 8.0.5 software, according to the regression equation to draw a response surface plot Fig. 1a and c. As can be seen from Fig. 1a-c opened downwardly, indicating the response value has a maximum value.

|
Download:
|
| Figure 1. Three-dimensional plots showing effects of (a) temperature and time, (b) temperature and molar ratio, and (c) time and molar ratio. | |
Fig. 1a graphs the effect of time and temperature on the conversion rate at a molar ratio of 3. It shows that time and temperature have significant interaction effects on conversion rate. Extraction rate increased gradually with increasing time at lower temperatures (e.g. 70 ℃), but with increasing temperature, extraction rate tended to increase and then decrease with time. Possible reasons are: higher temperature may make more molecules able to overcome the activation energy of the reaction, increasing the diffusion, leading to higher conversion. Above a certain temperature, the ligands are unstable, which resulted in reduced conversion.
Fig. 1b shows the molar ratio and temperature's influence on the conversion rate at a reaction time of 10 h. It shows that as temperature decreases, the conversion rate gradually increases with increasing liquid ratio. At higher temperatures, the conversion rate tended to decrease after an initial increase as the molar ratio increased.
Fig. 1c shows the effect of molar ratio and time on the conversion rate at a temperature of 90 ℃. It shows that molar ratio increases then decreases with molar ratio, and increases with time.
From the Design Expert 8.0.5 software analysis, we could conclude the optimum synthesis conditions: time 10.1 h, temperature 91.3 ℃, molar ratio 3.1:1, and the predicted conversion of the functional group of PAN-CS is 45.13%. To verify the credibility of the results, the process was repeated 5 times and the average conversion rate was 44.41%, consistent with the predicted value (relative error 1.59%), indicating that the developed model was adequate for predicting the synthesis conditions of PAN-CS.
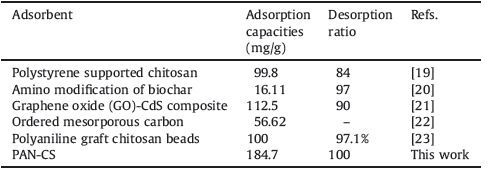
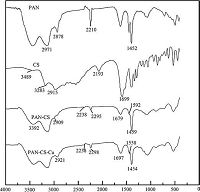
3.3. Characterization of PAN-CSThe structures of PAN, CS, and PAN-CS were confirmed using FTIR spectroscopy as shown in Fig. 2. In general, it is observed that there are significant changes in the IR spectra of PAN resin and PAN-CS resin. In the spectra of CS, the appearance of characteristic bands at 3489 and 3283 cm-1 is due to the stretching vibrations of N-H of amine and secondary amine groups. The FT-IR spectra of PAN with characteristic peaks at 2243 cm-1 was related to C≡N groups; 2971, 2878 and 1452 cm-1 were related to the antisymmetric stretching vibration band, symmetric stretching vibration band, and bending vibration band of -CH2 groups, respectively. Comparing the PAN and CS spectra, the remarkable decrease in peak intensity and a slight shift from 2210 cm-1 to 2238 cm-1 of C≡N band, the disappearance of the stretching vibrations of -NH2 group at 3489 and 3283 cm-1, along with the appearance of a new band at 3392 cm-1 related to secondary amino group (-N-H) in the spectra of PAN and CS, indicated that the reaction between C≡N and -NH2 occurred and the CS ligands have been grafted onto the PAN polymer surface successfully. The characteristics and elemental analysis of the resin are shown in Table 2. The increase of N content and the new appearance of S content confirmed the grafting of CS ligands on the surface of the PAN polymer. The potential synthesis routes of PAN-CS resin is presented in Scheme 1 on the basis of the FT-IR analysis.
|
Download:
|
| Figure 2. FT-IR spectra of PAN, CS, PAN-CS and PAN-CS-Cu. | |
|
|
Table 2 Characteristics and elemental analysis data of the resin. |
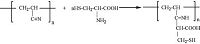
|
Download:
|
| Scheme 1. Synthesis routes for PAN-CS. | |
The FT-IR spectra of the Cu (II) adsorbed resin (PAN-CS-Cu) was also revealed to find out the adsorption mechanism. the disappearance of the peak at 1592 cm-1, the appearance of a new peak around 1=8 cm-1, A shift in the band at 2295 cm-1 (from 2295 cm-1 to 2298 cm-1) and at 2909 cm-1 (from 2909 to 2921 cm-1) were observed, indicating that N-H, -S-H, and O-H were involved in Cu (II) adsorption. In the PAN-CS-Cu complexes the peak of C=N was unchanged, suggesting that the endocyclic nitrogen is not involved in coordination.
3.4. Effect of pHpH value of aqueous solution is one of the most important factors for adsorption of metal ions. Adsorption capacity decreased by increasing or decreasing the pH. Cu (II) was present in the aqueous solution in the forms of Cu2+, Cu (OH)+, Cu (OH)2, Cu (OH)3-, Cu (OH)42- [15]. Since the zeta potentials of the PSDUs are positive at pH < 3.5 (point of zero charge, pHzpc, Fig. 3), the interactions between the adsorption sites on PAN-CSs and the copper ions are electrostatically repulsive. As the solution pH increases, the repulsion between adsorbent surface and metal ions becomes weaker, thus enhancing the adsorption capacity. In the pH range of pH 3.5-6, Cu (OH)3- and Cu (OH)42- were the dominant species, -COOH played a major role in the binding activity, and PAN-CS became deprotonated (-COO-), which may be electrostatically repulsive between adsorption sites on PAN-CS and Cu (OH)3-, Cu (OH)42-. As the solution pH increases, the repulsion between adsorbent surface and metal ions becomes stronger, thus reducing the adsorption capacity. The adsorption studies at pH > 6 were not conducted because of the precipitation of Cu (OH)2 from the solution.
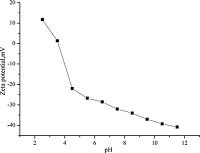
|
Download:
|
| Figure 3. Zeta potentials of PAN-CS resin at different pH. | |
The selective adsorption property was also investigated in a mixed metal solution in the same pH range. The result is shown Fig. 4. It showed that PAN-CS has much higher affinity toward Cu (II) than the other metal ions, which showed high selectivity and adsorption capacity for Cu (II) [16, 17]. The high adsorption capacity and selectivity for Cu (II) results from the different nitrogen groups in the PAN-CS that show different affinities to Cu (II) and the Ni (II), Hg (II), Cd(II), Pb (II), and Zn (II) metal ions. The -N-H and -C-N groups contribute to the high adsorption capacity toward Cu (II), and the -C=O group contributes to the high selectivity toward Cu (II), because it is a very soft basic group and has a superior affinity for Cu (II) ions.
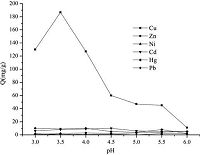
|
Download:
|
| Figure 4. The capacity of PAN-CS resin in different pH values. | |
3.5. Desorption and regeneration studies
In terms of practicality, reusability is a key factor for an advanced adsorbent. Such adsorbent has a higher adsorption capacity and better desorption performance which would reduce cost for the adsorbent. Cu (II) saturated PAN-CS were eluted with 30 mL HCl in various concentrations (0.5-2.5 mol/L) under 100 rpm at 298 K for 24 h. The desorption data shows that the adsorbed Cu (II) can be completely desorbed by 1 mol/L HCl solution with a desorption ratio of 100%. In order to show the reusability of the adsorbent, the adsorption-desorption cycle of metal ions was repeated five times using the same beads [18]. The results of the five adsorption-desorption cycles are shown in Table 3, indicating that the adsorption capacity was barely affected during the repeated adsorption-desorption operations and the adsorption capacity was maintained at 97.5% after five cycles, which suggests that PAN-CS could be repeatedly used in the removal of Cu (II) from waste solution.
|
|
Table 3 Reusing the PAN-CS for the adsorption of Cu (II). |
Data on the adsorption capacity and desorption ratio of Cu (II) by recently reported adsorbents [19-23] are listed in Table 4. It can be seen that a material with good comprehensive properties (high adsorption capacity, and high recovery rate) toward copper was seldom reported.
|
|
Table 4 Adsorption capacities of different adsorbents. |
4. Conclusion
In this research, a new composite chelating resin has been synthesized. FT-IR and elemental results indicate that the immobilization of CS onto PAN was accomplished. The influences of operating parameters such as temperature, molar ratio of reagents, and time for the synthesis conditions of PAN-CS were evaluated using RSM. Batch adsorption results showed that the resin possess an excellent selective adsorption ability of Cu (II) in the mixed metal solutions. Complete desorption of Cu (II) was achieved by using 1.0 mol/L HCl solution, and the regenerated adsorbents could be reused with little loss of adsorption capacity. In a word, PAN-CS resin can satisfactorily be considered as an alternative material for separation applications and recovery of Cu (II) from aqueous solutions. Furthermore, it can also be applied in the area of analysis and detection.
AcknowledgmentsThe work is supported by the National Natural Science Foundation of China (No. 21276235), Ph.D. Programs Foundation of Ministry of Education of China (No. 20133326110006), The Program of Science and Technology of Zhejiang Province, China (No. 2015C3704), Zhejiang Provincial Top Key Academic Discipline of Chemical Engineering and Technology, Zhejiang Sci-Tech University (No. YR2015002), and Zhejiang Provincial Key Laboratory of Fiber Materials and Manufacturing Technology (No. 2005002).
| [1] | Y.S. Petrova, A.V. Pestov, M.K. Usoltseva, L.K. Neudachina, Selective adsorption of silver (I) ions over copper (II) ions on a sulfoethyl derivative of chitosan. J. Hazard. Mater. 299 (2015) 696–701. DOI:10.1016/j.jhazmat.2015.08.001 |
| [2] | M.A.A. El-Ghaffar, Z.H. Abdel-Wahab, K.Z. Elwakeel, Extraction and separation studies of silver (I) and copper (II) from their aqueous solution using chemically modified melamine resins. Hydrometallurgy 96 (2009) 27–34. DOI:10.1016/j.hydromet.2008.07.008 |
| [3] | A.M. El-Menshawy, I.M. Kenawy, A.A. El-Asmy, Modification of chloromethylated polystyrene with 2-mercabtobenzothiazole for application as a new sorbent for preconcentration and determination of Ag+ from different matrices. J. Hazard. Mater. 173 (2010) 523–527. DOI:10.1016/j.jhazmat.2009.08.116 |
| [4] | C.K. Liu, R.B. Bai, Q.S. Ly, Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent:behaviors and mechanisms. Water Res. 42 (2008) 1511–1522. DOI:10.1016/j.watres.2007.10.031 |
| [5] | Z. Ioannou, J. Simitzis, Adsorption kinetics of phenol and 3-nitrophenol from aqueous solutions on conventional and novel carbons. J. Hazard. Mater. 171 (2009) 954–964. DOI:10.1016/j.jhazmat.2009.06.098 |
| [6] | J. Gao, F.Q. Liu, P.P. Ling, High efficient removal of Cu (II) by a chelating resin from strong acidic solutions:complex formation and DFT certification. Chem. Eng. J. 222 (2013) 240–247. DOI:10.1016/j.cej.2013.02.055 |
| [7] | Y. Sun, Z.C. Li, Y. Xu, Preparation and application of a novel orotic acid chelating resin for removal of Cu (II) in aqueous solutions. Chin. Chem. Lett. 24 (2013) 747–750. DOI:10.1016/j.cclet.2013.04.018 |
| [8] | C. Cheng, J.N. Wang, X. Yang, Preparation of novel chelating sponge as an adsorbent for the removal of Cu2+ from water. Chin. Chem. Lett. 24 (2013) 997–1000. DOI:10.1016/j.cclet.2013.06.012 |
| [9] | H. Chen, Y.G. Zhao, A.Q. Wang, Removal of Cu (II) from aqueous solution by adsorption onto acid-activated palygorskite. J. Hazard. Mater. 149 (2007) 346–354. DOI:10.1016/j.jhazmat.2007.03.085 |
| [10] | C.H. Xiong, Q. Jia, X.Y. Chen, G.T. Wang, C.P. Yao, Optimization of polyacrylonitrile-2-aminothiazole resin synthesis, characterization, and its adsorption performance and mechanism for removal of Hg (II) from aqueous solutions. Ind. Eng. Chem. Res. 52 (2013) 4978–4986. DOI:10.1021/ie3033312 |
| [11] | S.B. Deng, R.B. Bai, J.P. Chen, Aminated polyacrylonitrile fibers for lead and copper removal. Langmuir 19 (2003) 5058–5064. DOI:10.1021/la034061x |
| [12] | S.B. Deng, R.B. Bai, Removal of trivalent and hexavalent chromium with aminated polyacrylonitrile fibers:performance and mechanisms. Water Res. 38 (2004) 2424–2432. DOI:10.1016/j.watres.2004.02.024 |
| [13] | J.X. Han, Z.J. Du, W. Zou, H.Q. Li, C. Zhang, Fabrication of interfacial functionalized porous polymer monolith and its adsorption properties of copper ions. J. Hazard. Mater. 276 (2014) 225–231. DOI:10.1016/j.jhazmat.2014.05.035 |
| [14] | T. Chen, B. Li, L. Fang, Response surface methodology for optimizing adsorption performance of gel-type weak acid resin for Eu (III). Trans. Nonferrous Met. Soc. China 25 (2015) 4207–4215. DOI:10.1016/S1003-6326(15)64071-7 |
| [15] | A.Z.M. Badruddoza, A.S.H. Tay, P.Y. Tan, K. Hidajat, M.S. Uddin, Carboxymethyl-β-cyclodextrin conjugated magnetic nanoparticles as nano-adsorbents for removal of copper ions:synthesis and adsorption studies. J. Hazard. Mater. 185 (2011) 1177–1186. DOI:10.1016/j.jhazmat.2010.10.029 |
| [16] | E. Guibal, Interactions of metal ions with chitosan-based sorbents:a review. Sep. Purif. Technol. 38 (2004) 43–74. DOI:10.1016/j.seppur.2003.10.004 |
| [17] | R.S. Vieira, M.L.M. Oliveira, E. Guibal, E. Rodríguez-Castellón, M.M. Beppu, Copper, mercury and chromium adsorption on natural and crosslinked chitosan films:an XPS investigation of mechanism. Colloids Surf. A 374 (2011) 108–114. DOI:10.1016/j.colsurfa.2010.11.022 |
| [18] | C.H. Xiong, Y.L. Li, G.T. Wang, Selective removal of Hg with polyacrylonitrile-2-amino-1, 3, 4-thiadiazole chelating resin:Batch and column study. Chem. Eng. J. 259 (2015) 257–265. DOI:10.1016/j.cej.2014.07.114 |
| [19] | W. Jiang, X.B. Chen, B.C. Pan, Spherical polystyrene-supported chitosan thin film of fast kinetics and high capacity for copper removal. J. Hazard. Mater. 276 (2014) 295–301. DOI:10.1016/j.jhazmat.2014.05.032 |
| [20] | G.X. Yang, H. Jiang, Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater. Water Res. 48 (2014) 396–405. DOI:10.1016/j.watres.2013.09.050 |
| [21] | T.S. Jiang, W.P. Liu, Y.L. Mao, Adsorption behavior of copper ions from aqueous solution onto graphene oxide-CdS composite. Chem. Eng. J. 259 (2015) 603–610. DOI:10.1016/j.cej.2014.08.022 |
| [22] | C.C. Huang, J.C. He, Electrosorptive removal of copper ions from wastewater by using ordered mesoporous carbon electrodes. Chem. Eng. J. 221 (2013) 469–475. DOI:10.1016/j.cej.2013.02.028 |
| [23] | E. Igberase, P. Osifo, A. Ofomaja, The adsorption of copper (II) ions by polyaniline graft chitosan beads from aqueous solution:equilibrium, kinetic and desorption studies. J. Environ. Chem. Eng. 2 (2014) 362–369. DOI:10.1016/j.jece.2014.01.008 |
 2017, Vol. 28
2017, Vol. 28