b Department of Chemistry, C. U. Shah University, Wadhwan 363030, India
In the last two decades, the use of metal nanoparticles has been gaining impetus because they possess significantly distinct physical, chemical, and biological properties from their bulk counterparts. Silver nanoparticles (AgNps) offer excellent surface plasmon resonance (SPR) properties, emission intensity, and a sharp color-change in the presence of analytes. Therefore, AgNps have found applications as colorimetric sensors [1-4], catalysts [5], antibacterial [6-9], cytotoxic and genotoxicagents [10], disinfectant for pathogens [11].
The rapid growth of calixarene systems and their use in nanoscience and nanotechnology [12] can be attributed to their non-toxicity, non-immunogenicity, and chemical and biological stability, as well as their ease of production by known methodologies [13]. There are many methods for the synthesis of metal nanoparticles, such as electrochemical [14], photochemical [15], sonochemical [16, 17], polyol method [18] and chemical reduction of the corresponding metal salts [19, 20]. The chemical reduction methods are probably the most versatile, economical, and simple to control the shape and size of metal nanoparticles. Generally, sodium borohydride [21], hydrazine hydrate [22], ascorbic acid [23], poly (vinyl alcohol) [24], and poly (vinylpyrrolidone) [25] are used as reducing agents. Under the broader category of calixsystems, we have observed that their hydrazide derivatives have similar properties to hydrazine as a reducing agent, and these compounds possess web-like structures with an inherent hollow cavity making them very effective stabilizing agents. We have previously reported that gold nanoparticles protected by calix[4]-pyrrole octahydrazide [26] act as colorimetric and fluorometric chemosensors for selective detection of Co (II) ions [27, 28]. AgNps are also increasingly being explored as possible sensors for amino acids [1, 29]. Cysteine-modified AgNps have been used as colorimetric probes for histidine [30]. Para-sulfonatocalix[4]arene-modified and lysine-functionalized AgNps have been used for the colorimetric detection of histidine [31, 32], while 4, 4-bipyridine-functionalized AgNps were used as a colorimetric sensor for tryptophan [33]. Pu et al. reported that the coordination complex of terpyridine-CuCl2 is an efficient fluorescent sensor for histidine in aqueous solutions [34].
Among naturally occurring amino acids, histidine and tryptophan each have a distinct significance. Tryptophan plays an important role in different biological processes like protein biosynthesis and plant development [35, 36]. In the human body, tryptophan is also utilized as a precursor for serotonin (a potent neurotransmitter in the brain) and melatonin, known to regulate appetite, sleep, and impulse control. Additionally, both amino acids act as mood elevators [37]. Histidine is required to support growth and repair tissue, to maintain the protective myelin sheath of nerve cells, for proliferation and maturation of red and white blood cells, and to protect the body from heavy metal toxicity [38]. Therefore, a technology for the selective detection of histidine and tryptophan can have many useful applications.
Metallic nanoparticles show good antibacterial properties due to their large surface area to volume ratio, which provides better contact with microorganisms. Currently, there is significant interest in this aspect of metallic nanoparticles due to the growing concern of microbial resistance against traditional antibiotics, including metal ions [6, 8, 9, 39]. Generally Ag+ ions show an efficient antimicrobial effect due to their interaction with thiol groups found in the respiratory enzymes of bacterial cells where Ag+ ions bind to the bacterial cell wall and cell membrane and inhibits cellular respiration [40]. Although silver in its metallic state is inert reacts with moisture on the skin surface and fluids in skin wounds and becomes ionized. Ionized silver is highly reactive and binds to tissue proteins. AgNps interact with sulfur-containing proteins in the bacterial membrane as well as with phosphoruscontaining compounds like DNA and RNA. Nanoparticles attach to, and efficiently penetrate, bacterial cell membranes bringing structural changes to the bacterial cell wall and nuclear membrane leading to cell distortion and death [7, 41].
In this study, we report the one pot synthesis of thiacalix [4] are netetrahydrazide [42] protected silver nanoparticles (TCTHAgNps) in aqueous media under ambient conditions using amine chemistry for reduction and surface modification. Duly characterized TCTH-AgNps are explored for their sensitive and selective behavior toward histidine and tryptophan by spectrophotometry and spectrofluorimetry. We demonstrate that TCTH-AgNps can also be applied effectively in the control of microorganisms.
2. ExperimentalMaterial and reagents: Silver nitrate was purchased from Sigma-Aldrich (St. Louis, MO, USA). Other reagents, including the amino acids, were purchased from SRL (Sisco Research Laboratory, Mumbai, India). Analytical-grade solvents were purchased from commercial sources and used without further purification. All aqueous solutions were prepared using Millipore water (resistivity, 18 ΩX; Ettlingen, Germany). TLC plates activated by fluorescence (F-2009) were obtained from Merck (Billerica, MA, USA).
Methods: Melting points (uncorrected) were obtained using a VEEGO apparatus (VMP-DS, VEEGO Instruments Corporation, Mumbai, India). The FT-IR (Fourier transform-infrared) spectra of samples prepared as KBr pellets were recorded on a Tensor 27 infrared spectrometer (Bruker Optik GmbH, Mumbai, India). 1HNMR spectra were recorded on a Fourier transform-nuclear magnetic resonance (FT-NMR) Avance II spectrometer (Bruker Optik) (500 MHz) at 298 K with trimethylsilane as the internal reference. Electrospray ionization-mass spectrometry (ESI-MS) readings were recorded on a Quarter2 mass spectrometer (Micromass Ltd., Manchester, UK). Absorption spectra were measured on a Jasco V-570 UV-vis recording spectrophotometer (Jasco Inc., Easton, MD, USA). The pH concentrations of the prepared solutions were measured using a pH analyzer (LI-614, Elico Ltd., Hyderabad, India). A Zetasizer ZEN3600 (Malvern Instruments Ltd., Worcestershire, UK) was used to estimate the particle size (e.g., hydrodynamic diameter) and to quantify the zeta potential by laser Doppler electrophoresis without dilution. Transmission electron microscopy (TEM) images were recorded using a MACK/model JEOL JEM-2100 (Jeol USA, Inc., Peabody, MA, USA) at an accelerated voltage of 200 kV. A drop of sample solution diluted in water was placed on a carbon-coated copper grid, dried in a vacuum, and directly observed by TEM. Fluorescence spectra were recorded on a Jasco FP-6500 spectrofluorometer.
Synthesis of TCTH-AgNps: 5 mL of a 1 μmol/L solution of AgNO3 was heated to boiling, transferred to a 30 mL conical flask containing 10 mL of water, and rapidly added 5 mL of a 1 μmol/L aqueous solution of TCTH [40] with vigorous stirring. TCTHstabilized silver nanoparticles (TCTH-AgNps) were produced immediately, but vigorous stirring was continued for 8 h to ensure complete homogenization. The transparent, colorless solution was converted to a characteristic yellowish-orange color, indicating the formation of silver nanoparticles (SI-1). This TCTH-AgNps solution was then subjected to centrifugation at 5000 rpm and the absence of any unreacted silver and TCTH in centrifugate was evaluated by inductively coupled plasma-atomic emission spectrophotometry (ICP-AES) and UV-vis spectrophotometry, respectively. The residue was redispersed in deionized water for further studies.
General procedure for the UV-vis and Fluorescence measurements: A 0.0054% stock solution of TCTH-AgNps and 2 μmol/L solutions of various amino acids including valine (Val), proline (Pro), arginine (Arg), cysteine (Cys), aspartic acid (Asp), glutamic acid (Glu), glutamine (Gln), leucine (Leu), methionine (Met), phenylalanine (Phe), tryptophan (Trp), isoleucine (Ile), and histidine (His) were prepared in deionized water. Equal amounts (2.5 mL) of TCTH-AgNps and each amino acid solution were combined in a 5 mL volumetric flask so that the effective concentration of each amino acid was 1 μmol/L. UV-vis and fluorescence spectra of the resulting solutions were compared with the surface plasmon resonance band (425 nm) and emission spectra (579 nm) of TCTH-AgNps.
3. Results and discussionTCTH has been synthesized by a reported procedure [43, 44]. A simple, one-step process has been used for the synthesis of AgNps by using TCTH. Silver ions were reduced to zero-valent nuclei by the reducing agent (TCTH) and during the growth phase the reducing agent was oxidized on to the silver surface [45]. The reduction of AgNO3 occurred through the transfer of electrons from the amine group of TCTH to the Ag+ ion leading to the formation of Ag0. Thus, the terminal -NH-NH2 group of TCTH was responsible for the reduction of silver ions. TCTH also acted as a stabilizing agent for silver nanoparticles by adsorbing on their surface and separated by electrostatic repulsion.
TCTH is an amphiphilic molecule and therefore contains an hydrophobic and a hydrophilic regions. The hydrophobic part, i.e., the tert-butyl part of TCTH outside to the silver particles and the hydrophilic part, i.e., the -NHNH2 part of TCTH is toward the silver particles. Moreover, it is proposed that the TCTH forms a doublelayer structure on the surface of AgNps providing excellent stability to the TCTH-AgNps [46]. The fluorescent nature of TCTHAgNps can be attributed to localized surface plasmon resonance (LSPR) that offers unique luminescence [47, 48].
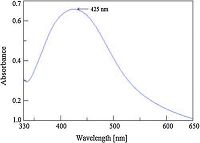
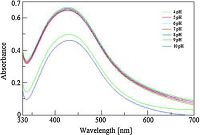
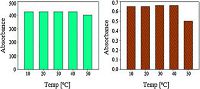
The formation of silver nanoparticles from TCTH and silver nitrate is shown in Scheme 1. A strong surface plasma resonance band identifying the formation of AgNps was observed at 425 nm (Fig. 1). In the primary state of nanoparticle formation the colorless solution was converted to a yellowish-orange colored product. The color intensity was dependent upon the stoichiometry of silver nitrate and TCTH concentration, as slight changes in stoichiometry resulted in a dark yellow color (i.e., aggregation of nanoparticles). The stability of TCTH-AgNps was measured at 30, 60, 90, and 120 days. The stability study revealed that TCTH-AgNps were stable for 90 days without any change in observed SPR (Fig. 2). To evaluate the influence of pH and temperature on stability, we tested a range of pH 4-10 and temperatures (10-50 ℃). The spectra indicated that the AgNps were most stable between pH 5-9 (Fig. 3) and 10-40 ℃ (Fig. 4). Based on thesefindings, we performed the subsequent spectrophotometric studies at pH 7 and at room temperature.

|
Download:
|
| Scheme 1. Formation of silver nanoparticles from TCTH and silver nitrate. | |
|
Download:
|
| Figure 1. Surface plasmon resonance band of TCTH-AgNps. | |

|
Download:
|
| Figure 2. Effect of time on stability of TCTH-AgNps. | |
|
Download:
|
| Figure 3. Effect of pH on absorbance and maximum wavelength of TCTH-AgNps. | |
|
Download:
|
| Figure 4. Effect of temperature on absorbance and maximum wavelength of TCTHAgNps. | |
3.1. Determination of the selectivity and sensitivity of TCTH-AgNps to histidine and tryptophan
The SPR absorption maxima of TCTH-AgNps failed to demonstrate a significant shift in the presence of most amino acids tested (2 × 10-4 mol/L; Val, Pro, Arg, Cys, Asp, Glu, Gln, Leu, Met, Ile, and Phe) with the notable exception of histidine (His) and tryptophan (Trp). Only His and Trp caused a blue shift of 27 nm toward the shorter wavelength region, and a red shift of 11 nm toward the longer wavelength region, respectively (Fig. 5).

|
Download:
|
| Figure 5. Absorption spectra of TCTH-AgNps with different amino acids. | |
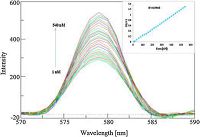
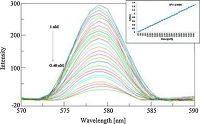
The fluorescence emission of TCTH-AgNps was examined over a wide range of pH 4-10 (Fig. 6), and no apparent changes of the fluorescence spectra were observed at pH values 5-9. The stable fluorescence and good selectivity of TCTH-AgNps at a pH value of approximately 7.0 was favorable for subsequent applications. The emission spectra of the TCTH-AgNps were recorded at 579 nm following excitation at 426 nm. The interaction behavior of various amino acids, including like Val, Pro, Arg, Cys, Asp, Glu, Gln, Leu, Met, Ile, and Phe, Try and His (2 × 10-6 mol/L) concentration with TCTH-AgNps was observed. The selectivity of our new approach for histidine and tryptophan separately with TCTH-AgNps was apparent through the observed enhancement and quenching of signal, respectively, compared to the other amino acids tested (Fig. 7). Fluorescence titrations of TCTH-AgNps with histidine and tryptophan were conducted under the same conditions using deionized water at pH 7.0. The fluorescence intensity of TCTHAgNps at 579 nm, gradually increases proportionately with the concentration of histidine, and decreases inversely with the concentration of tryptophan (Figs. 8 and 9). The detection range of tryptophan and histidine was 1 nmol/L to 0.48 μmol/L and 1 nmol/L to 0.54 μmol/L, respectively. The Stern-Volmer calibration curve showed good linearity of 5 nmol/L to 0.48 μmol/L for tryptophan and 4 nmol/L to 0.54 μmol/L for histidine, with correlation coefficients of 0.9965 and 0.9989, respectively (Insets, Figs. 8 and 9). As indicated in SI. 2 and 3, we also performed a competitive study involving the addition of TCTH-AgNps + histidine and TCTH-AgNps + tryptophan with other different amino acids, but did not observe any significant changes in fluorescence intensity. No fluorescence changes were observed in the presence of other amino acids even when their concentration was up to 100-fold higher than that of histidine and tryptophan. The negativelycharged side chains of some amino acids exhibit a very weak electrostatic interaction with the positively-charged nanoparticles [49], but in cases of histidine and tryptophan strong electrostatic interactions were observed. The high selectivity of TCTH-AgNps for histidine and tryptophan compared to other amino acids may be attributed, in part, to the strong coordination ability of these amino acids (Figs. 10-11).

|
Download:
|
| Figure 6. Effect of pH on fluorescence intensity of TCTH-AgNps. | |

|
Download:
|
| Figure 7. Emission spectra of TCTH-AgNps with various amino acids. | |
|
Download:
|
| Figure 8. Emission titration of TCTH-AgNps with various concentration of histidine (inset graph shows Stern Volmer linearity plot). | |
|
Download:
|
| Figure 9. Emission titration of TCTH-AgNps with various concentration of tryptophan (inset graph shows Stern Volmer linearity plot). | |

|
Download:
|
| Figure 10. Visual color change in TCTH-AgNps by addition of different amino acids. | |

|
Download:
|
| Figure 11. Histogram, TEM and EDX of TCTH-AgNps. | |
To evaluate the applicability of using TCTH-AgNps to recognize amino acids by visual detection, TCTH-AgNps were separately titrated with various amino acids in water by maintaining a 1:1 molar concentration. The yellowish-orange color of TCTH-AgNps turns to orange with the addition of tryptophan and pale yellow 20±2 nmas shown in the histogram. Energy-dispersive X-ray (EDX) analysis of the TCTH-AgNpsspectrum recorded in the spot-profile mode from a densely populated region on the surface of the film showed strong signals from Ag atoms, while weaker signals from C, O, Si, Cu and Ca atoms were observed (Fig. S1). The Zeta potential, the overall charge of the particle in a particular medium, was found to be +13 mV (Fig. S2 in Supporting information). The positive charge indicated that the silver nanoparticles were properly stabilized with a positively-charged TCTH. We concluded that TCTH acted both as a reducing and a stabilizing agent. A comparison of the previous work in terms of better sensitivity is reported in Table 1.
|
|
Table 1 The comparison of the previous work in terms of better sensitivity. |
3.3. Antimicrobial activity of TCTH-AgNps
The antimicrobial effect of TCTH-AgNps on the micro-organisms, Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, and Aspergillus niger, was compared with AgNO3 and the ligand TCTH. No antimicrobial activity was found for TCTH. A marginal enhancement was observed in antimicrobial efficiency of TCTH-AgNps compared to AgNO3 at a minimum concentration of 25%. A subsequent quantitative analysis was performed to determine the antimicrobial activity of the TCTH-AgNps. The quantitative analysis was expressed as a log reduction which is a mathematical term (as is "log increase") and used to show the relative number of live microbes eliminated from a surface by disinfecting or cleaning as a log reduction. Based on our findings, TCTH-AgNps hold great potential for use as antibacterial agents in transdermal applications (Table 2).
|
|
Table 2 The antimicrobial activity of different compounds. |
The exact mechanism for the action of silver nanoparticles is yet not known, but reports suggest that the positive charge of silver ions plays a significant role in the exhibition of antimicrobial activity may be due to electrostatic attraction between the negative charge cell membrane of micro-organisms and positive charge nanoparticles [50, 51]. The nanoparticles after penetrating inside the cell membrane, interacts with the sulfur containing proteins and with phosphorous containing compounds like DNA. They form a low molecular weight region in the center of the bacteria, thus, the bacteria conglomerate and the DNA is protected from the silver ions. The respiratory chain is attacked preferably by the nanoparticles that inhibit the cell division and cell death results. Thus, the enhanced bactericidal activity can be attributed to the release of silver ions by the nanoparticles [51-53]. Further studies to evaluate the spectra of their antimicrobial properties using additional species may help to shape recommendations of these TCTH-AgNps for therapeutic use.
4. ConclusionIn this manuscript, we report the successful synthesis of aqueous silver nanoparticles using TCTH as a reducing and stabilizing agent. The TCTH-AgNps were found to be stable at room temperature, over varying pH range and over at least 90 days. Among the amino acids tested, only tryptophan and histidine showed fluorescence quenching and fluorescence enhancement, respectively. TCTH-AgNps are a selective and sensitive fluorescent sensor for tryptophan and histidine, within a linear detection range of 5 nmol/L to 0.48 μmol/L and 4 nmol/L to 0.54 μmol/L, respectively. TCTH-AgNps can be used as potent growth inhibitors of several micro-organisms significant to human disease, potentially offering novel applications as antimicrobial control systems, particularly for medical devices.
AcknowledgmentThe authors gratefully acknowledge the financial assistance provided by University Grant Commission (UGC), New Delhi. The authors also thank CDRI (Lucknow), GFSU (Gandhinagar), GUJCOST (Gandhinagar) and CSMCRI (Bhavanagar) for providing instrumental facilities and INFLIBNET, Ahmedabad, for e-journals.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.07.026.
| [1] | Y. Sun, L. Zhang, H.B. Li, Chiral colorimetric recognition of amino acids based on silver nanoparticle clusters, New J. Chem. 36(2012) 1442-1444. |
| [2] | A. Ravindran, V. Mani, N. Chandrasekaran, A. Mukherjee, Selective colorimetric sensing of cysteine in aqueous solutions using silver nanoparticles in the presence of Cr3+. Talanta 85 (2011) 533–540. DOI:10.1016/j.talanta.2011.04.031 |
| [3] | K. Farhadi, M. Forough, R. Molaei, S. Hajizadeh, A. Rafipour, Highly selective Hg2+ colorimetric sensor using green synthesized and unmodified silver nanoparticles, Sens. Actuators B:Chem. 161(2012) 880-885. |
| [4] | H.B. Li, Z.M. Cui, C.P. Han, Glutathione-stabilized silver nanoparticles as colorimetric sensor for Ni2+ ion. Sens. Actuators B:Chem. 143 (2009) 87–92. DOI:10.1016/j.snb.2009.09.013 |
| [5] | N. Pradhan, A. Pal, T. Pal, Silver nanoparticle catalyzed reduction of aromatic nitro compounds. Colloids Surf. A:Physicochem. Eng. Asp. 196 (2002) 247–257. DOI:10.1016/S0927-7757(01)01040-8 |
| [6] | Q.L. Feng, J. Wu, G.Q. Chen, A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 52 (2000) 662–668. DOI:10.1002/(ISSN)1097-4636 |
| [7] | A.B.G. Lansdown, Silver I:its antibacterial properties and mechanism of action. J. Wound Care 11 (2002) 125–130. DOI:10.12968/jowc.2002.11.4.26389 |
| [8] | J.R. Morones, J.L. Elechiguerra, A. Camacho, The bactericidal effect of silver nanoparticles. Nanotechnology 16 (2005) 2346–2353. DOI:10.1088/0957-4484/16/10/059 |
| [9] | I. Sondi, B. Salopek-Sondi, Silver nanoparticles as antimicrobial agent:a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 275 (2004) 177–182. DOI:10.1016/j.jcis.2004.02.012 |
| [10] | P.V. AshaRani, G. Low Kah Mun, M.P. Hande, S. Valiyaveettil, Cytotoxicity and genotoxicity of silver nanoparticles in human cells, ACS Nano 3(2009) 279-290. |
| [11] | G. Anjana, M. Gowri, C.S. Raja, et al., Silver nanoparticles as a non alcoholic hospital disinfectant to combat nosocomial pathogens, J. Bionanosc. 9(2015) 102-111. |
| [12] | B.A. Makwana, D.J. Vyas, K.D. Bhatt, S. Darji, V.K. Jain, Novel fluorescent silver nanoparticles:sensitive and selective turn off sensor for cadmium ions. Appl. Nanosci. 6 (2016) 555–566. DOI:10.1007/s13204-015-0459-x |
| [13] | A. Acharya, K. Samanta, C.P. Rao, Conjugates of calixarenes emerging as molecular entities of nanoscience, Coord. Chem. Rev. 256(2012) 2096-2125. |
| [14] | M.T. Reetz, W. Helbig, Size-selective synthesis of nanostructured transition metal clusters. J. Am. Chem. Soc. 116 (1994) 7401–7402. |
| [15] | A. Henglein, Physicochemical properties of small metal particles in solution:"microelectrode" reactions, chemisorption, composite metal particles, and the atom-to-metal transition. J. Phys. Chem. 97 (1993) 5457–5471. DOI:10.1021/j100123a004 |
| [16] | R.A. Caruso, M. Ashokkumar, F. Grieser, Sonochemical formation of colloidal platinum. Colloids Surf. A:Physicochem. Eng. Asp. 169 (2000) 219–225. DOI:10.1016/S0927-7757(00)00438-6 |
| [17] | T. Fujimoto, S.Y. Terauchi, H. Umehara, I. Kojima, W. Henderson, Sonochemical preparation of single-dispersion metal nanoparticles from metal salts. Chem. Mater. 13 (2001) 1057–1060. DOI:10.1021/cm000910f |
| [18] | K.L. Zhang, Y.G. Du, S.M. Chen, Facile one-pot polyol method for the synthesis of uniform size silver nanowires. J. Nanosci. Nanotechnol. 16 (2016) 480–488. DOI:10.1166/jnn.2016.12158 |
| [19] | I.D.G. Macdonald, W.E. Smith, Orientation of cytochrome c adsorbed on a citratereduced silver colloid surface. Langmuir 12 (1996) 706–713. DOI:10.1021/la950256w |
| [20] | Y.M. Chung, H.K. Rhee, Dendrimer-templated Ag-Pd bimetallic nanoparticles, J. Colloid Interface Sci. 271(2004) 131-135. |
| [21] | P. Selvakannan, S. Mandal, S. Phadtare, R. Pasricha, M. Sastry, Capping of gold nanoparticles by the amino acid lysine renders them water-dispersible, Langmuir 19(2003) 3545-3549. |
| [22] | S. Park, J. An, I. Jung, et al., Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents, Nano Lett. 9(2009) 1593-1597. |
| [23] | J.L. Zhang, H.J. Yang, G.X. Shen, et al., Reduction of graphene oxide vial-ascorbic acid, Chem. Commun. 46(2010) 1112-1114. |
| [24] | H. Hirai, Y. Nakao, N. Toshima, Preparation of colloidal rhodium in poly (vinyl alcohol) by reduction with methanol. J. Macromol. Sci. Chem. 12 (1978) 1117–1141. DOI:10.1080/00222337808063179 |
| [25] | Y.G. Sun, Y.D. Yin, B.T. Mayers, T. Herricks, Y.N. Xia, Uniform silver nanowires synthesis by reducing AgNO3 with ethylene glycol in the presence of seeds and poly (vinyl pyrrolidone), Chem. Mater. 14(2002) 4736-4745. |
| [26] | K.D. Bhatt, B.A. Makwana, D.J. Vyas, D.R. Mishra, V.K. Jain, Selective recognition by novel calix system:ICT based chemosensor for metal ions. J. Lumin. 146 (2014) 450–457. DOI:10.1016/j.jlumin.2013.10.004 |
| [27] | K.D. Bhatt, D.J. Vyas, B.A. Makwana, S.M. Darjee, V.K. Jain, Highly stable water dispersible calix. Spectrochim. Acta Part A:Mol. Biomol. Spectrosc. 121 (2014) 94–100. DOI:10.1016/j.saa.2013.10.076 |
| [28] | D.J. Vyas, B.A. Makwana, H.S. Gupte, K.D. Bhatt, V.K. Jain, An efficient one pot synthesis of water-dispersible calix. J. Nanosci. Nanotechnol. 12 (2012) 3781–3787. DOI:10.1166/jnn.2012.5837 |
| [29] | Y. Zhou, J. Yoon, Recent progress in fluorescent and colorimetric chemosensors for detection of amino acids. Chem. Soc. Rev. 41 (2012) 52–67. DOI:10.1039/C1CS15159B |
| [30] | H.B. Li, Y.H. Bian, Selective colorimetric sensing of histidine in aqueous solutions using cysteine modified silver nanoparticles in the presence of Hg2+. Nanotechnology 20 (2009) 145502. DOI:10.1088/0957-4484/20/14/145502 |
| [31] | D.R. Bae, W.S. Han, J.M. Lim, Lysine-functionalized silver nanoparticles for visual detection and separation of histidine and histidine-tagged proteins. Langmuir 26 (2009) 2181–2185. |
| [32] | D.J. Xiong, M.L. Chen, H.B. Li, Synthesis of para-sulfonatocalix[4] arene-modified silver nanoparticles as colorimetric histidine probes, Chem. Commun. (2008) 880-882. |
| [33] | H.B. Li, F.Y. Li, C.P. Han, Highly sensitive and selective tryptophan colorimetric sensor based on 4, 4-bipyridine-functionalized silver nanoparticles. Sens. Actuators B:Chem. 145 (2010) 194–199. DOI:10.1016/j.snb.2009.11.062 |
| [34] | Z. Huang, J. Du, J. Zhang, X.Q. Yu, L. Pu, A simple and efficient fluorescent sensor for histidine. Chem. Commun. 48 (2012) 3412–3414. DOI:10.1039/c2cc17156b |
| [35] | J.E. Miller, C. Grădinaru, B.R. Crane, Spectroscopy and reactivity of a photogenerated tryptophan radical in a structurally defined protein environment. J. Am. Chem. Soc. 125 (2003) 14220–14221. DOI:10.1021/ja037203i |
| [36] | S. Diem, J. Bergmann, M. Herderich, Tryptophan-N-glucoside in fruits and fruit juices. J. Agric. Food Chem. 48 (2000) 4913–4917. DOI:10.1021/jf0003146 |
| [37] | A. Özcan, Y. Şahin, A novel approach for the selective determination of tryptophan in blood serum in the presence of tyrosine based on the electrochemical reduction of oxidation product of tryptophan formed in situ on graphite electrode, Biosens. Bioelectron. 31(2012) 26-31. |
| [38] | M.L. Bishop, E.P. Fody, L.E. Schoef, Clinical Chemistry:Principles, Techniques, and Correlations, 7th ed., Lippincott Williams & Wilkins, New York, 2013p. 205. |
| [39] | M. Rai, A. Yadav, A. Gade, Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 27 (2009) 76–83. DOI:10.1016/j.biotechadv.2008.09.002 |
| [40] | H.J. Klasen, A historical review of the use of silver in the treatment of burns II. Renewed interest for silver, Burns 26(2000) 131-138. |
| [41] | J.J. Castellano, S.M. Shafii, F. Ko, Comparative evaluation of silver-containing antimicrobial dressings and drugs. Int. Wound J. 4 (2007) 114–122. DOI:10.1111/iwj.2007.4.issue-2 |
| [42] | S.M. Darjee, D.R. Mishra, K.D. Bhatt, A new colorimetric and fluorescent chemosensor based on thiacalix[4] arene for fluoride ions. Tetrahedron Lett. 55 (2014) 7094–7098. DOI:10.1016/j.tetlet.2014.10.149 |
| [43] | E.A. Yushkova, I.I. Stoikov, P-tert-butyl thiacalix[4] arenes functionalized with amide and hydrazide groups at the lower rim in cone, partial cone, and 1, 3-alternate conformations are "smart" building blocks for constructing nanosized structures with metal cations of s-, p-, and d-elements in the organic phase, Langmuir 25(2009) 4919-4928. |
| [44] | I.I. Stoikov, E.A. Yushkova, I. Zharov, I.S. Antipin, A.I. Konovalov, Supramolecular self-assemblies of stereoisomers of p-tert-butyl thiacalix[4] arenes functionalized with hydrazide groups at the lower rim with some metal cations. Tetrahedron 65 (2009) 7109–7114. DOI:10.1016/j.tet.2009.06.045 |
| [45] | M. Grzelczak, L.M. Liz-Marzán, The relevance of light in the formation of colloidal metal nanoparticles. Chem. Soc. Rev. 43 (2014) 2089–2097. DOI:10.1039/C3CS60256G |
| [46] | M. Shen, W.F. Chen, Y. Sun, C.G. Yan, Synthesis and characterization of watersoluble gold colloids stabilized with aminoresorcinarene. J. Phys. Chem. Solids 68 (2007) 2252–2261. DOI:10.1016/j.jpcs.2007.06.007 |
| [47] | T.T. Li, N.Y. He, J.H. Wang, Effects of the i-motif DNA loop on the fluorescence of silver nanoclusters. RSC Adv. 6 (2016) 22839–22844. DOI:10.1039/C5RA22489F |
| [48] | D. Zhou, X.M. Lin, A.L. Wang, Fluorescence enhancement of Tb3+ complexes by adding silica-coated silver nanoparticles. Sci. China Chem. 58 (2015) 979–985. DOI:10.1007/s11426-014-5265-x |
| [49] | N. Wangoo, K.K. Bhasin, S.K. Mehta, C.R. Suri, Synthesis and capping of waterdispersed gold nanoparticles by an amino acid:bioconjugation and binding studies. J. Colloid Interface Sci. 323 (2008) 247–254. DOI:10.1016/j.jcis.2008.04.043 |
| [50] | S. Pal, Y.K. Tak, J.M. Song, Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 73 (2007) 1712–1720. DOI:10.1128/AEM.02218-06 |
| [51] | J.S. Kim, E. Kuk, K.N. Yu, Antimicrobial effects of silver nanoparticles. Nanomed.:Nanotechnol. Biol. Med. 3 (2007) 95–101. DOI:10.1016/j.nano.2006.12.001 |
| [52] | S.H. Jun, S. Cho, Y. Park, Functionalization of lysostaphin on gold and silver nanoparticles and their in vitro antibacterial activities against methicillin-resistant Staphylococcus aureus. Nanosci. Nanotechnol. Lett. 7 (2015) 433–440. DOI:10.1166/nnl.2015.1954 |
| [53] | S.P. Shukla, M. Roy, P. Mukherjee, Size selective green synthesis of silver and gold nanoparticles:enhanced antibacterial efficacy of resveratrol capped silver sol. J. Nanosci. Nanotechnol. 16 (2016) 2453–2463. DOI:10.1166/jnn.2016.10772 |
 2017, Vol. 28
2017, Vol. 28