b China-America Cancer Research Institute, Key Laboratory for Medical Molecular Diagnostics of Guangdong Province, Guangdong Medical University, Dongguan 523808, China;
c College of Chemistry & Environment Protection Engineering, Southwest University for Nationalities, Chengdu 610041, China
Carbon nanotubes (CNTs) have attracted much attention because of their unique electronic, mechanical, and thermal properties [1, 2]. They have been used in many fields including nanoscience, nanotechnology, and bioengineering [3-8]. The decoration of CNTs with magnetic nanoparticles, e.g., the coating or loading of CNTs with Fe/Co/Ni oxides, can improve or modify the optical, magnetic and electrochemical properties of the CNTs [9-16]. These advantages have motivated the expansion of studies on magnetic nano-composites, especially on magnetic CNTs. The exceptional electromagnetic properties and unique structures of magnetic nanotubes also have potential applications in magnetically guided drug delivery systems [17] and other fields.
Among the available magnetic materials, Fe oxides are particularly attractive because of their low cost, especially compared to Ni-and Co-based materials, and low toxicity. Fe oxides are strong candidates for the preparation of magnetic composites materials for industrial scale applications [18, 19]. To date, several approaches have been developed for the synthesis of magnetic Fe oxide/CNT composites including chemical precipitation [20], plasma treatment [21], chemical vapor deposition [22], combustion [23], spray pyrolysis [24], hydrothermal/solvothermal methods [25], and self-assembly methods [12]. The methods currently used for the synthesis of Fe oxide/CNTs, however, have some critical disadvantages including the following: (1) several methods suffer from high economic and energy costs [26, 27]; (2) other methods are relatively complicated and require the use of organic solvents, which are unsuitable for industrial applications [19, 28]. These disadvantages have likely slowed the widespread adoption of Fe oxide/CNT composites for practical applications. Therefore, the development of a simple, cost-effective, timesaving, and environmentally friendly method for the synthesis of magnetically separable Fe oxide/CNT composites is of vital practical significance.
Hydrothermal methods have been used to synthesize Fe3O4 nanoparticles in mild organic solvent-free systems [29]. This process is simple and has a low environmental impact. The following steps occur during the typical synthesis of a Fe3O4/CNT composite (Scheme 1): (Step 1) Fe2+ ions are first bound to the CNTs' functional groups, forming a nucleation site for iron oxide crystals. (Step 2) A precipitate gradually forms in an alkaline media. (Step 3) The solution is then sealed in an autoclave for hydrothermal reactions.
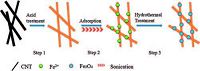
|
Download:
|
| Scheme 1. The illustration of Fe3O4/CNT composite synthesis. | |
To promote a more uniform dispersion of nucleation centers on the CNTs, ultrasonication was performed during the precipitation process (Step 2) in attempt to synthesize materials with appropriately sized crystalline phases [30]. This combined ultrasonication-assisted hydrothermal method (UAHM) is a straightforward and low-cost approach for the preparation of magnetite nanoparticles/CNT composites (Scheme 2). However, the effect of ultrasonication on Fe3O4/CNTs composite synthesis has not previously been determined. In this work, the effect of the duration of ultrasonicationon on the crystal structure, magnetic performance, and chemical composition of the composite was investigated. The morphological, microstructural, and magnetic properties of the obtained nanoparticles were studied using X-ray diffraction (XRD), transmission electron microscopy (TEM), and Fourier transform infrared spectroscopy (FTIR).
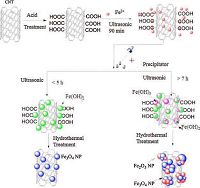
|
Download:
|
| Scheme 2. The formation mechanism of magnetite CNT composites. | |
2. Experimental
The CNTs were obtained from the Chengdu Organic Chemical limited Company in China, with nanotube's exterior diameter of 20-30 nm. All reactants were of analytical grade and were used without further purification.
The synthesis process for the decoration of magnetic components on CNT surfaces is as the follows: CNT samples were pretreated in concentrated nitric acid (68 wt%) at 140 ℃ for 2 h to remove the metal catalyst and amorphous carbon species. The purified CNTs were then washed with distilled water, dried at 60 ℃ under careful stirring in a water bath and finally dried under vacuum at 60 ℃ for 12 h before further use. Purified CNTs (0.5 g) were added to an aqueous solution of ferrous chloride (0.1 mol/L, 40 mL) and ultrasonicated for 90 min at room temperature to form a homogeneous solution. Aqua ammonia (3:1) was then added into a mixed solution in a drop-wise fashion under stirring until the pH value of the mixture was approximately 10. The mixture was ultrasonicated under stirring for a variable period of time (t=1, 3, 5, 7 and 9 h). The solution was then transferred to a Teflon-lined autoclave, which was heated to 160 ℃ in a blast oven for 12 h before being cooled to room temperature. The resulting black magnetic composites were then separated via several rounds of magnetic decantation. The composites were then washed with ethanol and deionized water until the solution reached a neutral pH. The product was dried at 60 ℃ under careful stirring in a water bath to prevent CNTs' aggregations and form bulks. Finally, the black powdered composites were dried under vacuum at 60 ℃ for 24 h. The as-prepared magnetic composites (MCs) are denoted as MC-1, 2, 3, 5, 7 and 9 for the different ultrasonication time of 1, 2, 3, 5, 7, and 9 h, respectively.
TEM were performed with a JEM-2000 FX transmission electron microscope operating at 200 kV (JEOL Ltd.). The samples were ultrasonically dispersed in ethanol and placed onto a carbon film supported by a copper grid. XRD analysis was conducted using a DX-2700 diffractometer operated at 40 kV and 30 mA (Philips Company). FTIR was performed using a Bruker Tensor 27 Fourier transform spectrometer (Bruker Corporation). The powdered samples were ground with KBr and compressed into a pellet. FTIR spectra from 4000-400 cm-1 were recorded in order to investigate the nature of the chemical bonds formed. Vibrating sample magnetometry (VSM) measurements were performed using a vibrating sample magnetometer (MPMS-XL-7 SQUID, The America Quantum Design Company). The magnetization measurements were carried out in an external field of up to 20 kOe at a temperature of 300 K.
3. Results and discussionThe microstructure of the as-prepared magnetite/CNT composites was characterized by TEM. Fig. 1a shows the purified CNT samples with an exterior diameter of 20-30 nm. Fig. 1b-f reveal the morphology of all magnetic composites: intertwined network of CNTs coated with nanoparticles, the particles were anchored onto the outwall of the CNTs. Fig. 1g-i show the high resolution transmission electron microscopy (HRTEM) images of sample MC-5, in which particles with diameters of 20-50 nm grew on the CNTs. The lattice spacing of the particles shown in Fig. 1i was 2.52 Å, which is in accordance with the typical lattice value of magnetite (3 1 1), revealing the crystalline nature of the Fe3O4 nanoparticles. The typical lattice value of 3.4 Å is ascribed to the graphite (0 0 2), revealing the nature of the CNT shell.
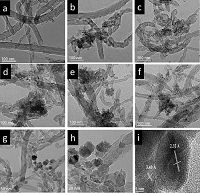
|
Download:
|
| Figure 1. The TEM micrographs of (a) purified CNTs in; (b-f) the samples of MC-1, 3, 5, 7, and 9, respectively; (g, h) the sample MC-5, in which nanoparticles grow along the CNTs walls; and (i) the crystalline interplanar spacing of the particles. | |
The field dependence of the magnetization was explored at room temperature (300 K), and the results are shown in Fig. 2a. The composites exhibited an "S" shape with a remnant magnetization (Mr=0 emu/g) and coercive field (Hc=0 Oe), suggesting that these samples were superparamagnetic [31]. The saturation magnetization of the as-prepared samples ranged from 3.73 to 30.88 emu/g. These values were smaller than that of the bulk magnetite (92 emu/g) [32], because the nanometer size of the Fe3O4 as well as the disordered spin and spin canting effects affected the magnetization [33, 34].
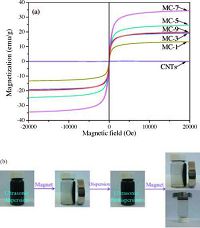
|
Download:
|
| Figure 2. (a) Magnetization-magnetic field curve of magnetite particles obtained at room temperature; (b) the photographs of CNTs/Fe3O4 nanocomposites dispersed in aqueous solution in the absence and presence of the application of an external magnet. | |
The saturation magnetization of CNT was 0.10 emu/g, which may be resulted from the residual catalysts, such as Ni, Fe species that exhibited weak magnetic responses. The saturation magnetization of samples MC-1, 3 and 5 were 23.45, 26.53 and 30.88 emu/ g, respectively. In comparison, the decoration of magnetic particles onto CNTs' walls could effectively improve the magnetic responses. However, the saturation magnetization decreased to 27.85 and 3.73 emu/g with the increasing of ultrasonication time to 7 and 9 h, respectively. It indicates that the duration of ultrasonication time lead to the changes in the magnetic performances of the MC samples.
Optical photographs of the magnetic CNT composites dispersed in water with or without a magnet are shown in Fig. 2b. The composites were easily dispersed in water to form a homogeneous black solution as shown in Fig. 2b. When an external magnetic field was applied, the composites were separated from the solution within ten seconds. After removing the magnetic field, the composites were placed into the ultraphonic batches and shaken for about 5-10 s, the composites were easily re-dispersed in solvents. The process of magnetism-driven aggregation and redispersion was repeatable, indicating that the aggregation and dispersion of the Fe3O4/CNT composites in water could be controlled using an external magnetic field.
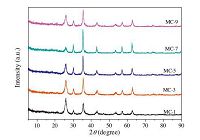
Fig. 3 shows the XRD patterns of the Fe3O4 nanoparticles, the Fe2O3 nanoparticles, and the as-prepared composites. For each sample, six characteristic peaks were observed at 2θ=30.2°, 35.5°, 43.3°, 53.7°, 57.4°, 62.7°, which can be indexed to the (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), (4 4 0) planes of face-centered cubic Fe3O4 (JCPDS 19-0629), suggesting that all of the composites contained Fe3O4 nanoparticles [35]. The diffraction peaks at 26.0° corresponded to the (0 0 2) plane of the hexagonal graphite structure of pure CNTs.
|
Download:
|
| Figure 3. XRD powder pattern of Fe3O4, α-Fe2O3 and the magnetic carbon nanotube composites. | |
Moreover, an interesting phenomenon was observed in the XRD pattern of MC-9, the additional peaks at 2θ=24.1°, 35.5°, 40.7°, 49.2°, and 63.9° in sample MC-9 were associated with the (0 1 2) (1 1 0) (1 1 3) (0 2 4) (4 4 1) planes of α-Fe2O3 (JCPDS 33-0664), suggesting that a longer ultrasonication time resulted in the formation of α-Fe2O3 [35]. The results suggests that the product MC-1, 3, 5, 7 is composed of two phases: α-Fe3O4 and CNTs; and MC-9 is composed of three phases: Fe3O4, α-Fe2O3, and CNTs.
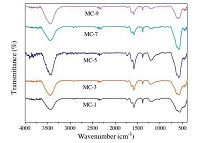
In addition, for all samples, the peaks located at -590 cm-1 corresponded to Fe-O stretching, which was indicative of the Fe-O-Fe in Fe3O4 [36]. For samples MC-7 and MC-9, the band at 460 cm-1 corresponded to Fe2O3. Therefore, samples MC-1, MC-3, and MC-5 were Fe3O4/CNT composites, while samples MC-7 and MC-9 were Fe3O4/a-Fe2O3/CNT composites.
The characteristic signals of Fe2O3 were not detected in the XRD spectra of MC-7, but they were observed in the FTIR spectra, which may be a result of the fact that the very small-sized particles did not form an obvious crystal phase, resulting in an atypical XRD spectrum of α-Fe2O3.
The FT-IR results and the XRD patterns proved that the increases of the ultrasonication time will lead to the gradual formation of the α-Fe2O3/Fe3O4/CNT composites rather than the Fe3O4/CNT composites (Fig. 4).
|
Download:
|
| Figure 4. FT-IR spectra of the magnetite/carbon nanotube composites. | |
In has been reported that in aqueous solutions of ferric salt, hydroxylation of the ferric ions occurred, which led to the formation of an amorphous ferrihydrite phase that transformed into aquo complexes ([Fe (H2O)6]2+) [37, 38]. The nitric acid-treated CNTs contained sufficient number of functional groups, e.g., hydroxyl, carboxyl, and carbonyl groups [39]. These functional groups on CNTs served as the nucleation sites for these complexes. After the addition of a precipitator, aquo complexes formed to produce Fe (OH)2. In our work, in the first 5 h of ultrasonication, an increase in the ultrasonication time caused Fe2+ complexes precipitates anchored onto the CNTs' walls. Iron oxide is formed by further condensation of Fe-OH to Fe-O-Fe and the crystals of Fe3O4 form during the hydrothermal reaction. However, when the ultrasonication time exceeded 7 h, the Fe2+ on the top layer of the precipitates was gradually oxidized into Fe3+ and the Fe2+/Fe3+ ratio decreased. Meanwhile, with the duration time of ultrasonication increases, more Fe2+ was transformed to Fe3+. Consequentially, Fe2O3 and Fe3O4 crystalline phases both appeared in samples MC-7 and MC-9.
4. ConclusionIn summary, the UAHM was shown to be a simple and environmentally friendly method to synthesize magnetite/CNT composites with potential industrial applications. However, the ultrasonication time was a critical factor that affected the structure and magnetic performance of the MC composites. By controlling the ultrasonication time, the crystal phase structure of Fe oxide could be selectively modulated and the magnetic performance of the MCs could be effectively tuned.
AcknowledgmentThis work was supported by The National Natural Science Foundation of China (Nos. 21406039, 21506174) and the Project of Postgraduate Degree Construction, Southwest University for Nationalities (No. 2015XWD-S0703).
| [1] | S. Iijima, Helical microtubules of graphitic carbon. Nature 354 (1991) 56–58. DOI:10.1038/354056a0 |
| [2] | M.F. Ran, W. Chu, J. Wen, Y.F. Li, Promoting effects of chromium on Ni/MgO catalysts for CNTs synthesis by chemical vapor deposition method. Chem. J. Chin. Univ. 30 (2009) 231–235. |
| [3] | J.F. Ren, S. Shen, D.G. Wang, The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials 33 (2012) 3324–3333. DOI:10.1016/j.biomaterials.2012.01.025 |
| [4] | Y.G. Wang, L. Shi, L. Gao, The removal of lead ions from aqueous solution by using magnetic hydroxypropyl chitosan/oxidized multiwalled carbon nanotubes composites. J. Colloid Interface Sci. 451 (2015) 7–14. DOI:10.1016/j.jcis.2015.03.048 |
| [5] | M. Yadav, K.Y. Rhee, S.J. Park, D. Hui, Mechanical properties of Fe3O4/GO/chitosan composites. Compos. Part B-Eng. 66 (2014) 89–96. DOI:10.1016/j.compositesb.2014.04.034 |
| [6] | P.F. Zong, S.F. Wang, Y.L. Zhao, Synthesis and application of magnetic graphene/iron oxides composite for the removal of U (VI) from aqueous solutions. Chem. Eng. J. 220 (2013) 45–52. DOI:10.1016/j.cej.2013.01.038 |
| [7] | Y. Zhang, Y.H. Bai, B. Yan, Functionalized carbon nanotubes for potential medicinal applications. Drug Discov. Today 15 (2010) 428–435. DOI:10.1016/j.drudis.2010.04.005 |
| [8] | M.H. Yeh, Y.S. Li, G.L. Chen, Facile synthesis of boron-doped Graphene nanosheets with hierarchical microstructure at atmosphere pressure for metalfree electrochemical detection of hydrogen peroxide. Electrochim. Acta 172 (2015) 52–60. DOI:10.1016/j.electacta.2015.01.210 |
| [9] | Y.J. Yao, S.D. Miao, S.Z. Liu, Synthesis, characterization, and adsorption properties of magnetic Fe3O4@graphene nanocomposite, Chem. Eng. J. 184 (2012) 326–332. |
| [10] | R.H. Wu, J.H. Liu, L.Q. Zhao, Hydrothermal preparation of magnetic Fe3O4@C nanoparticles for dye adsorption. J. Environ. Chem. Eng. 2 (2014) 907–913. DOI:10.1016/j.jece.2014.02.005 |
| [11] | N.S. Ye, Y.L. Xie, P.Z. Shi, T. Gao, J.C. Ma, Synthesis of magnetite/graphene oxide/chitosan composite and its application for protein adsorption. Mat. Sci. Eng. C 45 (2014) 8–14. DOI:10.1016/j.msec.2014.08.064 |
| [12] | M.A. Salam, R.M. El-Shishtawy, A.Y. Obaid, Synthesis of magnetic multi-walled carbon nanotubes/magnetite/chitin magnetic nanocomposite for the removal of Rose bengal from real and model solution. J. Ind. Eng. Chem. 20 (2014) 3559–3567. DOI:10.1016/j.jiec.2013.12.049 |
| [13] | S. Qu, J. Wang, J.L. Kong, P.Y. Yang, G. Chen, Magnetic loading of carbon nanotube/nano-Fe (3) O (4) composite for electrochemical sensing. Talanta 71 (2007) 1096–1102. DOI:10.1016/j.talanta.2006.06.003 |
| [14] | C. Luo, Z. Tian, B. Yang, L. Zhang, S.Q. Yan, Manganese dioxide/iron oxide/acid oxidized multi-walled carbon nanotube magnetic nanocomposite for enhanced hexavalent chromium removal. Chem. Eng. J. 234 (2013) 256–265. DOI:10.1016/j.cej.2013.08.084 |
| [15] | T. Hao, X.H. Rao, Z.J. Li, Synthesis of magnetic separable iron oxide/carbon nanocomposites for efficient adsorptive removal of Congo red. J. Alloys Comp. 617 (2014) 76–80. DOI:10.1016/j.jallcom.2014.07.111 |
| [16] | Q. Liu, J.Q. Tian, W. Cui, Carbon nanotubes decorated with CoP Nanocrystals:a highly active non-noble-metal nanohybrid electrocatalyst for hydrogen evolution. Angew. Chem. 126 (2014) 6828–6832. DOI:10.1002/ange.201404161 |
| [17] | C. Sengiz, G. Congur, E. Eksin, A. Erdem, Multiwalled Carbon Nanotubes-Chitosan Modified single-use biosensors for electrochemical monitoring of drug-DNA interactions. Electroanalysis 27 (2015) 1855–1863. DOI:10.1002/elan.v27.8 |
| [18] | J. Ma, F. Yu, Z.H. Wen, A facile one-pot method for synthesis of low-cost iron oxide/activated carbon nanotube electrode materials for lithium-ion batteries. Dalton Trans. 42 (2013) 1356–1359. DOI:10.1039/C2DT31887C |
| [19] | M.Y. Zhu, G.W. Diao, Review on the progress in synthesis and application of magnetic carbon nanocomposites. Nanoscale 3 (2011) 2748–2767. DOI:10.1039/c1nr10165j |
| [20] | Y. Cao, Preparation and magnetic properties of a multi-walled carbon nanotubeiron oxide nanoparticle composite. Fuller. Nanotub. Carbon Nanostruct. 23 (2014) 623–626. |
| [21] | P. Clément, I. Hafaiedh, E.J. Parra, Iron oxide and oxygen plasma functionalized multi-walled carbon nanotubes for the discrimination of volatile organic compounds. Carbon 78 (2014) 510–520. DOI:10.1016/j.carbon.2014.07.032 |
| [22] | Z.H. Wang, Z.D. Zhang, C.J. Choi, B.K. Kim, Structure and magnetic properties of Fe (C) and Co (C) nanocapsules prepared by chemical vapor condensation. J. Alloys Comp. 361 (2003) 289–293. DOI:10.1016/S0925-8388(03)00441-9 |
| [23] | J. Borysiuk, A. Grabias, J. Szczytko, Structure and magnetic properties of carbon encapsulated Fe nanoparticles obtained by arc plasma and combustion synthesis. Carbon 46 (2008) 1693–1701. DOI:10.1016/j.carbon.2008.07.011 |
| [24] | J.B. Park, S.H. Jeong, M.S. Jeong, J.Y. Kim, B.K. Cho, Synthesis of carbon-encapsulated magnetic nanoparticles by pulsed laser irradiation of solution. Carbon 46 (2008) 1369–1377. DOI:10.1016/j.carbon.2008.05.011 |
| [25] | W.X. Li, B.L. Lv, L.C. Wang, G.M. Li, Y. Xu, Fabrication of Fe3O4@C core-shell nanotubes and their application as a lightweight microwave absorbent. RSC Adv. 4 (2014) 55738–55744. DOI:10.1039/C4RA10172C |
| [26] | F. Batmanghelich, M. Ghorbani, Effect of pH and carbon nanotube content on the corrosion behavior of electrophoretically deposited chitosan-hydroxyapatitecarbon nanotube composite coatings. Ceram. Int. 39 (2013) 5393–5402. DOI:10.1016/j.ceramint.2012.12.046 |
| [27] | J. Ma, J.N. Wang, Purification of single-walled carbon nanotubes by a highly efficient and nondestructive approach. Chem. Mater. 20 (2008) 2895–2902. DOI:10.1021/cm8001699 |
| [28] | F. Yu, J.H. Chen, L. Chen, Magnetic carbon nanotubes synthesis by Fenton's reagent method and their potential application for removal of azo dye from aqueous solution. J. Colloid Interface. Sci. 378 (2012) 175–183. DOI:10.1016/j.jcis.2012.04.024 |
| [29] | D.H. Guan, Z. Gao, W.L. Yang, Hydrothermal synthesis of carbon nanotube/cubic Fe3O4 nanocomposite for enhanced performance supercapacitor electrode material. Mat. Sci. Eng. B 178 (2013) 736–743. DOI:10.1016/j.mseb.2013.03.010 |
| [30] | H. Sayahi, M.A. Kiani, S.H. Kazemi, Ultrasonic-assisted synthesis of magnetite/carbon nanocomposite for electrochemical supercapacitor. J. Solid State Electrochem. 18 (2014) 535–543. DOI:10.1007/s10008-013-2289-7 |
| [31] | Z.Y. Sun, Z.M. Liu, Y. Wang, Fabrication and characterization of magnetic carbon nanotube composites. J. Mater. Chem. 15 (2005) 4497–4501. DOI:10.1039/b509968d |
| [32] | H. Setyawan, F. Fajaroh, W. Widiyastuti, One-step synthesis of silica-coated magnetite nanoparticles by electrooxidation of iron in sodium silicate solution. J. Nanopart. Res. 14 (2012) 807. DOI:10.1007/s11051-012-0807-7 |
| [33] | K. Bubke, H. Gnewuch, M. Hempstead, J. Hammer, M.L.H. Green, Optical anisotropy of dispersed carbon nanotubes induced by an electric field. Appl. Phys. Lett. 71 (1997) 1906–1908. DOI:10.1063/1.119976 |
| [34] | J.Q. Wan, W. Cai, J.T. Feng, X.X. Meng, E.Z. Liu, In situ decoration of carbon nanotubes with nearly monodisperse magnetite nanoparticles in liquid polyols. J. Mater. Chem. 17 (2007) 1188–1192.. DOI:10.1039/b615527h |
| [35] | H.T. Cui, Y. Liu, W.Z. Ren, Structure switch between α-Fe2O3, γ-Fe2O3 and Fe3O4 during the large scale and low temperature sol-gel synthesis of nearly monodispersed iron oxide nanoparticles. Adv. Powder Technol. 24 (2013) 93–97. DOI:10.1016/j.apt.2012.03.001 |
| [36] | D.N. Huang, X.Y. Wang, C.H. Deng, Facile preparation of raisin-bread sandwich-structured magnetic graphene/mesoporous silica composites with C18-modified pore-walls for efficient enrichment of phthalates in environmental water. J. Chromatogr. A 1325 (2014) 65–71. DOI:10.1016/j.chroma.2013.12.025 |
| [37] | J.P. Jolivet, C. Chane ác, E. Tronc, Iron oxide chemistry. From molecular clusters to extended solid networks, Chem. Commun. (2004) 481-483. |
| [38] | W.J. Yu, L.L. Zhang, P.X. Hou, et al., High reversible lithium storage capacity and structural changes of Fe2O3 nanoparticles confined inside carbon nanotubes, Adv. Energy Mater. 6(2016), http://dx.doi.org/10.1002/aenm.201501755. |
| [39] | W.J. Sun, Z.Q. Liu, C.F. Jiang, Experimental and theoretical investigation on the interaction between palladium nanoparticles and functionalized carbon nanotubes for Heck synthesis. Catal. Today 212 (2013) 206–214. DOI:10.1016/j.cattod.2012.09.024 |
 2017, Vol. 28
2017, Vol. 28 


