b ICPEES, L'Institut de Chimie et Procedes pour l'energie, l'environnement et la santé, Université de Strasbourg, Strasbourg 67087, France;
c Department of Chemical Engineering, Sichuan University, Chengdu 610065, China
The hydrogenation of cinnamaldehyde (CAL) is a reaction of interest in the fine chemical industry because, when performed selectively, it can yield the unsaturated alcohol, which is widely used in for fragrance purposes.
Supported Au catalysts have been shown to be active and selective for the hydrogenation of cinnamaldehyde [1].The aspect concerning the Au particle size on the activity of unsaturated alcohol was found that CAL conversion and hydrogenation of C=O bond are strongly dependent on the Au particle size, normally, the higher catalytic performances of gold catalyst associated with the smaller active size [1, 2]. Supported Cu catalysts have attracted the researcher's interest due to the low cost of copper. It was found that Cu supported on reducible oxides (CeO2 and Fe2O3) are considered as suitable candidate to hydrogenate the C=O bond due to the presence of metal-support interfacial sites resulting from the presence of (Ce4+/Ce3+ or Fe2+/Fe3+) [3].
From thermodynamic considerations, hydrogenation of the C=C bond is favored as compared to hydrogenation of the C=O bond. In order to catalytically favor the selective hydrogenation of the C=O bond to the desired unsaturated alcohol product, attempts have been made to take advantage of synergistic effects of bimetallic catalysts. The incorporation of Fe into Ir/TiO2 with an optimized Fe/Ir atomic ratio led to an increase in activity [4]. A similar result with the addition of Co to Pt supported on graphite was observed, i.e. an improved selectivity toward cinnamyl alcohol and an enhanced cinnamaldehyde conversion [5]. Zhang et al. reported that Co-Pt and Cu-Pt bimetallic catalysts displayed a higher hydrogenation activity than the corresponding monometallic catalysts. Besides, they pointed out that bimetallic Co-Pt showed much higher selectivity toward C=O bond hydrogenation than Cu-Pt [6]. The enhanced catalytic behavior of PdAu/MCM-41 as compared with the monometallic Pd was ascribed to the synergistic effect between Pd and the added Au [7]. Few reports involve the utilization of Au-based bimetallic catalysts for CAL hydrogenation [7]. Therefore, in this work, we extended the application of the bimetallic Au-Cu systems described in the previous work [8] to the hydrogenation of CAL under a mild condition atmospheric pressure. We show that, although cinnamyl alcohol (COL) is never observed under our conditions[9-11], pretreatment and composition of the Au-Cu system affect the catalytic properties of the material for CAL hydrogenation by preparing three nominal compositions of the Au-Cu systems on CeO2.
2. Experimental 2.1. Catalyst preparationThe supported bimetallic Au-Cu catalysts were prepared by a two-step method as described in our previous work [8, 12]. The gold loading was fixed at 2% and the amount of copper was chosen in order to vary the Au/Cu atomic ratio, abbreviated as AuxCuy (Au/Cu=3/1, 1/1, and 1/3). In the first step, supported gold was prepared by Direct Anionic Exchange (DAE) as mentioned above. In a second step, the copper was loaded by the impregnation method. Finally, a fraction of catalyst precursor was calcined under air at 300 ℃ for 4 h. The samples were named as AuxCuy/CeO2-C. The other fraction of the catalyst precursor was reduced in a furnace under a flowing 50% H2/He gas mixture with a flow rate of 50 mL/min, heated to 300 ℃ at a rate of 10 ℃/min, and held for 2 h. The samples were named as AuxCuy/CeO2-R. Monometallic gold or copper was loaded by DAE and impregnation, respectively.
2.2. Catalyst characterizationInductively coupled plasma emission spectroscopy (ICP-AES) was performed at the CNRS center of Chemical Analysis (Solaize, France) to determine the gold and copper loadings of the calcined catalysts. ICP results reveal that the actual Au loading on CeO2 is very close to 2% in all cases.
The BET surface area, pore volume, and average pore size of the catalysts were determined by nitrogen adsorption-desorption at 77 K (liquid nitrogen temperature) using a Micromeritics ASAP 2010 apparatus. Prior to the nitrogen adsorption experiment, the samples were outgassed at 200 ℃ for 1-2 h in order to desorb impurities and moisture. The average pore size is found to be about 12 -2 nm for the catalysts. The total pore volume is accordingly in the ceria-based catalysts (0.25 -0.05 cm3/g). The surface area calculated by the BET method (Brunauer-Emmet-Teller) for the reduced bimetallics is c.a. 80 m2/g, generally higher than that of the calcined samples (70 m2/g), but systematically lower than that of the supported monometallics (90 m2/g).
Analyses of the core-shell structure were performed using a high-angle annular dark-field scanning TEM (HAADF-STEM) technique. Photographs and maps were taken on the same microscope at an accelerating voltage of 200 kV. In this case, few mg of catalyst were dispersed in ethanol and a drop was deposited on a holey Mo grid.
2.3. Catalytic measurementsThe hydrogenation of cinnamaldehyde was conducted in a round-bottom flask at atmospheric pressure and low temperature, i.e. 70 ℃. The concentration of reactants and the stirring speed were variable. Influence of solvents, such as ethanol and dioxane were investigated in order to increase the ability of reactant diffusion approaching the catalyst. Hydrogen was allowed to bubble through the stirred liquid-phase at a flow rate of 60 mL/min for 30 min at r.t. in order to remove traces of dissolved oxygen in the medium, before introducing the catalyst. The hydrogen stream was regulated by a Brooks 5850 TR mass flow meter linked to a Brooks 5876 control unit. In the meantime, an oil bath was heated to 70 ℃. After the temperature of the bath reached 70 ℃, the round-bottom flask was introduced into the heated oil, which determined the start (t=0) of the catalytic reaction.
The cinnamaldehyde concentration and the product distribution were followed as a function of time by gas chromatography (GC) analyses of microsamples (1 μL) periodically withdrawn and diluted with 2 mL of ethanol or dioxane. GC analysis was performed on a Varian 3400-CX gas chromatograph equipped with a PONA capillary column coated with methyl siloxane (Hewlett-Packard, length 50 m, 0.2 mmi.d., film thickness 0.5 mm) and a flame ionization detector (FID). Calibration was performed by injecting cinnamaldehyde (Alfa Aesar, purity > 98%), as well as the three different possible products of reaction, namely cinnamyl alcohol (ACROS, purity > 98%), 3-phenylpropyl alcohol (ACROS, purity > 98%), and 3-phenylpropionaldehyde (ACROS, purity > 98%). All compounds were diluted in a dioxane or ethanol solution (concentration). The error on quantification by using this external calibration method was estimated to be within 5%.
Conversion (X%) and selectivity to the various products observed were calculated from GC analysis, as follows:
| $X\%=100\text{ }\times \text{ }\left( 1-\text{ }\frac{{{\text{A}}_{\text{CAL}}}}{{{\text{A}}_{\text{CAL}}}\text{ }~\text{ + }~\text{ }{{\text{A}}_{\text{HCAL}}}\text{ }~\text{ + }~\text{ }{{\text{A}}_{\text{HCOL}}}\text{ }~\text{ + }~\text{ }{{\text{A}}_{\text{CO}}}} \right)$ |
| $S\%=\text{ }100\text{ }\times \text{ }\frac{{{\text{C}}_{\text{HCAL}}}}{X}~$ |
| ${{\text{C}}_{\text{HCAL}}}\%=\frac{\text{100 }\times \text{ }{{\text{A}}_{\text{HCAL}}}}{{{\text{A}}_{\text{CAL}}}\text{ }~\text{ + }{{\text{A}}_{\text{HCAL}}}\text{ }~\text{ + }{{\text{A}}_{\text{HCOL}}}\text{ }~\text{ + }{{\text{A}}_{\text{COL}}}}~$ |
CHCAL is the concentration of HCAL. It is noted that in our case, there is no identification of cinnamyl alcohol (COL) possibly due to the limited analysis conditions [13].
3. Results and discussion 3.1. Reaction parameters (stirring speed, initial concentration of cinnamaldehyde, and solvent) on the catalytic behavior of Au-Cu/ CeO2 catalystsThe dependence of the stirring speed on cinnamaldehyde conversion and selectivity toward HCAL is shown in Table 1. With an increase in the stirring speed from 400 rpm to 700 rpm, cinnamaldehyde conversion increases by about 45%, from about 9 to 13%. No further improvement but a slight decrease is observed with further increasing the stirring speed to 1000 rpm. Generally, the stirring speed has a major role in reactant diffusion. Hence, this study suggests that, while the conversion observed at 400 rpm is by diffusion limitations, 700 rpm is an optimum stirring speed in that it allows to achieve good mass transfer and to get a high CAL conversion. The gain in conversion observed between 400 rpm and 700 rpm is associated with an increase in selectivity toward HCAL, from 70% to 80%.
Table 1 presents the catalytic performances of Au/CeO2 at different initial concentration of the substrate. The conversion decreases as the initial concentration of cinnamaldehyde increases, which is consistent with the reported results reported by Kapteijn et al. who found that when the initial concentration of cinnamaldehyde increased from 0.7 mol/L to 3 mol/L, CAL conversion decreased largely on carbon supported Pt catalyst. In our case, when the initial concentration was varied from 0.008 mol/L to 0.4 mol/L, the reaction rate of CAL hydrogenation is different, changing from 48.2 molCAL/molmetal/h to 4.4 molCAL/molmetal/h. This indicates that the initial CAL concentration has a negative effect on CAL conversion and positive effect on reaction rate. Generally, the effect of reactant concentration on the reactivity including conversion and selectivity is depended upon the chosen range of substrate concentration and catalyst preparation. For CAL concentration at 3.78 × 10-1 and 4 × 10-2, the selectivity toward HCAL is almost the same, but it is higher than that of concentration at 8 × 10-3. This study reveals that the lower substrate concentration favors the contact of CAL with the catalyst by increasing the diffusion of reactants. Taking into account this point, a concentration at 8 × 10-3 was chosen for the subsequent studies. The hydrogenation of cinnamaldehyde was conducted in polaraprotic (dioxane) and protic (ethanol) solvents. The physical properties of solvents (dioxane and ethanol) lead to a marked effect on CAL conversion and HCAL selectivity. Reaction rate of cinnamaldehyde hydrogenation in dioxane is much higher than in ethanol. However, selectivity toward HCAL is higher in ethanol for the bimetallic Au1Cu1/CeO2-C catalyst. Because of the higher HCAL selectivity, ethanol was chosen as the solvent of choice in further studies.
|
|
Table 1 Cinnamaldehyde conversion and selectivity to HCAL at different stirring speeds and initial concentration of cinnamaldehyde (reaction conditions: 4 × 10-2 mol/L, 0.1 g catalyst, 100mL ethanol, T=75 ℃, t=7 h). |
3.2. Catalytic activities of AuxCuy/CeO2 catalysts
Fig. 1 shows the evolution of CAL conversion as a function of time for bimetallic AuxCuy/CeO2 catalysts with different atomic ratios. All the catalysts exhibit a similar trend, that is, the CAL conversion increases gradually with reaction time; the selectivity toward HCAL remain almost unchanged during the investigated reaction time. It can be seen that the conversion of CAL on monometallic Au/CeO2 (≈37%) is higher than that on CuO/CeO2 catalyst (≈27%) when reaction time is 7 h. However, catalyst Au/ CeO2 gives only about 60% selectivity toward HCAL, which is much lower than that on CuO/CeO2 catalyst (≈90%). Therefore, it seems that Au/CeO2 catalyst is a better candidate to perform good CAL conversion while CuO/CeO2 catalyst is more suitable to enhance the selectivity toward HCAL, that is to say, the promotion of the hydrogenation of C=C bond.

|
Download:
|
| Figure 1. CAL conversion and selectivity of HCAL on AuxCuy/CeO2 catalysts. (Reaction conditions: 700 rpm, 0.1 g catalyst, 100 mL ethanol, T=75 ℃.). | |
CAL conversion on a series of bimetallic Au-Cu/CeO2 catalyst seems a bit complex because it depends upon the Au/Cu atomic ratio and thermal pretreatments. However, the addition of Cu to Au leads to a decrease in CAL conversion and an increase in selectivity toward HCAL in spite of which type of pretreatment. This behavior is likely due to the addition of Cu leading to a decrease in a fraction of Au sites available for hydrogenation and consequently CAL conversion becomes lower. Conversely, because Cu itself can promote the selective hydrogenation of C=C bond, a higher selectivity toward HCAL comparing to monometallic Au/CeO2 is reasonably observed. The aspect concerning the influence of catalyst precursor pretreatment on CAL conversion was found that the three investigated atomic ratios of AuxCuy/CeO2-R always displays the higher CAL conversion than that of AuxCuy/CeO2-C catalysts during the measured reaction time. In terms of selectivity toward HCAL, only when atomic ratio of Au/Cu is 1/3, reduction pretreatment leads to a higher selectivity comparing to that of the calcined catalyst. The other two atomic ratio of Au/Cu (3/1 and 1/1) give almost the equal selectivity under both pretreatments.
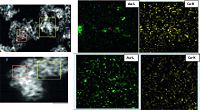
It has been previously demonstrated that pretreatments (calcination or reduction) lead to significant Au-Cu bimetallic structures [8]. XRD and HRTEM results of Au1Cu3/CeO2-R implied a new phase of a formation of Au-Cu alloy comparison to Au1Cu3/ CeO2-C. XPS results have revealed that a rich Cu phase on the calcined bimetallic Au1Cu3 nanoparticles, indicating that surface distribution of Au and Cu are not homogeneous. To further support this point, results of STEM-HAADF for Au1Cu3/CeO2 under calcination or reduction are shown in Fig. 2. It can be seen that in the chosen red zone, Au signal is weaker than that of Cu signal for Au1Cu3/CeO2-C sample, indicating a heterogeneous distribution of Au and Cu. On the contrary, in the case of Au1Cu3/CeO2-R catalyst, Au signal is enhanced and it is close to Cu signal resulting from a very homogeneous Au and Cu distribution. The higher CAL conversion and HCAL selectivity on reduced Au1Cu3/CeO2 could be related with formation of an Au-Cu alloy resulting in the more distribution of Au exposed on the catalyst external surface, not covered by copper comparing to the calcined bimetallic Au1Cu3/ CeO2.
|
Download:
|
| Figure 2. STEM-HAADF of Au1Cu3/CeO2-C (A, C, and D) and of Au1Cu3/CeO2-R (B, E, and F). | |
4. Conclusion
In this work, catalysts AuxCuy/CeO2 were evaluated for hydrogenation of cinnamaldehyde under a mild condition namely atmosphere. HCAL was obtained as the main selective product. CAL concentration, the stirring speed, nature of the solvent, and structure of the catalyst influence the catalytic activities. Pretreatments lead to a major effect on CAL conversion and HCAL selectivity. Catalysts AuxCuy/CeO2pretreated under reduction display higher CAL conversion than that of under calcination mainly due to the synergistic effect resulting in a formation of Au-Cu alloy.
AcknowledgmentWe gratefully acknowledge the Chinese Scholarship Council (CSC), French Eiffel Scholarship for financial supports of Xuemei Liao as well as the financial supported by the Open Research Subject of key laboratory (Research Base) of Grain and Oil Engineering and food safety (No. szjj2015-006), Agricultural and Forestry Talents in Food quality and safety, Key Research Fund of Xihua University (No. Z1520527).
| [1] | Z.M. Tian, X. Xiang, L.S. Xie, F. Li, Liquid-phase hydrogenation of cinnamaldehyde:enhancing selectivity of supported gold catalysts by incorporation of cerium into the support. Ind. Eng. Chem. Res. 52 (2013) 288–296. |
| [2] | H.N. Chen, D.A. Cullen, J.Z. Larese, Highly efficient selective hydrogenation of cinnamaldehyde to cinnamyl alcohol over gold supported on zinc oxide materials. J. Phys. Chem. C 119 (2015) 28885–28894. DOI:10.1021/acs.jpcc.5b07823 |
| [3] | V. Gutierrez, M. Alvarez, M.A. Volpe, Liquid phase selective hydrogenation of cinnamaldehyde over copper supported catalysts, Appl. Catal. A:Gen. 413-414(2012) 358-365. |
| [4] | P. Reyes, H. Rojas, J.L.G. Fierro, Effect of Fe/Ir ratio on the surface and catalytic properties in citral hydrogenation on Fe-Ir/TiO2 catalysts. J. Mol. Catal. A:Chem. 203 (2003) 203–211. DOI:10.1016/S1381-1169(03)00263-2 |
| [5] | W. Koo-amornpattana, J.M. Winterbottom, Pt and Pt-alloy catalysts and their properties for the liquid-phase hydrogenation of cinnamaldehyde. Catal. Today 66 (2001) 277–287. DOI:10.1016/S0920-5861(00)00654-4 |
| [6] | R.G. Zhang, H.Y. Liu, B.J. Wang, L.X. Ling, Insights into the effect of surface hydroxyls on CO2 hydrogenation over Pd/γ-Al2O3 catalyst:a computational study. Appl. Catal. B:Environ. 126 (2012) 108–120. DOI:10.1016/j.apcatb.2012.07.009 |
| [7] | X. Yang, D. Chen, S.J. Liao, High-performance Pd-Au bimetallic catalyst with mesoporous silica nanoparticles as support and its catalysis of cinnamaldehyde hydrogenation. J. Catal. 291 (2012) 36–43. DOI:10.1016/j.jcat.2012.04.003 |
| [8] | X.M. Liao, W. Chu, X.Y. Dai, V. Pitchon, Bimetallic Au-Cu supported on ceria for PROX reaction:effects of Cu/Au atomic ratios and thermal pretreatments, Appl. Catal. B:Environ. 142-143(2013) 25-37. |
| [9] | L. Truong-Phuoc, T. Truong-Huu, L. Nguyen-Dinh, Silicon carbide foam decorated with carbon nanofibers as catalytic stirrer in liquid-phase hydrogenation reactions. Appl. Catal. A:Gen. 469 (2014) 81–88. DOI:10.1016/j.apcata.2013.09.032 |
| [10] | I. Florea, M. Houllé, O. Ersen, Selective deposition of palladium nanoparticles inside the bimodal porosity of β-SiC investigated by electron tomography. J. Phys. Chem. C 113 (2009) 17711–17719. DOI:10.1021/jp905968n |
| [11] | J.P. Tessonnier, L. Pesant, G. Ehret, M.J. Ledoux, C. Pham-Huu, Pd nanoparticles introduced inside multi-walled carbon nanotubes for selective hydrogenation of cinnamaldehyde into hydrocinnamaldehyde. Appl. Catal. A:Gen. 288 (2005) 203–210. DOI:10.1016/j.apcata.2005.04.034 |
| [12] | X.M. Liao, V. Caps, W. Chu, V. Pitchon, Highly stable bimetallic Au-Cu supported on Al2O3 for selective CO oxidation in H2-rich gas:effects of Cu/Au atomic ratio and sensitive influence of particle size. RSC Adv. 6 (2016) 4899–4907. DOI:10.1039/C5RA22550G |
| [13] | C. Pham-Huu, N. Keller, G. Ehret, Carbon nanofiber supported palladium catalyst for liquid-phase reactions:an active and selective catalyst for hydrogenation of cinnamaldehyde into hydrocinnamaldehyde. J. Mol. Catal. A:Chem. 170 (2001) 155–163. DOI:10.1016/S1381-1169(01)00055-3 |
 2017, Vol. 28
2017, Vol. 28