b College of Chemistry and Materials Engineering, Quzhou University, Quzhou 324000, China
Dye-sensitized solar cells (DSSCs) have attracted much attention due to their low material cost, flexibility, easy manufacturing process, and high efficiency [1-3]. To date, DSSCs based on Ru complexes have an overall solar energy conversion efficiency (η) approaching 12%. On the other hand, organic dye sensitizers provide the advantages of high molar extinction efficiencies, customized molecular designs, cost effectiveness, and environmental friendliness, which have been extensively investigated. [2-5] Recently, novel organic dyes based on coumarin [6], carbazole [7], indoline [8], phenothiazine [9], and phenoxazine [10] have been investigated as sensitizers for DSSCs, and solar cells based on triphenylamine have been reported [11] with an energy conversion efficiency exceeding 10%.
The general strategy in the design of highly efficient metal-free dye sensitizers is to design novel structures of D-π-A molecules, especially in electron donor (D) and π-conjugated bridges. The structures of the chromophore donor, bridge linker, and acceptor can be modified independently to tune the energy levels of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) [5, 11]. In D-π-A systems, the use longer π-conjugation dye molecules is generally intended to achieve a larger absorption maximum and extend the absorption region. However, long conjugation dye molecules are unstable under irradiation with high-energy photons and prone to unfavorable π-π aggregation [12]. To avoid the aggregation of dyes, organic dyes with D-D-π-A structures, in which two D moieties are connected with a π-bridge, have recently been reported [4, 13]. These studies suggested that organic dyes with DD-π-A structures might achieve better performances than those with the simple D-π-A structures.
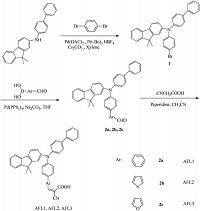
Electron donors containing triarylamine moieties have been widely investigated because of their prominent electron-donating ability and hole-transport properties; these donors also prevent the direct charge recombination between TiO2 and I3 -by covering TiO2 surfaces with a bulky aryl group [4, 11]. We [14] and other researchers [4, 15-17] reported novel organic dyes containing a triarylamine unit. Fluorene-based organic dyes are highly stable under UV irradiation and heating conditions; these dyes can also suppress dye aggregation and disrupt molecular stacking because of their nonplanarity [18-20]. In addition to the arylamine moiety (D), an electron-rich biphenyl unit acts as a secondary electron donor; as a result, a D-D-π-A molecular configuration is formed. The π-conjugated bridge connecting the donor and the acceptor in the dye sensitizer influences not only the region of light absorbed by DSSCs but also the degree of electron injection from the excited state of the dye to the TiO2 surface. Therefore, varying the π-bridges has been demonstrated as an effective strategy to obtain high short-circuit photocurrent density (JSC) and open-circuit voltage (VOC) [5, 18, 21-24]. This study synthesized a series of three novel organic dye sensitizers (AFL1-AFL3) consisting of fluorenyl, the biphenyl moieties of triarylamine, and the different π-conjugated linkers of benzene, thiophene, and furan to improve the power conversion efficiency and to investigate the influence of the π-bridge. This study also examined the photophysical and electrochemical properties and photovoltaic performance of DSSCs. The synthetic procedures are shown in Scheme 1.
|
Download:
|
| Scheme 1. Synthesis of AFL1, AFL2, and AFL3. | |
2. Experimental 2.1. Materials and instruments
1H and 13C NMR spectra were recorded by using a spectrometer at 400 and 125 MHz, respectively. Mass spectra were obtained using a Waters Xevo Q-Tof mass spectrometer. Absorption spectra were determined by using a Shimadzu UV spectrophotometer (model UV2=0). Fluorescence spectra were obtained using a Shimadzu RF-5301PC spectrofluorometer.
Reactions were performed under nitrogen atmosphere. The solvents and chemicals used in this study were of analytical grade and used without further purification.
2.2. Fabrication of DSSCsFTO glass slides were cleaned with a detergent solution in an ultrasonic bath for 15 min and then rinsed with water and ethanol. Two different titanium dioxide pastes were deposited by screen-printing to fabricate photoelectrodes; as a result, 0.16 cm2 TiO2 electrodes with light-scattering anatase particles were formed. The total thickness of the TiO2 film was 18 mm. The TiO2 electrodes were gradually heated in an air flow at 325 ℃ for 5 min, at 375 ℃ for 5min, at 450 ℃ for 15min, and at 500 ℃ for 15 min. Afterward, the electrodeswere cooled to 80 ℃, immersed in the dye solution in a mixture of THF and CHCl3 (THF:CHCl3=1:10), and kept at room temperature for 24 h to assure complete dye uptake. The electrodes were then rinsed with THF to remove excess dye. Counter electrodes were prepared by screen-printing a 50 nm Pt layer on the cleaned FTO plates. Open cellswere fabricated in air by clamping different photoelectrodes with platinized counter electrodes. The active area of DSSCswas 0.16 cm2. The electrolyte OPV-AN-I used in this study was contained 0.07 mmol L-1 I-(Yingkou Opvtech New Energy Co., Ltd.).
2.3. Photovoltaic characterizationThe photovoltaic performance of DSSCs was evaluated at AM 1.5 G illumination (100mW cm-2; Peccell-L15, Peccell, Japan) by using a Keithley digital source meter (Keithley 2601, USA). The incident light intensity was 100 mW/cm2 calibrated with a standard Si solar cell (BS-520, Japan). The action spectra of the monochromatic incident photon-to-current conversion efficiency (IPCE) of the solar cells were obtained by using a commercial setup (PEC-S20 IPCE Measurement System, Peccell, Japan). Electrochemical impedance spectroscopy (EIS) experiments were conducted using a computer-controlled potentiostat (Zenium Zahner, Germany). The measured frequency ranged from 100 to 1 MHz, and the AC amplitude was set to 10 mV. The bias of the EIS measurements was set to VOC of the corresponding dye sensitizer. Cyclic voltammetry was recorded with a computer-controlled electrochemical analyzer (IviumStat, Holland) at a constant scan rate of 100 mV s-1. Measurements were performed with a threeelectrode cell in 0.1 mol L-1 Bu4NClO4-N, N-dimethylformamide solution, Pt wire as a counter electrode, and an Ag/AgCl reference electrode; the measurements were then calibrated with ferrocene.
2.4. Detailed experimental procedures and characterization dataSynthesis of compound 2-amino-N-[(1, 10-biphenyl)-4-yl]-N-(4-bromophenyl)-9, 9-dimethylfluorene 1: A mixture of 1, 4-dibromobenzene (0.2 g, 0.8 mmol), 2-(4-biphenylyl) amino-9, 9-dimethylfluorene (0.36 g, 1 mmol), Pd (OAc)2 (0.046 g, 0.2 mmol), P (t-Bu)3 (0.04 g, 0.2 mmol), and Cs2CO3 (1.63 g, 5 mmol) in dry toluene (15 mL) was stirred and heated at 110 ℃ overnight. After the mixture was cooled to room temperature, saturated ammonium chloride solution was added to the reaction solution. The solution was extracted with dichloromethane, dried with MgSO4, and subjected to column chromatography (silica gel, dichloromethane/ hexanes=3:1). A yellow solid was obtained with the following properties: yield, 57% (0.24 g); mp 160.1-161.5 ℃; 1H NMR [400 MHz, (CD3)2CO]: δ 7.64 (d, 1H, J=8 Hz), 7.60-7.57 (m, 3H), 7.50-7.48 (m, 2H), 7.43-7.40 (m, 2H), 7.38-7.26 (m, 5H), 7.25 (s, 1H), 7.20 (s, 1H), 7.17 (s, 1H), 7.15 (s, 1H), 7.06-7.02 (m, 3H), 1.42 (s, 6H). HRMS: m/z calcd. for C33 H26 Br N: 516.1321 [M-H]-; Found: 516.1337.
Synthesis of compound 2a: A mixture of 1 (0.52 g, 0.1 mmol), 4-formylphenylboronic acid (0.18 g, 0.12 mmol), Pd (PPh3)4 (0.14 g, 0.012 mmol), K2CO3 (0.14 g, 1 mmol), and degassed water (2 mol/ L) in dry THF (15 mL) was refluxed overnight. After the mixture was cooled to room temperature, the solution was extracted with dichloromethane, dried with MgSO4, and subjected to column chromatography (silica gel, PE:EA=5:1). A light yellow solid was obtained. Yield: 68% (0.37 g). mp 95.9-97.7 ℃. 1H NMR [400 MHz, (CD3)2CO]: δ 10.0 (s, 1H), 7.92 (d, 2H, J=8 Hz), 7.75 (d, 2H, J=8 Hz), 7.64-7.52 (m, 7 H), 7.40 (q, 2H, J=4 Hz and 12 Hz), 7.32-7.23 (m, 10H), 7.13-7.11 (m, 1H), 1.44 (s, 6H); HRMS: m/z calcd. for C40 H31 NO: 542.2478 [M-H]-; Found: 542.2488.
Synthesis of compound 2b: A mixture of 1 (0.52 g, 0.1 mmol), 5-formylthiophene-2-boronic acid (0.19 g, 0.12 mmol), Pd (PPh3)4 (0.14 g, 0.012 mmol), K2CO3 (0.14 g, 1 mmol), and degassed water (2 mol/L) in dry THF (15 mL) was refluxed overnight. After the mixture was cooled to room temperature, the solution was extracted with dichloromethane, dried with MgSO4, and subjected to column chromatography (silica gel, PE:EA=15:1). A yellow solid was obtained. Yield: 49% (0.27 g).mp102.8-104.1 ℃. 1H NMR[400 MHz, (CD3)2CO]: δ 9.85 (s, 1H), 7.71 (δ, 1H, J=4 Hz), 7.67-7.53 (m, 7H), 7.43 (q, 3H, J=4 Hz and 12 Hz), 7.34-7.22 (m, 8H), 7.17 (d, 2H, J=8 Hz), 7.12 (d, 1H, J=8 Hz), 1.45 (s, 6H); HRMS: m/z calcd. for C38 H29 NOS: 548.2043 [M-H]-; Found: 548.2019.
Synthesis of compound 2c: A mixture of 1 (0.52 g, 0.1 mmol), 2-formylfuran-5-boronic acid (0.16 g, 0.12 mmol), Pd (PPh3)4 (0.14 g, 0.012 mmol), K2CO3 (0.14 g, 1mmol), anddegassedwater (2 mol/L) in dry THF (15 mL) was refluxed overnight. After the mixture was cooled to room temperature, the solution was extracted with dichloromethane, dried with MgSO4, and subjected to column chromatography (silica gel, PE:EA=3:1). A yellow solid was obtained. Yield: 47% (0.25 g).mp101.6-102.5 ℃. 1HNMR [400MHz, (CD3)2CO]: δ 9.59 (s, 1H), 7.69 (d, 4H, J=8 Hz), 7.66 (d, 1H, J=8 Hz), 7.61 (d, 2H, J=8 Hz), 7.53 (d, 2H, J=8 Hz), 7.42 (q, 2H, J=8 Hz and 16 Hz), 7.34-7.22 (m, 7H), 7.19 (s, 1H), 7.16 (s, 1H), 7.11 (d, 1H, J=8 Hz), 6.72 (d, 1H, J=4 Hz), 1.43 (s, 6H); HRMS: m/z calcd. for C38 H29NO2: 532.2271 [M-H]-; Found: 532.2278.
Synthesis of compound AFL1: 2a (0.54 g, 0.1 mmol) was mixed with cyanoacetic acid (0.02 g, 0.23 mmol) in dry acetonitrile (15 mL) and piperidine (0.1 mL). The solution was refluxed overnight. The solvent was removed in vacuo. The pure product AFL1 was obtained through column chromatography (silica gel, CH2Cl2:CH3OH:HAc=300:10:1). The following properties were observed: yield, 45% (0.27 g). mp 206.8-208.6 ℃. 1H NMR [400 MHz, (CD3)2CO]: δ 7.96 (s, 1H), 7.94 (d, 2H, J=4 Hz), 7.82-7.72 (m, 5H), 7.68-7.65 (m, 4H), 7.53 (d, 1H, J=4 Hz), 7.45 (t, 2H, J=8 Hz), 3.37-7.26 (m, 5H), 7.16 (q, 4H, J=8 Hz and 16 Hz), 7.10-7.07 (m, 1H), 1.40(s, 6H); 13C NMR (125 MHz, CDCl3) : δ 24.96, 26.97, 46.72, 95.00, 107.47, 112.46, 114.61, 119.53, 119.53, 119.91, 121.58, 122.23, 122.91, 122.92, 122.98, 123.30, 123.31, 124.27, 124.30, 126.43, 126.44, 127.35, 127.95, 127.95, 127.99, 128.06, 129.16, 129.16, 130.29, 131.90, 134.81, 134.87, 138.32, 139.64, 141.77, 144.97, 145.03, 146.29, 146.61, 150.91, 153.49, 1=.26, 165.70; HRMS: m/z calcd. for C43H31N2O2: 607.2453 [M-H]-; Found: 607.2477.
Synthesis of compound AFL2: 2b (0.=g, 0.1 mmol) was mixed with cyanoacetic acid (0.02 g, 0.23 mmol) in dry acetonitrile (15 mL) and piperidine (0.1 mL). The solution was refluxed overnight. The solvent was removed in vacuo and the residue. The pure product AFL2 was obtained through column chromatography (silica gel, CH2Cl2:CH3OH:HAc=300:10:1). Yield: 37% (0.23 g). mp 207.9-208.6 ℃. 1H NMR (125 MHz, CDCl3) : δ 7.65 (d, 1H, J=8 Hz), 7.62(d, 1H, J=8 Hz), 7.60-7.57 (m, 3H), 7.52 (d, 2H, J=8 Hz), 7.44-7.38 (m, 4H), 7.33-7.28 (m, 2H), 7.26 (m, 6H), 7.21 (s, 1H), 7.16 (s, 1 H), 7.13 (s, 1H), 7.11 (q, 1H, J=4 Hz and 8 Hz), 1.43 (s, 6H); 13C NMR (100MHz, CDCl3) : δ 24.90, 26.93, 46.84, 94.99, 108.85, 119.51, 119.57, 119.60, 119.89, 119.91, 119.96, 121.53, 121.57, 122.87, 122.90, 122.94, 122.96, 124.53, 124.66, 126.40, 126.40, 126.83, 127.16, 127.19, 127.21, 127.94, 127.96, 129.15, 129.15, 134.98, 135.56, 138.26, 139.26, 146.01, 146.31, 147.92, 148.54, 154.42, 153.49, 1=.26, 164.20; HRMS: m/z calcd. for C41H29N2O2S: 607.2453 [M-H]-; Found: 607.2477.
Synthesis of compound AFL3: A mixture of 2c (0.=g, 0.1 mmol) and cyanoacetic acid (0.02 g, 0.23 mmol) in dry acetonitrile (15 mL) and piperidine (0.1 mL) was prepared. The solution was refluxed overnight. The solvent was removed in vacuo and the residue. Then, the pure product AFL3 was obtained through column chromatography (silica gel, CH2Cl2:CH3OH:AcOH=400:20:1). Yield: 48% (0.28 g). mp 238.6-240.18C. 1H NMR (400 MHz, CDCl3) : δ 7.66-7.64 (m, 2H), 7.63 (s, 2H), 7.60 (s, 2H), 7.58 (s, 1H), 7.52 (s, 1H), 7.50 (s, 1H), 7.44-7.38 (m, 4H), 7.33-7.26 (m, 3H), 7.22 (s, 1H), 7.20 (s, 1H), 7.18 (s, 1 H), 7.16 (s, 1H), 7.10 (d, 1H, J=8 Hz), 7.02 (s, 1H), 6.76 (d, 1H, J=4 Hz), 1.42 (s, 6H); 13C NMR (125 MHz, CDCl3) : δ 22.99, 26.84, 46.57, 107.70, 107.90, 119.40, 119.47, 119.58, 119.85, 119.89, 121.44, 121.47, 121.48, 122.66, 122.82, 124.42, 124.42, 125.79, 125.81, 125.82, 126.34, 126.34, 127.06, 127.22, 127.23, 127.85, 127.87, 129.04, 129.04, 134.97, 135.07, 138.21, 139.56, 145.91, 146.20, 147.83, 148.36, 154.42, 1=.20, 156.11, 162.47; HRMS: m/z calcd. for C41H29N2O3: 597.2184 [M-H]-; Found: 597.2209.
3. Results and discussion 3.1. SynthesisThree novel triarylamine dye sensitizers consisting of cyanoacetic acid as an electron acceptor and fluorenyl and biphenyl moieties in the donor with various conjugated linkers were prepared. The synthetic routes are shown in Scheme 1. In a typical Ullmann reaction, 1 was obtained from 2-(4-biphenylyl) amino-9, 9-dimethylfluorene and 1, 4-dibromobenzene with a good yield. Then, the following Suzuki coupling reaction between 1 and arylboronic acids yielded the corresponding aldehydes 2a-2c. The three dye sensitizers were produced from 2a to 2c after Knoevenagel condensation reaction occurred with cyanoacetic acid in the presence of piperidine. The compounds and three new organic sensitizers (AFL1-AFL3) were confirmed through NMR and MS.
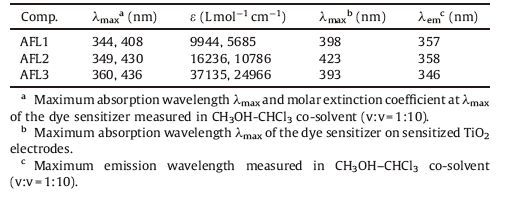
3.2. Photophysical propertiesThe UV-vis absorption spectra of AFL1-AFL3 recorded in CHCl3-CH3OH at room temperature are displayed in Fig. 1a, and the corresponding data are summarized in Table 1. The absorption spectra of the triarylamine dye sensitizers display two distinct absorption peaks. The band located at the shorter wavelength was attributed to the π-π* electron transition of the chromophore. The absorption band at 300-391 nm can be ascribed to localized aromatic π-π* transitions. The longest absorption peak at approximately 391-600 nm could be attributed to the intramolecular charge transfer (ICT) from the triarylamine donor to the cyanoacetic acid acceptor mixed with delocalized π-π* transition.

|
Download:
|
| Figure 1. UV-vis spectra of triarylamine dyes. (a) The UV-vis absorption spectra of AFL1-AFL3 recorded in CHCl3-CH3OH at room temperature. (b) The absorption spectra of AFL1-AFL3 adsorbed on thin transparent films. | |
|
|
Table 1 Optical properties of triarylamine dyes. |
The maximum absorption peaks of the dye sensitizers are observed in the following order: AFL3 > AFL2 > AFL1. AFL3 displays the broadest absorption band and evident red-shifted absorption spectra compared with the other dye sensitizers. This finding could occur possibly because the introduction of thiophene (or benzene) and triphenylamine may twist the molecule; thus, dihedral angles were formed between the donor and the thiophene (or benzene) moiety, and the conjugation of the whole molecule was decreased. Among these dye sensitizers, AFL3 exhibits the highest molar extinction coefficient; this finding could be obtained because the resonance energy of furan (16 kcal mol-1) is smaller than that of thiophene (29 kcal mol-1) and benzene (36 kcal mol-1). As a result, the molecule undergoes effective conjugation and decreases the energy of ICT [18, 22, 25, 26].
The absorption spectra of AFL1-AFL3 adsorbed on thin transparent films are shown in Fig. 1b. The maximum absorption peaks of AFL1-AFL3 on the TiO2 films are located at 398, 423, and 393 nm that correspondingly blue shifted at 10, 7, and 43 nm from the solution spectra, respectively. The blue shift of the absorption spectra of AFL1-AFL3 on TiO2 could be ascribed to the deprotonation of carboxylic acid once these sensitizers adsorbed on the TiO2 surface and H-aggregates formed.
Compared with AFL1 and AFL2, AFL3 exhibits a longer blueshift. This may be caused by the increase of coplanarity between the triarylamine moiety and the electron acceptor due to the introduction of the furan unit. Similar phenomena have been observed for several other organic dyes [26-28]. Similar to the findings in the absorption in the solution, the results revealed that AFL3 yielded the highest absorbance. AFL2 showed lower absorbance than AFL1 does when bound to the TiO2 film, probably because of AFL2 aggregation, which may reduce the light absorptivity. AFL2 exhibited a broader absorption band on TiO2.
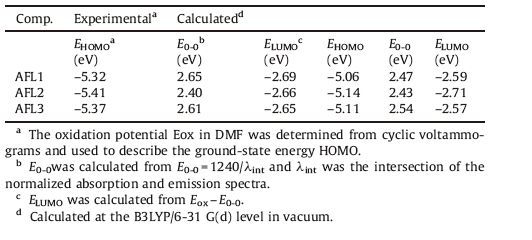
Cyclic voltammograms (CV) were performed to evaluate the thermodynamically allowed electron transfer processes from the excited dye molecule to the conduction band of TiO2, and the related data were shown in Table 2. As we can see, the lowest unoccupied molecular orbital (LUMO) levels of these dyes are in the range of -2.69 eV to -2.65 eV, which are higher than the TiO2 conduction band (CB) energy level (-4.0 eV), implying that electron injection from the excited dye into the conduction band of TiO2 is energetically permitted. In addition, the highest occupied molecular orbital (HOMO) level of dyes, ranging from -5.41 to -5.32 eV, which are lower than the energy level of the redox couple I-/I3- (-4.9 eV), indicating that the oxidized dyes formed after electron injection into the conduction band of TiO2 could thermodynamically accept electrons from I-ions.
|
|
Table 2 Electrochemical properties of triarylamine dyes. |
3.3. Computational studies
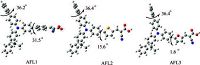
The geometry of the dye sensitizer was optimized by the B3LYP hybrid density functional method in conjunction with the dpolarized 6-31G (d) basis set implemented in the Gaussian 03 program [29] to obtain insights into the relationship between structural and photophysical properties. The wave function was then analyzed by Multiwfn [30]. The fully optimized geometrical structures of the three dye sensitizers are shown in Fig. 2. In the ground state, the geometry of the dye sensitizer is not planar. Fig. 2 displays the dihedral angles between the neighboring units in the conjugated spacer. For AFL1-AFL3, the ground-state structures of the dyes between two benzene units in the donor are characterized by twist angles of 36.28, 36.48, and 36.48. The twist angle in the donor corresponds to a less planar configuration. The dihedral angles between a donor and a bridge (benzene, thiophene, or furan) are 31.58, 15.68, and 1.68, respectively. Inserting a furan unit instead of a thiophene (or benzene) moiety close to the acceptor can twist the whole molecule to a less extent, because the steric hindrance of a small oxygen atom is less pronounced [31, 32]. Thus, the conjugation of AFL3 is increased to some extent.
|
Download:
|
| Figure 2. Optimized geometries (side view) of triarylamine dyes at the B3LYP/6-31G (d) level. | |
The HOMO energy level shown in Fig. 3 is located at -5.14 eV to -5.06 eV, which is lower than the potential of the redox couple I-/I3- (-4.9 eV). The LUMO energy level is concentrated on -2.71 eV to -2.57 eV and higher than the TiO2 CB energy level (-4.0 eV). This means that these dyes can favorably inject the electrons into the CB edge of TiO2, and the oxidized dyes can be reduced by the electrolyte successfully. The HOMOs of AFL2 and AFL3 are farther from the TiO2 CB when compared to that of AFL1, which will reduce charge recombination between the oxidized sensitizer and the injected electrons in TiO2 conduction band. Further, theLUMOlevel of AFL3 is higher than that of AFL1 and AFL2, indicating that AFL3 dye has more efficient electron injection, in accordance with the geometrical and experimental result.

|
Download:
|
| Figure 3. The energy of the frontier molecular orbitals of triarylamine dyes at B3LYP/6-31G (d) in vacuo. | |
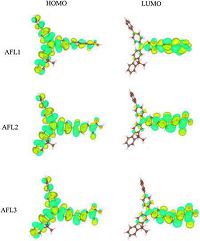
The electronic distribution in the frontier molecular orbitals HOMO and LUMO is shown in Fig. 4. The HOMO of the dye sensitizer is delocalized over the whole molecule. Conversely, the LUMO of the dye sensitizer is mainly found on the π-bridge and the acceptor; the latter favors an efficient electron transfer from the excited state of the dye sensitizer to the TiO2 conduction band edge.
|
Download:
|
| Figure 4. Electronic distributions in the frontier molecular orbitals of the triarylamine dyes. | |
3.4. Electrochemical properties
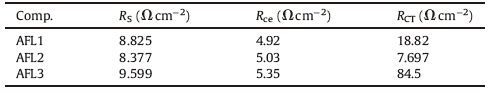
Electrochemical impedance spectroscopy (EIS) analysis was performed to investigate the electron recombination in the DSSCs sensitized by the triarylamine dye sensitizer. The Nyquist plots and Bode plots of the DSSCs in forward bias with a frequency range of 0.1 Hz-100 kHz are shown in Fig. 5a. The Bode phase plots are also shown in Fig. 5b. The reaction resistance of DSSCs was analyzed with ZSimpWin by using an equivalent circuit (Fig. 5c) containing a constant phase element (CPE) and resistance (R). In the equivalent circuit, RS corresponds to the overall series resistance; Rct and Rce represent charge transfer resistances at the photoanode/electrolyte interface and the counter electrode (CE), respectively. Rs and Rct are listed in Table 2. The DSSCs show similar series resistances of approximately 8.8-9.6 Ω because of the same surface area, electrolyte, and electrode in the materials.

|
Download:
|
| Figure 5. Electrochemical impedance spectra of DSSCs based on triarylamine dyes. (a) The Nyquist plots and Bode plots of the DSSCs in forward bias. (b) The Bode phase plots. (c) The reaction resistance of DSSCs. | |
Two semicircles were observed in the Nyquist plots. The small semicircle at a high frequency (1-100 kHz) is assigned to the redox charge transfer response at the Pt/electrolyte interface. The large semicircle at the intermediate frequency (1 Hz --1 kHz) represents the charge transfer resistances (Rct) at the photoanode/ electrolyte interface; a large Rct corresponds to a more difficult electron recombination from the conduction band to the electrolyte [14]. In Fig. 5a and Table 2, the semicircle of AFL3 at intermediate frequency is the largest among the semicircles of the three triarylamine dye sensitizers. This finding indicated that AFL3 exhibited the longest charge recombination lifetime and the highest VOC with furan as a bridge. Although AFL2 with a thiophene bridge yielded the least charge recombination, this sensitizer obtained only a moderate VOC because of its lower electron injection efficiency. Therefore, we conjecture that electron injection efficiency rather than charge recombination play the primary role in VOC among these dyes [33].
In the Bode phase plots, the peak position of the middle frequency is related to the lifetime of electrons; for example, a shift to a low frequency corresponds to a long electron lifetime. The Bode phase plots shown in Fig. 5b support the differences in the electron lifetime of TiO2 films derived using the three dyes. These plots further support the order of VOC of DSSCs based on these dye sensitizers.
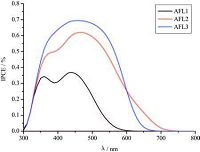
3.5. Photovoltaic performance of DSSCsThe IPCE action spectra of DSSCs based on triarylamine dye sensitizers were obtained in an AM 1.5 G irradiation of 100 mW cm-2 (Fig. 6). AFL3-sensitized DSSCs display a higher IPCE than AFL1 does because of the introduction of furan to the pconjugation system. AFL3-sensitized DSSC exhibits an IPCE exceeding 60% from 380 to 539 nm and a maximum of 70% at 460 nm; its photocurrent signal reaches approximately 700 nm possibly because of its highest absorbance and broad absorption spectrum compared to the other tested dyes. The IPCE values of AFL3 are the highest in awide range of spectra; thisfinding indicated that thisdye likely generates a large photocurrent. With the highest IPCE and almost the broadest spectra of the IPCE values, AFL3-sensitized TiO2 electrode possibly exhibits a higher conversion yield than the other dye sensitizersdo.However, AFL2possesses a broader spectral range than AFL1 and AFL3, which confirms that the electron-rich thiophene is beneficial to the broadening of the spectrum [34].
|
Download:
|
| Figure 6. IPCE curves of the DSSCs based on triarylamine dyes. | |
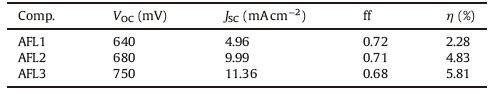
The photocurrent density-photovoltage (J-V) curves of the DSSCs based on triarylamine dye sensitizers are shown in Fig. 7. Key parameters, including short-circuit current density (JSC), open-circuit voltage (VOC), fill factor (ff), and overall power conversion efficiency (η), are summarized in Table 3. JSC is related to the molar extinction coefficient of the dye molecule, with a higher molar extinction coefficient showing good light-harvesting ability and yielding a higher short-circuit current. As mentioned, the short-circuit current density of the dyes assume the following order: AFL3 > AFL2 > AFL1. Less charge recombination and more efficient electron injection lead to higher Voc. Accordingly, AFL3 has superior Voc to AFL1. AFL3 shows the highest Voc among three triarylamine dye (Table 4). Because of its highest JSC and VOC, AFL3 exhibits the most efficient photoelectricity conversion efficiency and maximum η of 5.81% (VOC=760 mV, JSC=11.36 mA cm-2, and ff=0.68) under the simulated AM 1.5 G irradiation (100 mW cm-2).
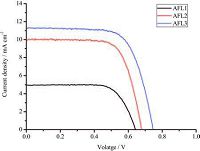
|
Download:
|
| Figure 7. Current-potential curves (J-V) of the DSSCs based on triarylamine dyes. | |
|
|
Table 3 Parameters obtained by fitting the impedance spectra of DSSCs based on triarylamine dyes with the equivalent circuit. |
|
|
Table 4 Parameters of the DSSCs based on triarylamine dyes. |
4. Conclusion
We synthesized three novel triarylamine dye sensitizers containing fluorenyl and biphenyl moieties in a triarylamine donor unit, a conjugate bridge with benzene, thiophene, and furan, and a cyanoacrylic acid acceptor. We applied the synthesized dye sensitizer to DSSCs. The spectral, photovoltaic, and electrochemical properties of the synthesized dye sensitizers were systematically investigated and compared on the basis of the differences in pbridges. Compared with the benzene bridge, the thiophene and furan bridges improved the spectral properties of the sensitizers. The light absorptivity of the sensitizers was improved. AFL2 and AFL3 possessed a long absorption maximum and a broad lightharvesting region.
The introduction of thiophene and furan linkers increased the short circuit photocurrent (JSC) and reached high efficiency. Among the three triarylamine dye sensitizers, the cell-based on the furan (AFL3) linkers exhibited the highest photoelectricity conversion efficiency of 5.81% (VOC=760 mV, JSC=11.36 mA cm-2, and ff=0.68). The results reveal that these organic triarylamine dyes are promising in the development of DSSCs and the optimization of their chemical structure and the device is in progress to further improve their energy conversion efficiency. In order to broaden the absorption spectrum and decrease the LUMO energy of the dyes, D-D-A-π-A system can be designed.
AcknowledgmentsThe authors gratefully acknowledge for funding: National Natural Science Foundation of China (No. 21176223); National Natural Science Foundation of China (No. 21406202) and Natural Science Foundation of Zhejiang province (No. LY15B020009).
| [1] | A. Mishra, M.K.R. Fischer, P. Ba üerle, Metal-free organic dyes for dye-sensitized solar cells:from structure:property relationships to design rules. Anew. Chem. lnt. Ed. Engl. 48 (2009) 2474–2499. |
| [2] | R.K. Kanaparthi, J. Kandhadi, L. Giribabu, Metal-free organic dyes for dye-sensitized solar cells:recent advances. Tetrahedron 68 (2012) 8383–8393. DOI:10.1016/j.tet.2012.06.064 |
| [3] | S. Mathew, A. Yella, P. Gao, Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 6 (2014) 242–247. DOI:10.1038/nchem.1861 |
| [4] | Z. Ning, H. Tian, Triarylamine:a promising core unit for efficient photovoltaic materials, Chem. Commun. (2009) 5483-5495. |
| [5] | S.B. Wang, J.C. Guo, L. He, Influence of thiophene and benzene unit in triphenylamine dyes on the performance of dye-sensitized solar cells. Synth. Met. 168 (2013) 1–8. DOI:10.1016/j.synthmet.2013.02.010 |
| [6] | L. Alibabaei, J.H. Kim, M. Wang, Molecular design of metal-free D-π-A substituted sensitizers for dye-sensitized solar cells. Energy Environ. Sci. 3 (2010) 1757–1764. DOI:10.1039/c0ee00218f |
| [7] | K. Hara, Z.S. Wang, Y. Cui, A. Furube, N. Koumura, Long-term stability of organicdye-sensitized solar cells based on an alkyl-functionalized carbazole dye. Energy Environ. Sci. 2 (2009) 1109–1114. DOI:10.1039/b907486d |
| [8] | T. Horiuchi, H. Miura, K. Sumioka, S. Uchida, High efficiency of dye-sensitized solar cells based on metal-free indoline dyes. J. Am. Chem. Soc. 126 (2004) 12218–12219. DOI:10.1021/ja0488277 |
| [9] | W.J. Wu, J.B. Yang, J.L. Hua, Efficient and stable dye-sensitized solar cells based on phenothiazine sensitizers with thiophene units. J. Mater. Chem. 20 (2010) 1772–1779. DOI:10.1039/b918282a |
| [10] | H.N. Tian, X.C. Yang, R.K. Chen, A. Hagfeldt, L.C. Sun, A metal-free "black dye" for panchromatic dye-sensitized solar cells. Energy Environ. Sci. 2 (2009) 674–677. DOI:10.1039/b901238a |
| [11] | Y. Numata, I. Ashraful, Y. Shirai, L.Y. Han, Preparation of donor-acceptor type organic dyes bearing various electron-withdrawing groups for dye-sensitized solar cell application. Chem. Commun. 47 (2011) 6159–6161. DOI:10.1039/c1cc11130b |
| [12] | Y.Z. Wu, M. Marszalek, S.M. Zakeeruddin, High-conversion-efficiency organic dye-sensitized solar cells:molecular engineering on D-A-π-A featured organic indoline dyes. Energy Environ. Sci. 5 (2012) 8261–8272. DOI:10.1039/c2ee22108j |
| [13] | S. Namuangruk, R. Fukuda, M. Ehara, D-D-π-A-type organic dyes for dyesensitized solar cells with a potential for direct electron injection and a high extinction coefficient:synthesis, characterization, and theoretical investigation. J. Phys. Chem. C 116 (2012) 25653–25663. DOI:10.1021/jp304489t |
| [14] | C.J. Zhong, J.R. Gao, Y.H. Cui, T. Li, L. Han, Coumarin-bearing triarylamine sensitizers with high molar extinction coefficient for dye-sensitized solar cells. J. Power Sources 273 (2015) 831–838. DOI:10.1016/j.jpowsour.2014.09.163 |
| [15] | W.X. Gao, M. Liang, Y.L. Tan, New triarylamine sensitizers for high efficiency dye-sensitized solar cells:recombination kinetics of cobalt (III) complexes at titania/dye interface. J. Power Sources 283 (2015) 260–269. DOI:10.1016/j.jpowsour.2015.02.121 |
| [16] | X.Z. Wang, J. Yang, H. Yu, A benzothiazole-cyclopentadithiophene bridged D-A-π-A sensitizer with enhanced light absorption for high efficiency dyesensitized solar cells. Chem. Commun. 50 (2014) 3965–3968. DOI:10.1039/c4cc00577e |
| [17] | W.D. Zeng, Y.M. Cao, Y. Bai, Efficient dye-sensitized solar cells with an organic photosensitizer featuring orderly conjugated ethylenedioxythiophene and dithienosilole blocks. Chem. Mater. 22 (2010) 1915–1925. DOI:10.1021/cm9036988 |
| [18] | K.S.V. Gupta, S.P. Singh, A. Islam, L. Han, M. Chandrasekharam, Simple fluorene based triarylamine metal-free organic sensitizers. Electrochimi. Acta 174 (2015) 581–587. DOI:10.1016/j.electacta.2015.05.158 |
| [19] | K.R.J. Thomas, N. Kapoor, C.P. Lee, K.C. Ho, Organic dyes containing pyrenylaminebased cascade donor systems with different aromatic π linkers for dye-sensitized solar cells:optical, electrochemical, and device characteristics. Chem. Asian J. 7 (2012) 738–750. DOI:10.1002/asia.v7.4 |
| [20] | F. Wu, J.L. Liu, L.T.L. Lee, Dye-sensitized solar cells based on functionalized truxene structure. Chin. Chem. Lett. 26 (2015) 955–962. DOI:10.1016/j.cclet.2015.03.008 |
| [21] | S.Y. Qu, B. Wang, F.L. Guo, New diketo-pyrrolo-pyrrole (DPP) sensitizer containing a furan moiety for efficient and stable dye-sensitized solar cells. Dyes Pigm. 92 (2012) 1384–1393. DOI:10.1016/j.dyepig.2011.09.009 |
| [22] | J.X. He, F.L. Guo, X. Li, New bithiazole-based sensitizers for efficient and stable dye-sensitized solar cells. Chem. Eur. J. 18 (2012) 7903–7915. DOI:10.1002/chem.201103702 |
| [23] | P. Shen, X.P. Liu, S.H. Jiang, Effects of aromatic π-conjugated bridges on optical and photovoltaic properties of N, N-diphenylhydrazone-based metal-free organic dyes. Org. Electron. 12 (2011) 1992–2002. DOI:10.1016/j.orgel.2011.08.010 |
| [24] | J.B. Yang, F.L. Guo, J.L. Hua, Efficient and stable organic DSSC sensitizers bearing quinacridone and furan moieties as a planar π-spacer. J. Mater. Chem. 22 (2012) 24356–24365. DOI:10.1039/c2jm31929b |
| [25] | J.X. He, J.L. Hua, G.X. Hu, Organic dyes incorporating a thiophene or furan moiety for efficient dye-sensitized solar cells. Dyes Pigm. 104 (2014) 75–82. DOI:10.1016/j.dyepig.2013.12.025 |
| [26] | M. Liang, W. Xu, F.S. Cai, New triphenylamine-based organic dyes for efficient dye-sensitized solar cells. J. Phys. Chem. C 111 (2007) 4465–4472. DOI:10.1021/jp067930a |
| [27] | K.R.J. Thomas, Y.C. Hsu, J.T. Lin, 2, 3-disubstituted thiophene-based organic dyes for solar cells. Chem. Mater. 20 (2008) 1830–1840. DOI:10.1021/cm702631r |
| [28] | Z.S. Wang, K. Hara, Y. Dan-oh, Photophysical and (photo) electrochemical properties of a coumarin dye. J. Phys. Chem. B 109 (2005) 3907–3914. DOI:10.1021/jp044851v |
| [29] | R.G. Parr, W.T. Yang, Density-functional theory electronic structure of molecules. Annu. Rev. Phys. Chem. 46 (1995) 701–728. DOI:10.1146/annurev.pc.46.100195.003413 |
| [30] | Z.M. Wu, C.Y. Rong, T. Lu, P.W. Ayers, S.B. Liu, Density functional reactivity theory study of SN2 reactions from the information-theoretic perspective. Phys. Chem. Chem. Phys. 17 (2015) 27052–27061. DOI:10.1039/C5CP04442A |
| [31] | S. Chaurasia, Y.C. Chen, H.H. Chou, Y.S. Wen, J.T. Lin, Coplanarindenofluorene-based organic dyes for dye-sensitized solar cells. Tetrahedron 68 (2012) 7755–7762. DOI:10.1016/j.tet.2012.07.045 |
| [32] | S.Y. Cai, X.H. Hu, J.L. Han, Efficient organic dyes containing dibenzo heterocycles as conjugated linker part for dye-sensitized solar cells. Tetrahedron 69 (2013) 1970–1977. DOI:10.1016/j.tet.2012.12.074 |
| [33] | L. Han, X.Y. Zu, Y.H. Cui, Novel D-A-π-A carbazole dyes containing benzothiadiazole chromophores for dye-sensitized solar cells. Org. Electron. 15 (2014) 1536–1544. DOI:10.1016/j.orgel.2014.04.016 |
| [34] | K. Hara, M. Kurashige, Y. Dan-oh, Design of new coumarin dyes having thiophene moieties for highly efficient organic-dye-sensitized solar cells. New J. Chem. 27 (2003) 783–785. DOI:10.1039/b300694h |
 2017, Vol. 28
2017, Vol. 28