b Department of Chemistry, Faculty of Science, Gonbad Kavous University, P. O. Box 163, Gonbad, Iran
Over the last decade, an important trend has developed in chemistry of developing environmentally benign reactions for numerous pathways and materials [1]. Green chemistry demonstrates considerable potential not only for reduction of byproducts, reduction in the waste products, and reduction of energy costs, but also in the development of new methodologies towards previously unattainable materials using available technologies [2]. All areas of chemistry, including medicinal and pharmaceutical chemistry, with their typically large waste/product ratio, are ideal targets for greening [3].
Designing and advancing favorable catalysts is an important strategy in green chemistry. One of the improvements in the field of catalysts is the development of nanocatalysts [4]. Some organic reactions offer high yields and good selectivity in the presence of nanocatalysts, which exhibit a better catalytic activity than their bulk-sized forms [5, 6]. The ZnO nanostructure as a low-cost and non-toxic catalyst has been applied in organic reactions [7-10].
Since the introduction of ferrocene, composed of two cyclopentadiene rings are bound to a transition metal [11], the cyclopentadiene ring and/or cyclopentadienyl system has garnered considerable research attention over more than half a century in organic chemistry and other research fields [11-18]. For example, some widely investigated reactions include the coordination toward metal ions [13, 14, 19], formation of cyclopentadienyl cations, anions, and radicals [14, 15], rearrangement of cyclopentadiene ring [17], and substitution/diene-addition reactions [17, 20]. In almost all of these cases, the cyclopentadiene moiety preserves its five-membered ring during different reaction processes [21]. In addition, 1, 3-cyclopentadienes are a key diene applied in Diels-Alder reactions. There are a great number of reactions in which 1, 3-cyclopentadiene is employed both for the synthetic work and studying the mechanistic facets of cycloaddition [22]. Therefore, numerous studies have been focused on the evolution of efficient synthetic procedures to functionalize cyclopentadienes [23].
Compounds with antioxidant activity which are mainly due to their redox properties and chemical structure can play important roles as transitional metals chelators, quenching singlet and triplet oxygen molecules, and protecting against the negative effect of free radicals. They can prevent or decrease many oxidative-stress related diseases such as cardiovascular, inflammatory bowel syndrome, cancer, ageing, atherosclerosis, and Alzheimer's disease [24-26]. Recently, medicinal chemists, food chemists, and biologists have investigated and tested new and efficient synthetic antioxidants as a protective strategy against these diseases.
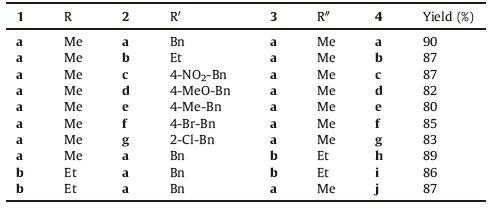
Herein, we investigated a "green" procedure for the synthesis of some cyclopentadiene derivatives via an efficient three component tandem reaction between alkyl propiolate 1, dialkyl acetylenedicarboxylate 3, and primary amines 2 in the presence of a catalytic amount of ZnO-nanorods (ZnO-NRs) under solvent-free conditions at 50 ℃ with very good yields (Scheme 1 and Table 1). Moreover, the antioxidant activities of the newly synthesized derivatives were investigated by DPPH radical scavenging and ferric ion reducing power assays.

|
Download:
|
| Scheme 1. Reaction of activated acetylenic compounds and primary amines in the presence of ZnO-NR for generation of highly functionalized cyclopentadienes. | |
|
|
Table 1 Reaction of alkyl propiolate, primary alkylamines, and acetylenic esters. |
2. Experimental
All the chemicals used in this work were purchased from Fluka (Buchs, Switzerland), Merck, Lolitech, and Aldrich Chemical Companies and used without further purification. ZnO nanorods were synthesized according to a previously reported method [27]. The morphology of ZnO nanorods was characterized by scanning electron microscopy (SEM) using a Holland Philips XL30 microscope. Crystalline structure of ZnO nanorods was identified by Xray diffraction (XRD) analysis at room temperature using a Holland Philips Xpert X-ray powder diffractometer, with CuKα radiation (λ=0.15406 nm), with 2θ ranging from 20 to 80°. The elemental analyses for the determination of C, H, and N were performed using a Heraeus CHNO-Rapid analyzer. The mass spectra were recorded on a FINNIGAN-MAT 8430 spectrometer operating at an ionisation potential of 70 eV. IR spectra were measured on a Shimadzu IR-460 spectrometer. The 1H and 13C NMR spectra were measured using a Bruker DRX-500 Avance spectrometer at 500.1 MHz and 125.8 MHz, respectively. The 1H and 13C spectra were obtained for CDCl3 solutions using TMS as the internal standard or 85 mass % H3PO4 as external standard; chemical shifts (δ) are given as parts per million (ppm).
2.1. General procedure for preparation of ZnO nanorodsSodium hydroxide (0.44 g) was dissolved in 75 mL of distilled water under vigorous stirring at ambient temperature. Subsequently, sodium dodecyl sulphate (SDS) (1.57 g) and Zn (AcO)2-2H2O (0.6 g) were added to the mixture. The solution was refluxed at 80 ℃ (pH 14) for 1.5 h. The solid phase was filtered and washed several times with distilled water and ethanol (96%) [27a, b].
2.2. General procedure for preparation of compounds 4a-gA mixture of dialkyl acetylenedicarboxylate 3 and primary amine 2 (2 mmol) was added to the magnetically stirred mixture of alkyl propiolate 1 (2 mmol) and ZnO-NR (15 mg) at 50 ℃. The reaction mixture was then stirred for 5 h. After completion of the reaction [TLC monitoring] (AcOEt/hexane 1:4), water (15 mL) was poured in the mixture. The solid phase was filtered and washed with ethyl acetate. In this stage, the catalyst was separated. Then, the solvent was evaporated and the viscous residue was purified by column chromatography on silica gel (Merck 230-400 mesh) using n-hexane-EtOAc (4:1) as eluent to afforded pure compounds 4.
Methyl 4-[benzyl (methoxycarbonyl) amino]-3-methoxy-1, 3-cyclopentadiene-1-carboxylate (4a): Yellow oil; yield (90%). IR (KBr) (vmax/cm-1): 1721, 1701, 1654, 1546, 1507, 1456, 1360, 12=, 1174, 1083, 1028, 897, 750. 1H NMR (500.1 MHz, CDCl3): δ 3.54 (s, 2H, CH2), 3.60, 3.78, 3.85 (s, 9H, 3 MeO), 5.51 (s, 2H, NCH2), 6.95 (s, 1H, CH), 6.98 (d, 2H, 3J=7.3 Hz, CH), 7.15-7.40 (m, 3H, CH). 13C NMR (125.7 MHz, CDCl3): δ 31.9 (CH2), 48.9 (MeO), 52.1 (MeO), 52.3 (MeO), 63.1 (NCH2), 113.7 (C), 120.1 (C), 121.2 (C), 124.5 (2 CH), 127.5 (CH), 128.7 (2 CH), 136.7 (CH), 143.9 (C), 161.1 (C=O), 165.0 (C=O). MS: m/z (%)=317 (M+, 32), 150 (100), 104 (100), 128 (31), 92 (49), 76 (71). Anal. calcd. for C17H19NO5 (317.34): C, 64.34; H, 6.03; N, 4.41. Found: C, 64.25; H, 6.01; N, 4.62.
The spectral data of 4b-g are given in Supporting information.
2.3. 1, 1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assayRadical scavenging activity of 4a, 4b, 4c, and 4d was measured by DPPH (2, 2-diphenyl-1-picrylhydrazyl) radical scavenging assay according to the reported method by Shimada et al. [28]. Different concentrations of 4a, 4b, 4c, and 4d (200-1000 ppm) were added to an equal volume of methanolic solution of DPPH (1 mmol/L). The mixtures were well shaken and then placed in a dark room. After 30 min at room temperature, the absorbance was recorded at 517 nm. In the control sample, 4a, 4b, 4c, and 4d were replaced with 3 mL methanol. Butylated hydroxytoluene (BHT) and 2-tertbutylhydroquinone (TBHQ) were used as standard controls. The percentage inhibition of the DPPH radical was calculated according to the formula of Yen and Duh [29].
I=[(AB-AS)/AB]×100
Where, I=DPPH inhibition (%), AB=absorbance of control sample (0 min), and AS=absorbance of a tested sample at the end of the reaction (after 30 min).
2.4. Reducing power assayThe ability of 4a, 4b, 4c, and 4d to reduce iron (III) was evaluated by the method of Yildirim et al. [30]. Samples (1 mL) were mixed with 2.5 mL of phosphate buffer (0.2 mol/L, pH 6.6) and 2.5 mL of potassium ferricyanide (K3Fe (CN)6; 10g/L) and incubated for 30 min at 50 ℃. Then, 2.5 mL of trichloroacetic acid (10% w/v) were added to the solution and centrifuged for 10 min. Finally, 2.5 mL of supernatant was combined with 2.5 mL of distilled water and 0.5 mL FeCl3 (1 g/L). The absorbance of samples was measured at 700 nm. Higher absorbance means higher reducing power.
2.5. Statistical analysisEach measurement was performed in triplicate. The data obtained were analyzed by running one way analysis of variance (ANOVA) using SPSS software version 18.0. A one way ANOVA was used to evaluate difference in the mean value of samples and control. All mean separations were performed by Duncan multiple range test using the significance level of 95% (P < 0.05).
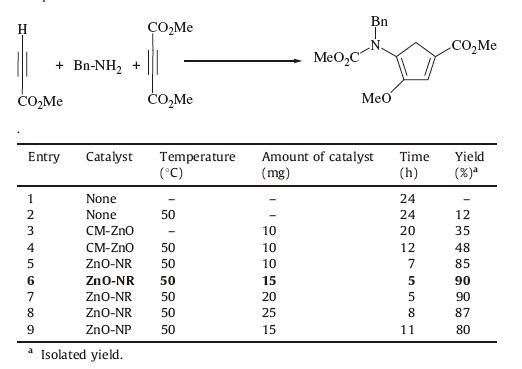
3. Result and discussion 3.1. ZnO nanorodsThe ZnO nanorods were produced by SDS [27]. SDS was applied as a directing agent to control the ZnO nanostructure morphologies. The morphology of ZnO nanorod was confirmed by SEM (Fig. 1). The length and diameter of the nanorods were 300-600 nm and 50-70 nm, respectively. The XRD image of ZnO-NR is shown in Fig. 2. The average crystallite size (D) for ZnO-NR was calculated based on the peak with the strongest intensity using the Debye-Scherrer's equation (D=Kλ/βcosθ); where D is the grain size, β is full-width at half-maximum or half-width (FWHM) in radians, and h is the position of the maximum of diffraction peak, K is the socalled shape factor (0.89), θ is Bragg's diffraction angle and λ is the X-ray wavelength used (1.5406 A8 for CuKα). The average crystal size for ZnO-NR is about 30 nm [27, 31]. All the prominent peaks were in agreement with the reference pattern, which can be indexed on the basis of JCPDS file No. 36-1451.

|
Download:
|
| Figure 1. SEM image of ZnO nano rod. | |

|
Download:
|
| Figure 2. XRD pattern of NR-ZnO. | |
3.2. Catalytic activity of ZnO nanostructures in synthesis of functionalized cyclopentadienes
In the starting point of this work, condensation reaction of methyl propiolate 1a, dimethyl acetylenedicarboxylate 3a, and benzyl amine 2a (molar ratio 1:1:1) under solvent free condition in room temperature was used as a model reaction to achieve the optimum conditions. Under this condition, no product was detected even after 24 h (entry 1, Table 2). By increasing the reaction temperature to 50 ℃, a trace amount of 4a was obtained after 24 h (entry 2, Table 2). In order to improve this process, 10 mg commercial ZnO (CM-ZnO) was added to the reaction mixture. After 12 h, 48% yield of 4a was obtained (entry 4, Table 2). Then, the reaction was carried out in the presence of 10 mg of ZnO-NR as catalyst. As expected, in these conditions, the desired product 4a was obtained in 85% yield after 7 h (entry 5, Table 2). Subsequently, to find the optimal catalyst loading, various amounts (15-25 mg) of Zn-NR were used. The results showed that 15 mg of catalyst is sufficient to obtain an excellent yield (entry 6, Table 2). In order to further evaluate the catalytic activity, ZnO nanoparticles were applied in this reaction, and 80% yield of 4a was obtained (entry 9, Table 2). Consequently, these results revealed the important role of ZnO-NR as catalyst in this reaction. Therefore, morphology of the nano ZnO has a dramatic effect on the reaction yield.
|
|
Table 2 Effect of catalyst, its loading and temperature on the condensation reaction of compound 4a. |
In this research the effects of some solvents was also investigated on the production of 4a in presence of 15 mg of ZnO-NR catalyst The results tabulated in Table 3 show that under solvent-free conditions the yields are higher than in solvents. According to the outcomes of optimization reported in the Tables 2 and 3, ZnO-NR (15 mg) as catalyst, solvent free condition, and 50 ℃ temperature were estimated to be the optimum reaction conditions.
|
|
Table 3 Effects of solvent and temperature on generation of 4a compound in presence of 15mg of ZnO-NR catalyst. |
The reusability of the catalyst was proved in the model reaction (the synthesis of compound 4a). The results revealed that the catalyst can be reused four times without considerable loss of activity (Fig. 3). After each run, the catalyst was removed by filtration and washed with ethyl acetate; it was then dried at ambient temperature for 24 h and used for the next catalytic cycle.
The structures of compounds 4 were characterized by IR, 1H NMR, 13C NMR, and mass spectral data. For example, the 1H NMR spectrum of 4a revealed one singlet for methylene at δ 3.54 (2H, s, CH2), tree singlet for tree methoxy groups at δ 3.60, 3.78, and 3.85 ppm, one singlet for at 5.51 (2 H, s, NCH2), and one singlet at δ 6.95 ppm for methyne. Also, characteristic signals for the aromatic moiety appeared at δ 6.98-7.40. The 13C NMR spectrum of 4a displayed 15 distinct resonances confirming once again the proposed structure. In the 13C NMR spectrum, the signals corresponding to the ester carbonyl groups of 4a were observed at δ 161.1 and δ 165.0. The mass spectrum of 4a displayed the molecular ion peak at m/z 317. The IR spectrum of 4a was displayed characteristic C=O bands.
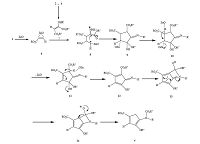
Although there are no data regarding the mechanistic details, the reaction can be explained as occurring by the mechanism proposed in Scheme 2. Presumably, alkyl propiolate 1 activated by ZnO-NR leads to complex 6, which is attacked by the enaminoester 7, generated in situ from the corresponding amine 2 and dialkyl acetylenedicarboxylate 3, to form the zwitterion 8. Intramolecular cycloaddition reaction of 8 afforded imino-cyclopentene intermediate 9. The product 4 is formed after two stages of elimination in intermediate 10 and 11, followed by cyclisation and 1, 3-acyl migration in intermediate 13 (Scheme 2) [23].

|
Download:
|
| Figure 3. Activity of reused catalyst for synthesis of compound 4a. | |
|
Download:
|
| Scheme 2. Proposed mechanism for the formation of 4. | |
Yavari and Bayat have reported the synthesis of functionalized cyclopenta-1, 3-dienes via a tandem reaction between Ph3P, primary alkylamines, and dialkyl acetylenedicarboxylates in CH2Cl2 at r.t. after 6-12 h with low atom economy (56%) [23]. The main advantages of our approach in comparison with the method of Yavari consist of high atom economy (95.2%), solventfree reaction conditions, low loading of nanocatalyst, higher yield, shorter reaction times, and easy work-up, which are in good accordance with many of the 12 principles of green chemistry [1].
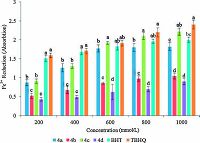
3.3. Antioxidant evaluationsDPPH radical scavenging activity: Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay is widely used to evaluate compounds' ability to scavenge free radicals and their antioxidant activity in foods and biological systems [32, 33]. The DPPH assay assesses hydrogen atom (or one electron) donating activity and provides an evaluation of antioxidant activity due to free radical scavenging. The antioxidant activity of structures 4a, 4b, 4c, and 4d, was studied by testing their ability to reduce the stable DPPH radical. In its radical form, DPPH radical absorbs at 517 nm, but upon reduction by an antioxidant or a radical species its absorption decreases. The hydrogen atom donating ability of the products was determined by the decolorization (absorption decreases) of methanol solution of 2, 2-diphenyl-1-picrylhydrazyl (DPPH) [32, 34]. In this study, the antioxidant activity of the structures 4a, 4b, 4c, and 4d was compared to BHT and TBHQ at different concentrations from 200 mmol/L to 1000 mmol/L (Fig. 4). The results revealed that the free radical scavenging activity of 4a-4d was weaker than BHT and TBHQ and the scavenging power of the samples was in the following order: TBHQ>BHT>4c>4a>4d>4b. Both composition and concentration are important factors in DPPH scavenging activity (P < 0.05) (Fig. 4).

|
Download:
|
| Figure 4. Radical scavenging activity (RSA) of compounds 4a, 4b, 4c and 4d. Values in the same concentration followed by different letters are significantly different (P < 0.05). | |
Ferric ions (Fe3+) reducing potential (FRAP): Reducing ability of the synthesized compounds was investigated by measuring the extent of conversion of Fe3+/ferricyanide complex to the Fe2+/ ferrous form at 700 nm [32]. The reducing ability of a compound may serve as a significant indicator of its potential antioxidant activity. The reducing capacity of synthesized compounds 4a, 4b, 4c, and 4d was compared with synthetic antioxidants (BHT and TBHQ) and the results are shown in Fig. 5. Higher absorbance value indicates a stronger reducing power of the samples. The reducing activity of compounds 4a, 4b, 4c, and 4d exhibited the following order: TBHQ>BHT>4c>4a>4b>4d (Fig. 5). All compounds showed concentration-dependent reducing power. The reducing power activity of 4a and 4c were significantly higher than that the standards (BHT and TBHQ). Moreover, 4b and 4d had medium reducing activity compared to the standards. The reducing power activities of these compounds indicate that they are electron donors and can act as antioxidants.
|
Download:
|
| Figure 5. Ferric ions (Fe3+) reducing antioxidant power (FRAP) of compounds 4a, 4b, 4c and 4d. Values in the same concentration followed by different letters are significantly different (P < 0.05). | |
4. Conclusion
In summary, we report an efficient, green, and environmentally benign procedure involving acetylenic compounds and primary amines in the presence of ZnO-NR at 50 ℃ under solvent-free conditions which provides a new path to the synthesis of functionalized cyclopentadienes. The present method has many advantages such as high atom economy (95%) and yield, mild and clean solvent free reaction condition, low catalyst loading, and short reaction time. Also, the antioxidant activities of 4a, 4b, 4c, and 4d compounds were evaluated by DPPH radical scavenging and ferric reducing power assays. The compounds 4a, 4b, 4c, and 4d do not demonstrate good DPPH radical scavenging activity, but revealed desirable FRAP compared to synthetic antioxidants BHT and TBHQ.
AcknowledgmentThe authors gratefully acknowledge the support of the Research Council of Iran University of Science and Technology, Tehran, Iran.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.09.011.
| [1] | P. Anastas, T.C. Williamson, Green Chemistry:Frontiers in Benign Chemical Syntheses and Processes, Oxford Science Publications: New York, 1998 . |
| [2] | G.W.V. Cave, C.L. Raston, J.L. Scott, Recent advances in solventless organic reactions:towards benign synthesis with remarkable versatility. Chem. Commun. (2001) 2159–2169. |
| [3] | R.A. Sheldon, Catalysis and pollution prevention. Chem. Ind. 1 (1997) 12–15. |
| [4] | S.B. Kalidindi, B.R. Jagirdar, Nanocatalysis and prospects of green chemistry. ChemSusChem 5 (2012) 65–75. DOI:10.1002/cssc.201100377 |
| [5] | D. Beydoun, R. Amal, G. Low, S. McEvoy, Role of nanoparticles in photocatalysis. J. Nanopart. Res. 1 (1999) 439–458. DOI:10.1023/A:1010044830871 |
| [6] | S. Rostamizadeh, M. Nojavan, R. Aryan, E. Isapoor, M. Azad, Amino acid-based ionic liquid immobilized on α-Fe2O3-MCM-41:an efficient magnetic nanocatalyst and recyclable reaction media for the synthesis of quinazolin-4(3H)-one derivatives. J. Mol. Catal. A 374-375 (2013) 102–110. DOI:10.1016/j.molcata.2013.04.002 |
| [7] | Z. Mirjafary, H. Saeidian, A. Sadeghi, F.M. Moghaddam, ZnO nanoparticles:an efficient nanocatalyst for the synthesis of (β-acetamido ketones/esters via a multi-component reaction. Catal. Commun. 9 (2008) 299–306. DOI:10.1016/j.catcom.2007.06.018 |
| [8] | F.M. Moghaddam, H. Saeidian, Controlled microwave-assisted synthesis of ZnO nanopowder and its catalytic activity for O-acylation of alcohol and phenol. Mater. Sci. Eng.:B 139 (2007) 265–269. DOI:10.1016/j.mseb.2007.03.002 |
| [9] | L. Lietti, E. Tronconi, P. Forzatti, G. Busca, Surface properties of ZnO-based catalysts and related mechanistic features of the higher alcohol synthesis by FT-IR spectroscopy and TPSR. J. Mol. Catal. 55 (1989) 43–54. DOI:10.1016/0304-5102(89)80241-X |
| [10] | M. Gupta, S. Paul, R. Gupta, A. Loupy, ZnO:a versatile agent for benzylic oxidations. Tetrahedron Lett. 46 (2005) 4957–4960. DOI:10.1016/j.tetlet.2005.05.104 |
| [11] | T.J. Kealy, P.L. Pauson, A new type of organo-iron compound. Nature 168 (1951) 1039–1040. |
| [12] | G. Wilkinson, F.G.A. Stone, E.W. Abel, Comprehensive Organometallic Chemistry, Pergamon, Oxford, 1982, pp. 3-7. |
| [13] | U. Siemeling, Chelate complexes of cyclopentadienyl ligands bearing pendant Odonors. Chem. Rev. 100 (2000) 1495–1526. DOI:10.1021/cr990287m |
| [14] | H. Butenschön, Cyclopentadienylmetal complexes bearing pendant phosphorus, arsenic, and sulfur ligands. Chem. Rev. 100 (2000) 1527–1564. DOI:10.1021/cr940265u |
| [15] | A.D. Allen, T.T. Tidwell, Antiaromaticity in open-shell cyclopropenyl to cycloheptatrienyl cations, anions, free radicals, and radical ions. Chem. Rev. 101 (2001) 1333–1348. DOI:10.1021/cr990316t |
| [16] | V. Nair, R.S. Menon, P.B. Beneesh, V. Sreekumar, S. Bindu, A novel multicomponent reaction involving isocyanide, dimethyl acetylenedicarboxylate (DMAD), and electrophilic styrenes:facile synthesis of highly substituted cyclopentadienes. Org. Lett. 6 (2004) 767–769. DOI:10.1021/ol036491k |
| [17] | A. Gutnov, B. Heller, H.J. Drexler, A. Spannenberg, G. Oehme, Tartrate-derived cyclopentadienes for the synthesis of chiral substituted (η5-cyclopentadiene) (η4-cycloocta-1, 5-diene) cobalt (I) complexes. Organometallics 22 (2003) 1550–1553. DOI:10.1021/om020982b |
| [18] | O.T. Beachley Jr., D.J. MacRae, M.R. Churchill, A.Y. Kovalevsky, E.S. Robirds, Cyclopentadiene elimination reactions for the preparation of organoindium (III) derivatives. Crystal and molecular structures of Me2In (acac), (Me3CCH2)2In (acac), (Me)(Me3CCH2) In (acac), and[Me2InNH (t-Bu)]2, Organometallics 22(2003) 3991-4000. |
| [19] | P.A. Deck, M.M. Konaté, B.V. Kelly, C. Slebodnick, C-F activation reactions of (pentafluorophenyl) cyclopentadiene and 3-(pentafluorophenyl) indene with tetrakis (dimethylamido) titanium (IV). Organometallics 23 (2004) 1089–1097. DOI:10.1021/om034385g |
| [20] | B.Y. Lee, H. Moon, Y.K. Chung, N. Jeong, Generation of cyclopentadienyl ligands via the Pauson-Khand and retro-Diels-Alder reactions. J. Am. Chem. Soc. 116 (1994) 2163–2164. DOI:10.1021/ja00084a074 |
| [21] | W.T. Gong, G.L. Ning, X.C. Li, L. Wang, Y. Lin, A facile oxidation and oxygen insertion of the cyclopentadiene ring by molecular oxygen in solution. J. Org. Chem. 70 (2005) 5769–5770. |
| [22] | A. Kumar, S.S. Pawar, AlCl3-catalysed dimerization of 1, 3-cyclopentadiene in the chloroaluminate room temperature ionic liquid. J. Mol. Catal. A:Chem. 208 (2004) 33–37. DOI:10.1016/S1381-1169(03)00510-7 |
| [23] | I. Yavari, M.J. Bayat, Ph3P-mediated tandem synthesis of functionalized cyclopentadienes from primary alkylamines and acetylenic esters. Synlett (2010) 2293–2295. |
| [24] | (a) B. Halliwell, Antioxidant defence mechanisms:from the beginning to the end (of the beginning), Free Radical Res. 31(1999) 261-272; (b) F. Ahmadi, M. Kadivar, M. Shahedi, Antioxidant activity of Kelussia odoratissima Mozaff. in model and food systems, Food Chem. 105(2007) 57-64. |
| [25] | M.A. Babizhayev, A.I. Deyev, V.N. Yermakovea, I.V. Brikman, J. Bours, Lipid peroxidation and cataracts:N-acetylcarnosine as a therapeutic tool to manage age-related cataracts in human and in canine eyes. Drugs R D 5 (2004) 125–139. DOI:10.2165/00126839-200405030-00001 |
| [26] | L. Liu, M. Meydani, Combined vitamin C and E supplementation retards early progression of arteriosclerosis in heart transplant patients. Nutr. Rev. 60 (2002) 368–371. DOI:10.1301/00296640260385810 |
| [27] | (a) M. Sabbaghan, A. Anaraki Firooz, V. Jan Ahmadi, The effect of template on morphology, optical and photocatalytic properties of ZnO nanostructures, J. Mol. Liq. 175(2012) 135-140; (b) R. Hajinasiri, Z. Hossaini, F. Sheikholeslami-Farahani, ZnO-nanorods as the catalyst for the synthesis of 1, 3-thiazole derivatives via multicomponent reactions, Comb. Chem. High Throughput Screen. 18(2015) 42-47. |
| [28] | K. Shimada, K. Fujikawa, K. Yahara, T. Nakamura, Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 40 (1992) 945–948. DOI:10.1021/jf00018a005 |
| [29] | G.C. Yen, P.D. Duh, Scavenging effect of methanolic extracts of Peanut Hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 42 (1994) 629–632. DOI:10.1021/jf00039a005 |
| [30] | A. Yildırım, A. Mavi, A.A. Kara, Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 49 (2001) 4083–4089. DOI:10.1021/jf0103572 |
| [31] | M. Hosseini-Sarvari, H. Sharghi, S. Etemad, Nanocrystalline ZnO for Knoevenagel condensation and reduction of the carbon, carbon double bond in conjugated alkenes. Helvet. Chim. Acta 91 (2008) 715–724. DOI:10.1002/(ISSN)1522-2675 |
| [32] | A.R. Saundane, K.N. Mathada, Synthesis, characterization, and biological evaluation of Schiff bases containing indole moiety and their derivatives. Monatsh. Chem. 146 (2015) 1751–1761. DOI:10.1007/s00706-015-1440-9 |
| [33] | A.M. Bidchol, A. Wilfred, P. Abhijna, R. Harish, Free radical scavenging activity of aqueous and ethanolic extract of Brassica oleracea L. var. italica. Food Bioprocess Tech. 4 (2011) 1137–1143. DOI:10.1007/s11947-009-0196-9 |
| [34] | M. Oyaizu, Studies on products of browning reaction:antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 44 (1986) 307–315. DOI:10.5264/eiyogakuzashi.44.307 |
 2017, Vol. 28
2017, Vol. 28