b Faculty of Science, Ain Shams University, Cairo 11566, Egypt
The surfactants used in the synthesis of nanoparticles span a wide range, owing to their ability to adsorb onto particle surfaces. The surfactant prevents the aggregation of the produced nanoparticles and increases their stability [1-3]. Surfactants are compounds that contain one part with an affinity to polar media and one part for non-polar media. Surfactants are characterized by the concentration at which they aggregate into micelles, the critical micelle concentration. At lower concentrations, the surfactants have the ability to adsorb at interfaces. Whether adsorption of micellization occurs depends on the chemical structure of the surfactants [4-6]. Surfactants are used in various applications such as detergents, paints, coatings, inks, and drugs, and in the petroleum industry as corrosion inhibitors, biocides, drilling mud, pour point depressants, and demulsifiers [7-12]. Cationic surfactants are characterized by their attraction to negatively charged surfaces, and their adsorption onto interfaces depends on the presence of some adsorption center like heteroatoms and multiple bonds. Because of that ability, cationic surfactants can be used in nanoparticle synthesis as capping agents to control the size and shape of the formed nanoparticles. Many studies examined the effect of concentration of the cationic surfactants on the size, stability, and shape of the produced nanoparticles [13-15]. This work is a continuation of previous work, confirming the effect of altering the hydrophobic tail on the stability and the size distribution of the produced nanoparticles [16, 17]. Silver nanoparticles exhibit strong biocidal activity against bacteria (gram positive and negative) and fungi [18, 19]. Because of the similarity in the chemical structure between cellular constituents and quaternary ammonium surfactant, the cationic surfactant shows a good biocidal activity. Because of increased prevalence of immunity in microorganism toward common biocides, the use of cationic surfactant and silver nanoparticles is greatly demanded [20]. This work aimed to prepare cationic surfactants based on Schiff base and to study their role in controlling the size distribution on the preparation of silver nanoparticles. The effect of altering the hydrophobic chain length on the stability and amount of the produced silver nanoparticles were studied. The surface activity of the synthesized cationic surfactant has been determined from surface tension measurements. The silver nanoparticles enhanced the biological activity of the synthesized cationic surfactants against bacteria and fungi.
2. Experimental 2.1. MaterialsAll the chemicals used in the synthesis of cationic surfactants and silver nanoparticles are of analytical grade and used without purification. Dimethylaminopropylamine (DMAPA), vanillin, decyl bromide, dodecyl bromide, hexadecyl bromide, and silver nitrate were purchased from Sigma-Aldrich Chemicals Co. The solvents (ethyl alcohol absolute and diethyl ether) are high grade and purchased from El-gomhoria Chemical Co.
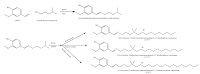
2.2. Synthesis of cationic surfactant3-Dimethylamino-1-propylamine (0.2 mol) was refluxed with vanillin (0.2 mol) in ethanol as a solvent for 6 h, to obtain the corresponding Schiff base. The reaction mixture was left to cool then filtered and recrystallized twice.
The obtained Schiff base (0.1 mol) was refluxed with decyl bromide (0.1 mol), dodecyl bromide (0.1 mol) and hexadecyl bromide (0.1 mol) individually in ethyl alcohol as solvent for 40 h. Then, the solvent was removed using a vacuum rotary evaporator. Subsequent purification was done by means of vacuum distillation to remove the excess and residual materials [21]. The obtained cationic surfactants were designated as C10V, C12V and C16V. Scheme 1 shows the synthetic route of the prepared cationic surfactant.
|
Download:
|
| Scheme 1. Preparation route of prepared cationic surfactants C10V, C12V and C16V. | |
2.3. Synthesis of silver nanoparticles
The silver nanoparticles were prepared by a simple one-step procedure. Aqueous solution of silver nitrate (1 × 10-3 mol/L) was mixed separately with the prepared cationic surfactants (1 × 10-3 mol/L). After the mixing process, the solution was exposed to sunlight [16]. A change in the solution color was observed after exposure, indicating the conversion of the silver ions to silver nanoparticles colloids. The solution color was fixed after 10 min maximum.
2.4. Cationic surfactant and their nanostructure characterizationThe chemical structure of the new prepared cationic surfactants was characterized by FTIR spectroscopy (using ATI Mattsonm Infinity SeriesTM, Bench top 961 controlled by Win FirstTM V2.01 Software, Egyptian Petroleum Institute), mass spectroscopy (using GC MS-OP1000EX, Micro Analytical Center, Cairo University) and 1H NMR spectroscopy (using Aspect Varian, GEMINI 200 in DMSO-d6, National Research Institute).
Transmission electron microscopy (TEM) was used to investigate the nanostructure of the produced silver nanoparticles. Small droplets of silver nanoparticle colloid were placed on carboncoated grid pre-covered with a very thin amorphous carbon film. A photographic plate of the transmission electron microscope employed in the present work to investigate the microstructure of the prepared silver nanoparticles was TEM model "Joel JeM-2100 (Japan)" (Egyptian Petroleum Research Institute EPRI).
The effect of the synthesizing cationic surfactant on the silver nanoparticles formation was estimated using a UV-visible spectrophotometer (Shimadzu, UV-2550, Japan) equipped with a quartz cell with optical path of 10 mm, and spectral resolution of 1 nmat a 25±1 ℃ in the wavelength range (190-700 nm). Dynamic light scattering (DLS) technique was used to determine the size distribution and stability of the synthesized silver nanoparticles using a Malvern Zetasizer Nano Instruments Ltd, Worcestershire, UK. The energy-dispersive X-ray (EDX) spectroscopy was recorded with an EDX detector (Oxford LINKISIS 300) equipped on a Transmission electron microscope (TEM, Hitachi S-520) operated at 10 kV accelerating voltage used to confirm the presence of metallic silver nanoparticle in the solution.
2.5. Surface activity measurementsSurface tension measurements of the three synthesized cationic surfactants were performed using a KrussK6 tensiometer by the platinum ring detachment method (±0.5 mN/m). Freshly prepared aqueous solutions of the synthesized cationic surfactant were used with a concentration range from 1 × 10-2 mol/L to 1 × 10-8 mol/L at 25 ℃, 40 ℃ and 60 ℃. The solutions were allowed to stabilize and complete adsorption on the solution surface, and then the apparent surface tension values were measured three times with 5 min intervals between each reading, and the recorded values were taken as the average of these values [22, 23]. The CMC values were determined from the abrupt change in the slope of surface tension (γ) versus (log c) plots.
2.6. Antimicrobial activityThe antibiotic activities of the prepared cationic surfactants and their silver nanostructure were estimated by a modified agar well diffusion method [24]. In this method, the tested microbial strains (Gram-positive bacteria (Bacillus pumilus and Micrococcus luteus), Gram-negative bacteria (Pseudomonas aeuroginosa and Sarcina lutea) and Fungi (Candida albicans and Penicillium chrysogenum)) were poured with the prepared medium in the plates (nutrient agar for the bacterial strains, wickerham agar for the yeast and czapek dox for the fungal strains). In each agar plate, a sterile 10 mmcork borer was used to cut three equidistant wells. In addition, a 0.1 mL of the prepared cationic surfactants (concentration, 5000 ppm) was introduced into the wells. The agar plates were incubated overnight at 37 ℃ for the tested bacterial strains or for 48 h at 30 ℃ for the yeast and the fungus strains. The antimicrobial activity was estimated by measuring the inhibition zone diameter (mm). Triplicates were maintained and the inhibition experiment was repeated thrice. For each replicate, the measurements were taken in three different fixed directions. Furthermore, sterile water was used as a negative control.
3. Results and discussion 3.1. Structure confirmation of the synthesized cationic surfactantThe FTIR spectra of the synthesized cationic surfactants show the disappearance of amino and carbonyl group of DMAPA and vanillin at 3300 cm-1 and 1730 cm-1, respectively. The synthesized C16V surfactant as an example, showing absorption band at 1675 cm-1 corresponding to imine group (C=N) formation. The prepared cationic surfactant showed stretching, vibration band of -C-H aliphatic symmetric and asymmetric at 2851 and 2922 cm-1, respectively. Some other bands at 3431 cm-1, 3018 cm-1, 1590 cm-1, 1377 cm-1, 1464 cm-1, and 1156 cm-1 correspond to a hydroxyl group, aromatic proton stretching, C=C aromatic stretching, -CH2 bending, -CH3 bending, and C-N bond, respectively (Fig. S1 in Supporting information).
The 1H NMR data of the synthesized surfactant (C10V) are: δ 0.834 (t, 3H, -CH3), 1.2 (m, 14H, -(CH2)7CH3), 1.674 (m, 2H, -CH2(CH2)7CH3), 2.028-2.18 (m, 2H, -CH2CH2CH2N=CH-Ph (OH)(OCH3)), 2.747 (s, 3H, =CH-Ph (OH)(OCH3)), 3.18-3.25 (t, 4H, -CH2N+(CH3)2CH2-), 3.413 (t, 2H, -CH2N=CH-Ph (OH)(OCH3)), 3.915 (s, 6H, -CH2N+ (CH3)2CH2-), 5.23 (s, 1H, -CH2N=CH-Ph (OH)(OCH3)), 7-7.38 (m, 3H, CH2N=CH-Ph (OH)(OCH3)), 8.138 (s, 1H, -N=CH-Ph (OH)(OCH3)) (Fig. S2 in Supporting information).
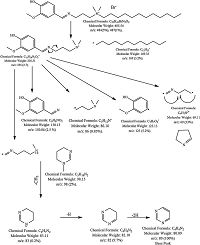
The mass spectra of N-(3-((4-hydroxy-3-methoxybenzylidene) amino) propyl)-N, N-dimethyldodecan-1-ammonium bromide (C12V) showed ion peaks at m/z 485 (5%) (Corresponding to molecular weight), m/z 487 (1%) (Corresponding to isotope peak) and at m/z 80 (100%) (corresponding to base peak) (Fig. S3 in Supporting information). The expected fragmentations are shown in Scheme 2.
|
Download:
|
| Scheme 2. The expected fragments of N-(3-((4-hydroxy-3-methoxybenzylidene) amino) propyl)-N, N-dimethyldodecan-1-ammonium bromide C12V). | |
3.2. Characterization the silver nanoparticles
A fast change in the color of the solution was observed when the solution containing silver nitrate and the prepared surfactant were exposed to sunlight as an indication for the conversion of the silver ions to its nano-form. In this method, the sunlight was used as a reducing agent while the synthesized cationic surfactants were used as the capping agent and accelerating the reaction rate. The solvated electron, which is produced from the hydrolysis of water by sunlight, acts as a strong reducing agent [25]. Some other species result from the photolysis of the water by sunlight like •H and •OH radicals. The presence of the synthesizing cationic surfactants in the reaction mixture scavenges the hydrolyzed water products, hence the hydrolysis of water increases, increasing the amount of the formed solvated electrons, so reaction rate increase [25, 26].
Fig. 1 shows the morphology of the synthesized silver nanoparticles colloid using the synthesized capping agents C10V, C12V, and C16V. The used method produced uniform and well dispersed AgNPs. C10V and C16V produced spherical AgNPs, while C12V produced cubic AgNPs. This may be attributed to the fact that the surfactant C12V is the more effective surfactants in the reduction of surface tension, as it is shown in Table 2. This is an indication of the high accumulation of C12V at the just formed silver nanoparticles surface (i.e. high molar ratio of C12V surfactant) which may be the main factor for controlling the size and producing the cubic shape [27, 28]. From Fig. 1 we can see that by increasing the chain length of the capping agent, the distance between the particles increases (this phenomena confirmed in the latter section using DLS).

|
Download:
|
| Figure 1. TEM image of prepared silver nanoparticle capped by (A) C10V, (B) C12V and (C) C16V. | |
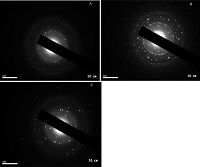
The selected area electron diffraction of synthesized silver nanoparticles (SAED) is shown in Fig. 2, indicating that the prepared silver nanoparticles using this method are polycrystalline [29, 30].
|
Download:
|
| Figure 2. SAED image of prepared silver nanoparticles capped by (A) C10V, (B) C12V and (C) C16V. | |
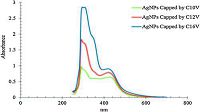
Fig. 2 shows the absorption band at λmax of 420, 420 and 422 nm for silver nanoparticles capped by C10V, C12V, and C16V surfactants, respectively, confirming the formation of silver nanoparticles in the solution. Fig. 3 shows the UV-Vis absorption spectra for the synthesized silver nanoparticles using the different capping agents. The synthesized AgNPs with differing capping agents nonetheless have nearly the same λmax, indicating that the obtained AgNPs have the same size distribution1. Also this conclusion as confirmed by dynamic light scattering technique [16, 25]. We note that by increasing the hydrophobic chain length of the capping agent, the absorption of AgNPs at the λmax increases as a result of increasing the amount of the produced AgNPs in the solution [17, 31, 32]. The absorbance of C10V, C12V, and C16V capping agent at λmax 420, 420, and 422 equals to 0.64, 0.79, and 0.91, respectively.
|
Download:
|
| Figure 3. UV spectra of prepared silver nanoparticles using C10V, C12V and C16V as capping agent. | |
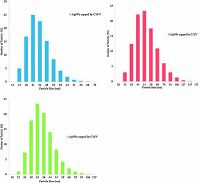
The size distribution of the synthesized silver nanoparticles colloids using different capping agents has been shown in Fig. 4 and Table 1. The obtained data in Table 1 and Fig. 4 reveal that the used method is suitable for synthesizing AgNPs with small size range. The use of sunlight as the reducing agent and C10V, C12V and C16V, as the capping agent produce AgNPs with a size distribution range from 24-44, 21-44 and 21-44 nm, respectively. Altering the alkyl chain of the capping agent does not effect on the size distribution, which confirmed by UV-Vis spectroscopy data in Fig. 3.
|
Download:
|
| Figure 4. Size distribution of synthesized silver nanoparticles using the prepared capping agents. | |
|
|
Table 1 Size distribution of prepared silver nanoparticles using prepared capping agents. |
Table 1 represents the zeta potential of synthesized silver nanoparticles colloids using the different synthesized capping agents. By inspection data in Table 1, the zeta potential of the AgNPs prepared by C10V, C12V and C16V capping agent was 38.4 mV, 45.1 mV and 57.8 mV, respectively. These data indicate that the obtained AgNPs colloid is stable against aggregation, where the charge on silver surface is high, so the electrostatic repulsion between colloid particles prevents aggregation. It is known that when the zeta potential is either higher than +30 mV or lower than -30 mV, the colloid is stable. The positive sign of the zeta potential was ascribed to the capping agent carrying positive charge. Increasing the hydrophobic chain length of the capping agent, the zeta potential of the obtained AgNPs increases, indicating that the stability increase [33-35].
Fig. S4 (Supporting information) shows energy dispersive X-ray spectroscopy analysis of the prepared silver nanoparticles, which confirm the presence of metallic silver in the prepared solutions. The presence of absorption band at 3 keV is typical for metallic silver due to surface plasmon resonance [36, 37]. There are some other absorption bands for C, O and Br elements characteristic for the synthesized cationic surfactants.
Fig. 5, shows the FTIR spectra of the surfactant C16V after the reduction process to confirm its role as capping agent. Fig. 5, shows the following bands at 3018 cm-1, 2922 cm-1, 2851 cm-1 and 1675 cm-1 indicating the presence of the capping agent with the silver nanoparticles. On comparing FTIR spectra of the surfactant C16V before and after the AgNPs formation we note an increasing in the intensity of the band around 1375 cm-1 for the surfactant C16V after the reduction process than before (surfactant alone), which indicate the presence of a co-ordination bond between CH2 of hydrophobic chain length of capping agent and silver nanoparticles surface [17, 25]. This co-ordination bond enhances the role of capping agent's chain lengths in shaping, sizing and distribution as indicated by both transmission electron microscope, UV-Vis spectroscopy and dynamic light scattering. On comparing between the figure in supporting information (S1) and Fig. 5 we note disappearance of band at 3431 cm-1 which correspond to stretching vibration of -OH group, this can attain to the proton scavenger of hydroxyl group by OH• radicals and H• produced from the photolysis of water by sunlight irradiation during silver nanoparticles formation which also confirm that the used cationic surfactants act as capping agent participate in the reduction process by accelerating the reduction process.

|
Download:
|
| Figure 5. FTIR spectrum C16V capped silver nanoparticle. | |
3.3. Surface activity 3.3.1. Critical micelle concentration and surface parameters
The critical micelle concentration (CMC) of the prepared cationic surfactant was obtained graphically from surface tension measurements. Fig. 6 (Fig. S5, Fig. S6 in Supporting information) represents the relation between the surface tension of the cationic surfactant aqueous solution and their concentration at three different temperatures. The abrupt change in the curve corresponds to the critical micelle concentration of the specified cationic surfactant at the specified temperature. The obtained critical micelle concentrations of the synthesized cationic surfactants were listed in Table 2. By analyzing the data in Table 2, it was found that the critical micelle concentration is inversely proportional to the hydrophobic character and temperatures. Increasing the hydrophobic tail of the synthesized cationic surfactant increases the hydrophobicity and thus the free energy of the system. Hence, the surfactant monomers tend to migrate to the interface of the system and/or aggregate in micelles. The migration of surfactant monomers to the surface is followed by a decrease in the surface tension, till surface saturation then the monomers aggregate into clusters which correspond to a steady state in the surface tension. The migration of surfactant to the surface or aggregation in clusters is a way to minimize the free energy of the system [38-41]. Hence, by increasing the hydrophobic tail of the laboratory synthesized cationic surfactant, the concentration at which the monomers aggregated was decreased. Raising the temperature was accompanied by a decrease in CMC as shown in Fig. 7 and Table 2. The temperature has two opposing effects, the first is decreasing the hydration around the hydrophilic head by which the surfactant favor aggregate in clusters; while the second effect is disruption of the water structure around the hydrophobic alkyl tail by which the surfactants disfavor micellization process; therefore, the net effect is the sum of the two opposing effect. From the obtained data in Table 2, the major effect is a decreasing the CMC [42-44].

|
Download:
|
| Figure 6. The surface tension against log concentration of compound C10V at different temperatures. | |

|
Download:
|
| Figure 7. Effect of temperatures and hydrophobic chain length on critical micelle concentration values of prepared cationic surfactants. | |
|
|
Table 2 The surface properties of synthesized cationic surfactant at different temperatures. |
The effectiveness of the surfactant (πCMC) is the difference in the surface tension values of blank distilled water (γo) and surface tension at critical micelle concentration (γCMC) as indicated in the following equation:
| ${{\pi }_{CMC}}={{\gamma }_{0}}-{{\gamma }_{CMC}}$ |
The efficiency of the surfactant (C20) is the concentration of the surfactant capable of the suppressing the surface tension by 20 dyne/cm.
Both calculated efficiency and effectiveness values were listed in Table 2. The data reveal that the surfactant C12V is the more effective surfactant in the reduction of surface tension at the CMC. The effectiveness values of C10V, C12V and C16V surfactant at 25 ℃ were 38.5 mN/m, 40.36 mN/m, and 33.57 mN/m, respectively. The values of CMC/C20 is responsible for determining the most effective surfactant, the greater value of CMC/C20, the greater ability of surfactant to reduce the surface tension at critical micelle concentration (adsorption on the surface is easier than micellization). The surfactant C12V is the more effective surfactant which ascribed to the greater CMC/C20 values at all the tested temperatures 25 ℃, 40 ℃ and 60 ℃, which equal to 120.77, 287.75 and 168.19, respectively [45-47].
By inspection of the efficiency values of the laboratory synthesized cationic surfactant in Table 2, it was found that by increasing both the hydrophobic character and raising the solution temperature, the concentration required to reduce the surface tension decreased by 20 mN/m. Increasing the hydrophobic chain length, followed by increasing the free energy of the system, so the surfactant monomers migrate more rapidly to the surface, hence a higher reduction in the surface tension attained at lower concentration [48, 49].
The maximum surface excess is the surfactant concentration at the interface per unit area, which depends mainly on the hydrophobic chain length and the temperature. The values of maximum surface excess Γmax could be calculated from surface tension measurements using the slope below the critical micelle concentration (δγ/δ log c) in Gibb's adsorption equation [50].
| ${{\mathit{\Gamma }}_{\rm{max}}}=\left( \frac{1}{2.303nRT} \right){{\left( \frac{\delta \gamma }{\delta \rm{log}c} \right)}_{T}}$ |
where R is the gas constant, n is the number of active species (n equal 2 for monovalent cationic surfactant with monovalent counter ion) and T is the absolute temperature.
The minimum average surface area is the average area (in square angstrom) occupied by each monomer adsorbed at the system interface [21].
The minimum surface area, Amin of the synthesized cationic surfactant has been calculated using Gibb's adsorption equation:
| ${{A}_{\rm{min}}}=\frac{{{10}^{6}}}{{{\mathit{\Gamma }}_{\rm{max}}}N}$ |
The calculated Γmax and Amin values are listed in Table 2. By inspecting the data in Table 2, it is revealed that the Γmax decreases with increasing the solution temperature and the hydrophobic character. Increasing both temperature and hydrophobic character causes an increase in the free energy, so the surfactant monomers are directed toward the surface at lower concentrations, so Γmax decreases and so the packing densities of synthesized surfactants at the surface decreased. This forces the organization of surfactant molecules to be less perpendicular. In addition, the long chain of the tail paired with the head group's lower rigidity makes it more prone to coil, so the average minimum surface area increases [51, 52].
3.3.2. Micellization and adsorption thermodynamic studyThe adsorption and micellization thermodynamic parameters of the laboratory synthesized cationic surfactant have been calculated by the Gibb's adsorption equation [51, 53-56]:
| $\begin{align} &\Delta G_{mic}^{0}=RT\rm{ln}\left( {{X}_{CMC}} \right) \\ &\Delta G_{ads}^{0}=\Delta G_{mic}^{0}-\left( \frac{{{\pi }_{CMC}}}{{{\mathit{\Gamma }}_{\rm{max}}}} \right) \\ &\Delta H_{mic}^{0}=\frac{d\left( \Delta G_{mic}^{0}/\Delta T \right)}{d\left( 1/T \right)}=-R{{T}^{2}}\left[ \frac{d\rm{ln}{{X}_{CMC}}}{dT} \right] \\ &\Delta H_{ads}^{0}=\frac{d\left( \Delta G_{ads}^{0}/\Delta T \right)}{d\left( 1/T \right)}=-{{T}^{2}}d\left( \Delta G_{ads}^{0}/T \right)/dT \\ &\Delta {{S}_{mic}}=\left( \frac{\Delta H_{mic}^{0}-\Delta G_{mic}^{0}}{\Delta T} \right) \\ &\Delta {{S}_{ads}}=\left( \frac{\Delta H_{ads}^{0}-\Delta G_{ads}^{0}}{\Delta T} \right) \\ \end{align}$ |
The micellization and adsorption behavior have been studied at 25 ℃, 40 ℃ and 60 ℃ and the calculated thermodynamic parameters were listed in Table 3. The values of the change in the free energy of adsorption ΔGadso and micellization ΔGmico are negative, indicating that the two processes are spontaneous. Therefore, the adsorption at the interface or aggregation in clusters is more favorable than the presence of the surfactant monomers in the bulk solution. From the data in Table 3, we note that the change in free energy of adsorption and micellization processes increases in the negative direction by increasing the hydrophobicity and raising the solution temperature. Increasing the chain length of laboratory-synthesized surfactants was accompanied by increasing the hydrophobicity of the aqueous system, hence destroying the water structure and increasing the free energy of the system. So, the surfactant monomers migrate to the surface and/or aggregates into clusters: this behavior decreases the energy of the system, so the change in the free energy of the prepared surfactant-solvent system will be decreased and increased in the negative direction [57, 6, 58].
|
|
Table 3 Micellization and adsorption thermodynamic parameters of the prepared cationic surfactants. |
Increasing the temperature of the surfactant aqueous system causes a decrease of hydration around the hydrophilic group, so the hydrophobicity of the system increases, and accompanied by increasing the energy of the system, so molecules of surfactant tend to adsorb and form micelles to decrease the energy of the system.
By comparing ΔGmico and ΔGadso in Table 3, we note that the change in the free energy of adsorption of the synthesized cationic surfactant is higher, which indicates that the adsorption process tends to adsorb on the surface till complete surface coverage, after which the surfactant monomers aggregates in clusters.
The change in the entropy of both micellization ΔSmic and adsorption ΔSads values was listed in Table 3. The positive sign indicates the disruption of the water structure around the cationic surfactant when they transfer from the aqueous bulk to the airwater interface or to the micellar interior. The change in the entropy of adsorption ΔSads is more positive than that of micellization ΔSmic. This reflects greater freedom of the hydrophobic part through motion to the interface than to form micelle [57, 59].
3.4. Antimicrobial activityThe antibiotic effects of the prepared cationic surfactants (C10V, C12V and C16V) and their corresponding silver nanostructures were determined against Gram-positive bacteria, Gram-negative bacteria, and fungi. From the obtained data listed in Table 4, it was found that the synthesized surfactant C12V has the maximum antibiotic effect against all the tested strains. The antibiotic effect increased as the hydrophobic chain length increased to 12 carbon atoms (C12V), then decreased again as the length increased to 16 carbon atoms (C16V). This effect is known as the cut-off effect and was observed in the surfactant alone and the surfactant with their silver nanostructure [59-64]. The cut-off behavior depends on some properties, like the change in the free energy of adsorption of prepared cationic surfactant on the bacteria cell membrane, critical micelle concentration of the used surfactant, the hydrophobic character, and the size of diffused surfactant. The magnitude of all these parameters governs the cut-off point. The obtained data in Table 4 reveal that the prepared cationic surfactant C12V and their AgNPs have the maximum effect on Gram positive and Gram-negative bacteria and on fungi. By comparing the antibiotic effect, we found that the silver nanoparticles made an enhancement for the antibiotic effect of the synthesized cationic surfactants. The inhibition zone of C12V on Sarcina lutea bacteria was 33 mm while it is becomes 37 when it incorporated with silver nanoparticles. This can be attributed to the high surface area of synthesized silver nanoparticles, which has biological activity in addition to the acquired positive charge, which facilitates adsorption at the negative cell wall of bacteria.
|
|
Table 4 Antimicrobial activity of synthesized surfactants and their silver nanostructure against pathogenic bacteria and fungi. |
The target of cationic surfactants antibiotic and their silver nanostructure is the cytoplasmic membrane, which is composed of a phospholipid bilayer where proteins are anchored. The outer surface of the Gram-positive bacterial cell wall carries a negative charge due to the presence of some Mg2+ and Ca2+ divalent cations. Cationic antimicrobial surfactants interact with the cell surface and migrate into the cytoplasmic membrane of the cell. Such an interaction disorganizes growth, which is sufficient to cause fluidity loss of the membrane, causing cell death [65-70].
4. ConclusionWe can conclude that the chemical structure of the laboratory synthesized cationic surfactant affects of the size distribution, stability, and the quantity of the produced silver nanoparticles. Increasing the length of the hydrophobic chain length of the used capping agent increased the stability of the AgNPs, which is indicated by the increased zeta potential. The amount of the produced AgNPs increased as the hydrophobic tail length of the capping agent increased. The reported method for the synthesis of AgNPs produces a narrow size distribution. The synthesized cationic surfactant showed a good tendency to adsorb at the surface and aggregate into micelles. The chemical structure of the synthesized cationic surfactant governs two processes. The synthesized cationic surfactant molecules tend to adsorb first on the surface until complete surface coverage, after which they aggregate into micelles. The silver nanoparticles enhanced the biological activity of the synthesized cationic surfactant. The synthesized C12V surfactant and its AgNPs have the best antibiotic effect against the tested strains.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.09.010.
| [1] | N.A. Negm, A.A. Abd-Elaal, D.E. Mohamed, A.F. El-Farargy, S. Mohamed, Synthesis and evaluation of silver nanoparticles loaded with Gemini surfactants:surface and antimicrobial activity. J. Ind. Eng. Chem. 24 (2015) 34–41. DOI:10.1016/j.jiec.2014.09.006 |
| [2] | T. Mishra, R.K. Sahu, S.H. Lim, L.G. Salaman-Riba, S. Bhattacharjee, Hexadecylamine capped silver and gold nanoparticles:comparative study on formation and self-organization. Mater. Chem. Phys. 123 (2010) 540–545. DOI:10.1016/j.matchemphys.2010.05.011 |
| [3] | S.S. Wei, X.Y. Xu, Y.J. Liu, J.M. Yang, Preparation of hydrophobic nano-silver colloid and aqueous nano-silver colloid by phase transfer. Mater. Chem. Phys. 126 (2011) 12–15. DOI:10.1016/j.matchemphys.2010.11.012 |
| [4] | A.M. Al Sabagh, Surface and thermodynamic properties of p-alkylbenzene polyethoxylated and glycerated sulfonate derivative surfactants. Colloids Surf. A:Physicochem. Eng. Asp. 134 (1998) 313–320. DOI:10.1016/S0927-7757(97)00205-7 |
| [5] | V.I. Martín, R.R. de la Haba, A. Ventosa, Colloidal and biological properties of cationic single-chain and dimeric surfactants. Colloids Surf. B 114 (2014) 247–254. DOI:10.1016/j.colsurfb.2013.10.017 |
| [6] | I. Ahmad, P. Patial, C. Kaur, S. Kaur, Cationic imidazolium monomeric surfactants:their synthesis and surface active properties. J. Surf. Deterg. 17 (2014) 269–277. DOI:10.1007/s11743-013-1527-4 |
| [7] | S.M. Shaban, I. Aiad, M.M. El-Sukkary, E.A. Soliman, M.Y. El-Awady, Inhibition of mild steel corrosion in acidic medium by vanillin cationic surfactants. J. Mol. Liq. 203 (2015) 20–28. DOI:10.1016/j.molliq.2014.12.033 |
| [8] | M.I. Abdou, A.M. Al-sabagh, M.M. Dardir, Evaluation of Egyptian bentonite and nano-bentonite as drilling mud. Egypt. J. Petrol. 22 (2013) 53–59. DOI:10.1016/j.ejpe.2012.07.002 |
| [9] | S.M. Shaban, I. Aiad, M.M. El-Sukkary, E.A. Soliman, M.Y. El-Awady, Evaluation of some cationic surfactants based on dimethylaminopropylamine as corrosion inhibitors. J. Ind. Eng. Chem. 21 (2015) 1029–1038. DOI:10.1016/j.jiec.2014.05.012 |
| [10] | W. Zieliński, K.A. Wilk, G. Para, Synthesis, surface activity and antielectrostatic properties of new soft dichain cationic surfactants. Colloids Surf. A:Physicochem. Eng. Asp. 480 (2015) 63–70. DOI:10.1016/j.colsurfa.2015.01.050 |
| [11] | M. Potempa, M. Hafner, C. Frech, Mechanism of Gemini disulfide detergent mediated oxidative refolding of lysozyme in a new artificial chaperone system. Protein J. 29 (2010) 457–465. DOI:10.1007/s10930-010-9279-8 |
| [12] | C.C. Lai, K.M. Chen, Dyeing properties of modified Gemini surfactants on a disperse dye-polyester system. Text. Res. J. 78 (2008) 382–389. DOI:10.1177/0040517507087676 |
| [13] | R. Janardhanan, M. Karuppaiah, N. Hebalkar, T.N. Rao, Synthesis and surface chemistry of nano silver particles. Polyhedron 28 (2009) 2522–2530. DOI:10.1016/j.poly.2009.05.038 |
| [14] | Z. Khan, S.A. Al-Thabaiti, A.Y. Obaid, A.O. Al-Youbi, Preparation and characterization of silver nanoparticles by chemical reduction method. Colloids Surf. B 82 (2011) 513–517. DOI:10.1016/j.colsurfb.2010.10.008 |
| [15] | Z.L. Yang, D.D. Zhai, X. Wang, J. Wei, In situ synthesis of highly mono dispersed non aqueous small-sized silver nano-colloids and silver/polymer nanocomposites by ultraviolet photo polymerization. Colloids Surf. A:Physicochem. Eng. Asp. 448 (2014) 107–114. DOI:10.1016/j.colsurfa.2014.02.017 |
| [16] | I. Aiad, M.M. El-Sukkary, E.A. Soliman, M.Y. El-Awady, S.M. Shaban, In situ and green synthesis of silver nanoparticles and their biological activity. J. Ind. Eng. Chem. 20 (2014) 3430–3439. DOI:10.1016/j.jiec.2013.12.031 |
| [17] | S.M. Shaban, I. Aiad, M.M. El-Sukkary, E.A. Soliman, M.Y. El-Awady, Preparation of capped silver nanoparticles using sunlight and cationic surfactants and their biological activity. Chin. Chem. Lett. 26 (2015) 1415–1420. DOI:10.1016/j.cclet.2015.06.006 |
| [18] | N.M. Zaina, A.G.F. Stapley, G. Sham, Green synthesis of silver and copper nanoparticles using ascorbic acid and chitosan for antimicrobial applications. Carbohydr. Polym. 112 (2014) 195–202. DOI:10.1016/j.carbpol.2014.05.081 |
| [19] | C.J. Lee, K.Y. Nam, D.Y. Kim, New routes to the preparation of silver-soft liner nanocomposites as an antibacterial agent. J. Ind. Eng. Chem. 20 (2014) 1276–1279. DOI:10.1016/j.jiec.2013.07.004 |
| [20] | M.E. Young, H.I. Alakomi, I. Fortune, Development of a biocidal treatment regime to inhibit biological growths on cultural heritage:BIODAM. Environ. Geol. 56 (2008) 631–641. DOI:10.1007/s00254-008-1455-1 |
| [21] | I. Aiad, M.M. El-Sukkary, E.A. Soliman, M.Y. El-Awady, S.M. Shaban, Characterization, surface properties and biological activity of new prepared cationic surfactants. J. Ind. Eng. Chem. 20 (2014) 1633–1640. DOI:10.1016/j.jiec.2013.08.010 |
| [22] | S. Chavda, P. Bahadur, V.K. Aswal, Interaction between nonionic and Gemini (cationic) surfactants:effect of spacer chain length. J. Surf. Deterg. 14 (2011) 353–362. DOI:10.1007/s11743-011-1263-6 |
| [23] | S.M. Shaban, I. Aiad, M.M. El-Sukkary, E.A. Soliman, M.Y. El-Awady, Synthesis, surface, thermodynamic properties and biological activity of dimethylaminopropylamine surfactants. J. Ind. Eng. Chem. 20 (2014) 4194–4201. DOI:10.1016/j.jiec.2014.01.020 |
| [24] | D.N. Muanza, B.W. Kim, K.L. Euler, L. Williams, Antibacterial and antifungal activities of nine medicinal plants from Zaire. Int. J. Pharm. 32 (1994) 337–345. DOI:10.3109/13880209409083012 |
| [25] | J.W.T. Spinks, R.J.Y. Woods, An Introduction to Radiation Chemistry, 3rd ed., Wiley Interscience, New York, 1990p. 255. |
| [26] | S.M. Shaban, I. Aiad, M.M. El-Sukkary, E.A. Soliman, M.Y. El-Awady, One step green synthesis of hexagonal silver nanoparticles and their biological activity. J. Ind. Eng. Chem. 20 (2014) 4473–4481. DOI:10.1016/j.jiec.2014.02.019 |
| [27] | B. Wiley, T. Herricks, Y.G. Sun, Y.N. Xia, Polyol synthesis of silver nanoparticles:use of chloride and oxygen to promote the formation of single-crystal, truncated cubes and tetrahedrons. Nano Lett. 4 (2004) 1733–1739. DOI:10.1021/nl048912c |
| [28] | A. Tao, P. Sinsermsuksakul, P.D. Yang, Polyhedral silver nanocrystals with distinct scattering signatures. Angew. Chem. Int. Ed. 45 (2006) 4597–4601. DOI:10.1002/(ISSN)1521-3773 |
| [29] | K. Yvon, W. Jeitschko, E. Parthé, LAZY PULVERIX, a computer program, for calculating X-ray and neutron diffraction powder patterns. J. Appl. Crystallogr. 10 (1977) 73–74. DOI:10.1107/S0021889877012898 |
| [30] | G.A. Bhaduri, R. Little, R.B. Khomane, Green synthesis of silver nanoparticles using sunlight. J. Photochem. Photobiol. A:Chem. 258 (2013) 1–9. DOI:10.1016/j.jphotochem.2013.02.015 |
| [31] | M.V. Roldán, L.B. Scaffardi, O. de Sanctis, N. Pellegri, Optical properties and extinction spectroscopy to characterize the synthesis of amine capped silver nanoparticles. Mater. Chem. Phys. 112 (2008) 984–990. DOI:10.1016/j.matchemphys.2008.06.057 |
| [32] | M. Chen, Y.G. Feng, X. Wang, Silver nanoparticles capped by oleylamine:formation, growth, and self-organization. Langmuir 23 (2007) 5296–5304. DOI:10.1021/la700553d |
| [33] | J. Hedberg, M. Lundin, T. Lowe, Interactions between surfactants and silver nanoparticles of varying charge. J. Colloids Interface Sci. 369 (2012) 193–201. DOI:10.1016/j.jcis.2011.12.004 |
| [34] | M. Zargar, K. Shameli, G.R. Najafi, F. Farahani, Plant mediated green biosynthesis of silver nanoparticles using Vitex negundo L. extract. J. Ind. Eng. Chem. 20 (2014) 4169–4175. DOI:10.1016/j.jiec.2014.01.016 |
| [35] | S. Elzey, V.H. Grassian, Agglomeration, isolation and dissolution of commercially manufactured silver nanoparticles in aqueous environments. J. Nanopart. Res. 12 (2010) 1945–1958. DOI:10.1007/s11051-009-9783-y |
| [36] | K.S. Lokesh, A. Shambhulinga, N. Manjunatha, M. Imadadulla, M. Hojamberdiev, Porphyrin macrocycle-stabilized gold and silver nanoparticles and their application in catalysis of hydrogen peroxide. Dyes Pigm. 120 (2015) 155–160. DOI:10.1016/j.dyepig.2015.04.002 |
| [37] | S.X.Hu ${referAuthorVo.mingEn}, Y.L.Hsieh ${referAuthorVo.mingEn}, Synthesis of surface bound silver nanoparticles on cellulose fibers using lignin as multi-functional agent. Carbohydr. Polym. 131 (2015) 134–141. DOI:10.1016/j.carbpol.2015.05.060 |
| [38] | S.M. Shaban, I. Aiad, M.M. El-Sukkary, E.A. Soliman, M.Y. El-Awady, Surface and biological activity of N-(dimethoxybenzylidene) amino) propyl)-N, N-dimethylalkyl-1-ammonium derivatives as cationic surfactants. J. Mol. Liq. 207 (2015) 256–265. DOI:10.1016/j.molliq.2015.03.043 |
| [39] | P. Patial, A. Shaheen, I. Ahmad, Synthesis, surface active and thermal properties of novel imidazolium cationic monomeric surfactants. J. Ind. Eng. Chem. 20 (2014) 4267–4275. DOI:10.1016/j.jiec.2014.01.032 |
| [40] | B. Gao, M.M. Sharma, A family of alkyl sulfate gemini surfactants. 1. Characterization of surface properties. J. Colloids Interface Sci. 404 (2013) 80–84. DOI:10.1016/j.jcis.2013.04.043 |
| [41] | X. Zhong, J.W. Guo, L.J. Feng, X.J. Xu, D.Y. Zhu, Cationic Gemini surfactants based on adamantane:synthesis, surface activity and aggregation properties. Colloids Surf. A:Physicochem. Eng. Asp. 441 (2014) 572–580. DOI:10.1016/j.colsurfa.2013.10.016 |
| [42] | S.M. Shaban, I. Aiad, H.A. Fetouh, A. Maher, Amidoamine double tailed cationic surfactant based on dimethylaminopropylamine:synthesis, characterization and evaluation as biocide. J. Mol. Liq. 212 (2015) 699–707. DOI:10.1016/j.molliq.2015.10.024 |
| [43] | M.A. Rub, M.S. Sheikh, F. Khan, S.B. Khan, A.M. Asiri, Bile salts aggregation behavior at various temperatures under the influence of amphiphilic drug imipramine hydrochloride in aqueous medium. Z. Phys. Chem. 228 (2014) 747–767. |
| [44] | C.C. Ruiz, Thermodynamics of micellization of tetradecyltrimethylammonium bromide in ethylene glycol-water binary mixtures. Colloids Polym. Sci. 277 (1999) 701–707. DOI:10.1007/s003960050443 |
| [45] | T. Yoshimura, T. Kusano, H. Iwase, M. Shibayama, T. Ogawa, H. Kurata, Starshaped trimeric quaternary ammonium bromide surfactants:adsorption and aggregation properties. Langmuir 28 (2012) 9322–9331. DOI:10.1021/la301220y |
| [46] | S.M. Shaban, I. Aiad, H.Y. Moustafa, A. Hamed, Amidoamine gemini surfactants based dimethylamino propyl amine:preparation, characterization and evaluation as biocide. J. Mol. Liq. 212 (2015) 907–914. DOI:10.1016/j.molliq.2015.10.048 |
| [47] | L.F. Zhi, Q.X. Li, Y.L. Li, Y.B. Song, Synthesis, adsorption and aggregation properties of new saccharide-cationic surfactants. Colloids Surf. A:Physicochem. Eng. Asp. 436 (2013) 684–692. DOI:10.1016/j.colsurfa.2013.08.009 |
| [48] | C.C. Ren, F. Wang, Z.Q. Zhang, Synthesis, surface activity and aggregation behavior of Gemini imidazolium surfactants 1, 3-bis (3-alkylimidazolium-1-yl) propane bromide. Colloids Surf. A:Physicochem. Eng. Asp. 467 (2015) 1–8. DOI:10.1016/j.colsurfa.2014.11.031 |
| [49] | M.T. Garcia, I. Ribosa, L. Perez, A. Manresa, F. Comelles, Self-assembly and antimicrobial activityof long-chain amide-functionalized ionic liquids in aqueous solution. Colloids Surf. B 123 (2014) 318–325. DOI:10.1016/j.colsurfb.2014.09.033 |
| [50] | R. Kamboj, S. Singh, V. Chauhan, Synthesis, characterization and surface properties of N-(2-hydroxyalkyl)-N'-(2-hydroxyethyl) imidazolium surfactants. Colloids Surf. A:Physicochem. Eng. Asp. 441 (2014) 233–241. DOI:10.1016/j.colsurfa.2013.08.063 |
| [51] | M.J. Rosen, Surfactants and Interfacial Phenomena, 2nd ed., Wiley, New York, 2nd ed., Wiley: New York, 1989 . |
| [52] | B. Dong, X.Y. Zhao, L.Q. Zheng, Aggregation behavior of long-chain imidazolium ionic liquids in aqueous solution:micellization and characterization of micelle microenvironment. Colloids Surf. A:Physicochem. Eng. Asp. 317 (2008) 666–672. DOI:10.1016/j.colsurfa.2007.12.001 |
| [53] | G.Z. Cao, X.F. Guo, L.H. Jia, X.H. Tian, Aggregation behaviours and bactericidal activities of novel cationic surfactants functionalized with amides and ether groups. RSC Adv. 5 (2015) 27197–27204. DOI:10.1039/C4RA14645J |
| [54] | Z.L. Zhao, X.F. Guo, L.H. Jia, Y.Y. Liu, Synthesis and properties of quaternary ammonium surfactants containing a methoxy benzyl substitute. RSC Adv. 4 (2014) 56918–56925. DOI:10.1039/C4RA07363K |
| [55] | V. Chauhan, S. Singh, R. Mishra, G. Kaur, Synthesis and bio-physicochemical properties of amide-functionalized N-methylpiperazinium surfactants. J. Colloids Interface Sci. 436 (2014) 122–131. DOI:10.1016/j.jcis.2014.08.029 |
| [56] | B. Kumar, D. Tikariha, K.K. Ghosh, N. Barbero, P. Quagliotto, Effect of polymers and temperature on critical micelle concentration of some gemini and monomeric surfactants. J. Chem. Thermodyn. 62 (2013) 178–185. DOI:10.1016/j.jct.2013.03.006 |
| [57] | A.A. Abd-Elaal, S.M. Tawfik, S.M. Shaban, Simple one step synthesis of nonionic dithiol surfactants and their self-assembling with silver nanoparticles:characterization, surface properties, biological activity. Appl. Surf. Sci. 342 (2015) 144–153. DOI:10.1016/j.apsusc.2015.03.038 |
| [58] | S. Chauhan, K. Sharma, Effect of temperature and additives on the critical micelle concentration and thermodynamics of micelle formation of sodium dodecyl benzene sulfonate and dodecyltrimethylammonium bromide in aqueous solution:a conductometric study. J. Chem. Thermodyn. 71 (2014) 205–211. DOI:10.1016/j.jct.2013.12.019 |
| [59] | S.M. Tawfik, A.A. Abd-Elaal, S.M. Shaban, A.A. Roshdy, Surface, thermodynamic and biological activities of some synthesized Gemini quaternary ammonium salts based on polyethylene glycol. J. Ind. Eng. Chem. 30 (2015) 112–119. DOI:10.1016/j.jiec.2015.05.011 |
| [60] | A.S. Janoff, M.J. Pringle, K.W. Miller, Correlation of general anesthetic potency with solubility in membranes. Biochim. Biophys. Acta 649 (1981) 125–128. DOI:10.1016/0005-2736(81)90017-1 |
| [61] | G. Viscardi, P. Quagliotto, C. Barolo, Synthesis and surface and antimicrobial properties of novel cationic surfactants. J. Org. Chem. 65 (2000) 8197–8203. DOI:10.1021/jo0006425 |
| [62] | M.J. Pringle, K.B. Brown, K.W. Miller, Can the lipid theories of anesthesia account for the cutoff in anesthetic potency in homologous series of alcohols. Mol. Pharmacol. 19 (1981) 49–55. |
| [63] | H. Nagamune, T. Maeda, K. Ohkura, Evaluation of the cytotoxic effects of bisquaternary ammonium antimicrobial reagents on human cells. Toxicol. In Vitro 14 (2000) 139–147. DOI:10.1016/S0887-2333(00)00003-5 |
| [64] | F. Devínsky, A. Kopecka-Leitmanová, F. Šeršeň, P. Balgavý, Cut-off effect in antimicrobial activity and in membrane perturbation efficiency of the homologous series of N, N-dimethylalkylamine oxides. J. Pharm. Pharmacol. 42 (1990) 790–794. DOI:10.1111/jphp.1990.42.issue-11 |
| [65] | C. Campanac, L. Pineau, A. Payard, G. Baziard-Mouysset, C. Roques, Interactions between biocide cationic agents and bacterial biofilms. Antimicrob. Agents Chemother. 46 (2002) 1469–1474. DOI:10.1128/AAC.46.5.1469-1474.2002 |
| [66] | A.M. Badawi, M.A. Mekawi, A.S. Mohamed, M.Z. Mohamed, M.M. Khowdairy, Surface and biological activity of some novel cationic surfactants. J. Surf. Deterg. 10 (2007) 243–255. DOI:10.1007/s11743-007-1040-8 |
| [67] | L. Pérez, A. Pinazo, R. Pons, M. Infante, Gemini surfactants from natural amino acids. Adv. Colloids Interface Sci. 205 (2014) 134–155. DOI:10.1016/j.cis.2013.10.020 |
| [68] | I. Sondi, B. Salopek-Sondi, Silver nanoparticles as antimicrobial agent:a case study on E. coli as a model for Gram-negative bacteria. J. Colloids Interface Sci. 275 (2004) 177–182. DOI:10.1016/j.jcis.2004.02.012 |
| [69] | H.M. Willemen, L.C.P.M. de Smet, A. Koudijs, Micelle formation and antimicrobial activity of cholic acid derivatives with three permanent ionic head groups. Angew. Chem. Int. Ed. 41 (2002) 4275–4277. DOI:10.1002/1521-3773(20021115)41:22<4275::AID-ANIE4275>3.0.CO;2-U |
| [70] | M. Tiecco, G. Cardinali, L. Roscini, R. Germani, L. Corte, Biocidal and inhibitory activity screening of de novo synthesized surfactants against two eukaryotic and two prokaryotic microbial species. Colloids Surf. B 111 (2013) 407–417. DOI:10.1016/j.colsurfb.2013.06.033 |
 2017, Vol. 28
2017, Vol. 28