b College of Pharmacy, East China University of Science & Technology, Shanghai 550025, China
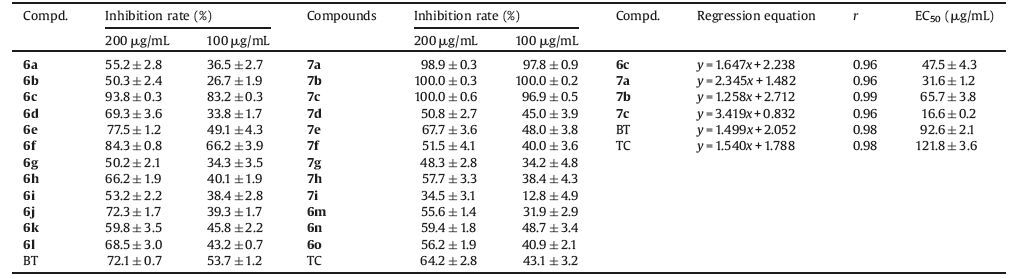
Heterocyclic substructures have been extensively studied for their powerful applications in construction of bioactive compounds [1-4]. Among them, pyrazole ring as an important functional group has already been used in the development of pharmaceuticals and agrochemicals due to its derivatives bearing multitudinous bioactivities, including anti-inflammatory, antitumor, herbicidal, insecticidal, antifungal, and antibacterial activities [5-13]. Furthermore, some pyrazole compounds have already been commercialized as fungicides, like sedaxane (Syngenta, 2005), isopyrazam (Syngenta, 2006), bixafen (Bayer, 2005), and fluxapyroxad (BASF, 2008) [14-17]. As another crucial scaffold, 1, 3, 4-oxadiazole, has exerted promising applications in creating new agrochemicals on account of the diverse bioactivities of its derivatives [18-21]. In our previous work, we had found a series of new 1, 3, 4-oxadiazole sulfone compounds (structure depicted in Fig. 1, lead compound) with high antibacterial/fungicidal bioactivities [22-24]. In order to find new structures with antibacterial/antifungal bioactivities, the two functional moieties of pyrazole and 1, 3, 4-oxadiazole were combined into one molecule by replacing the phenyl group to pyrazole moiety at the 5-position of the lead compound, as shown in Fig. 1. All the title compounds were bioassayed against pathogenic bacteria Xanthomonas oryzae pv. oryzae (Xoo) and five phytopathogenic fungi.
|
Download:
|
| Figure 1. Design strategy of the target compounds. | |
2. Experimental
All the chemicals were purchased from Aladdin and used as received. The organic solvents were distilled before used. NMR spectra were obtained by using a JEOL-ECX-500 apparatus. Chemical shifts were reported in parts per million (ppm) down field from TMS with the solvent resonance as the internal standard. Coupling constants (J) were reported in Hz and referred to apparent peak multiplications. MS data were recorded on an Agilent ESI-MSD Trap (VL) mass instrument.
2.1. General synthetic procedures for the target compounds (6a-6o) and (7a-7i)A solution of carbon disulfide (0.015 mol) in ethanol (10 mL) was added dropwise to the mixture of compound 4 (0.01 mol) and potassium hydroxide (0.012 mol) in ethanol (40 mL) at room temperature. Then, the reaction mixture was heated under reflux with stirring for 8 h. After the reaction was completed, ethanol was evaporated to give unpurified intermediate 5. An appropriate halohydrocarbon (0.01 mol) was added to the solution of unpurified intermediate 5 in water (20 mL) and the mixture was stirred for 1 h at room temperature. The solid was filtered, purified by column chromatography using a mixture of petroleum ether and ethyl acetate (10:1) as the eluent, and then the pure target compounds (6a-6o) were obtained.
The compound (6a-6i) (5 mmol) and acetic acid (15 mL) were added to a 50 mL three-neck round-bottom flask equipped with a magnetic stirrer. The resulting solution was stirred for 10 min when a clear solution was obtained, and then 7% KMnO4 solution (5 mmol) was added dropwise at room temperature and the progress of the reaction was monitored by thin layer chromatography (TLC) using petroleum ether:ethyl acetate (3:1). After the reaction was completed, 10% NaHSO3 solution was added to deoxidize the unreacted KMnO4. The resulted solid was filtered, washed with water, from which the pure compounds (7a-7i) can be obtained by column chromatography using a mixture of petroleum ether and ethyl acetate (15:1) as the eluent.
2.2. in vitro antibacterial bioassay (turbidimeter test)In our study, all the synthesized target compounds were evaluated for their antibacterial activities against Xoo by the turbidimeter test in vitro. Dimethylsulfoxide in sterile distilled water served as a blank control, Bismerthiazol and Thiodiazole Copper served as the positive controls. Approximately 40 μL of solvent NB (1.5 g beef extract, 2.5 g peptone, 0.5 g yeast powder, 5.0 g glucose, and 500 mL distilled water; pH 7.0-7.2) containing Xoo, incubated on the phase of logarithmic growth, was added to 5 mL of solvent NB containing the test compounds and positive control. The inoculated test tubes were incubated at 28±1 ℃ and continuously shaken at 180 rpm for 24-48 h until the bacteria were incubated on the logarithmic growth phase. The growth of the cultures was monitored on a microplate reader by measuring the optical density at 595 nm (OD595) given by turbidity corrected values=ODbacterial wilt-ODno bacterial wilt, and the inhibition rate I was calculated by I=(C -T)/C × 100%. C is the corrected turbidity values of bacterial growth on untreated NB (blank control), and T is the corrected turbidity values of bacterial growth on treated NB. The experiment was repeated three times.
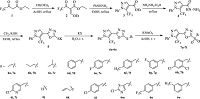
3. Results and discussionThe synthesis and structures of (6a-6o), and (7a-7i) are shown in Scheme 1. Briefly, ethyltrifluoroacetoacetate (1) was treated with triethoxymethane to give intermediate (E)-2-trifluoroacetyl-3-ethoxy-2-propenoate (2), followed by the cyclocondensation reaction to provide an important intermediate ethyl 1-phenyl-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate (3) containing pyrazole group in 82% yield. Next, the hydrazide 4 was obtained through refluxing 3 in hydrazine hydrate with the yield of 94%. A subsequent reaction with carbon disulfide in the presence of potassium hydroxide leaded to the formation of the crucial intermediate 5 containing 1, 3, 4-oxadiazole. Finally, the corresponding target thioethers (6a-6o) were achieved via thioetherification with halogenated agents in good yields ranging from 76% to 85%, and subsequently converted into the corresponding sulfones (7a-7i) by oxidizing the related thioether at room temperature. All the structures were confirmed by 1H NMR, 13C NMR, and MS (detailed information see Supplementary data).
|
Download:
|
| Scheme 1. Synthetic route of 2-(thioether/sulfone)-5-pyrazolyl-1, 3, 4-oxadiazole derivatives (6a-6o) and (7a-7i). | |
In our study, we first evaluated the antibacterial activity of all the title compounds via turbidmeter test [25-27] against pathogenic bacteria Xanthomonas oryzae pv. oryzae (Xoo), which was considered as one of devastative bacteria against rice in ricegrowing countries. Meanwhile, the commercial agricultural antibacterial bismerthiazol (BT) and thiodiazole copper (TC) were employed for the comparison of bioactivity in vitro. Preliminary bioassays revealed that most of the target compounds exerted appreciable inhibition bioactivity against Xoo in the dosage of 200 or 100 μg/mL (Table 1). Among them, compounds 6c, 6e, 6f, 6j, 7a, 7b, and 7c gives the inhibition rate above 72.3% against Xoo in the dosage of 200 μg/mL, which were better than that of BT (72.1%) and TC (64.2%); while compounds 6c, 6f, 7a, 7b, and 7c offersbetter inhibition rate above 66.2% against Xoo than that of BT (53.7%) and TC (43.1%) in the dosage of 100 μg/mL. The half-maximal effective concentration (EC50) values of 6c, 7a, 7b, and 7c were detected as 47.5, 31.6, 65.7, and 16.6 μg/mL, respectively, which were obviously better than that of commercial bactericides (92.6 or 121.8 μg/mL). Based on the above results, among all the thioether compounds (6a-6o), the isopropyl group compound (6c) exhibited the best bioactivity against Xoo than the other groups, while for benzyl thioether compounds, 4-methylbenzyl thioether (6f) gives superior activity than the other substituted benzyl in the dosage of 200 μg/mL or 100 μg/mL. For sulfone compounds, the antibacterial activity of alkyl sulfone compounds (such as 7a-7c) was dramatically better than the benzyl derivatives.
|
|
Table 1 Inhibition effect of sulfides/sulfones against Xoo. |
The antifungal activity of (6a-6o) and (7a-7i) was examined via the poisonplate technique [28] against fivephytopathogenic fungi, Gibberella zeae (G. z.), Fusarium oxysporum (F. o.), Cytospora mandshurica (C. m.), Sclertinia sclerotiorum (S. s.), and Rhizoctonia solani (R. s.) at the concentrate of 100 μg/mL, Meanwhile, the commercial agricultural antifungal Hymexazol (HM) and Carbendazim (CB) were employed for the comparison of bioactivity. As shown in Table 2, compounds 7a and 7c were observed having comprehensive antifungal activity with the inhibition rate ranging from 53.8% to 75.5% against the five kinds of fungi, which were comparable to the commercial fungicide HM. It is worth pointing out that compound 6j exerted good antifungal activity with the inhibition rate of 86.4% against S. sclerotiorum. In comparison of 6a and 7a, 6b and 7b, 6c and 7c, 6d and 7d, 6f and 7f, the antifungal activity was improved after oxidizing the thioether into the sulfone, further suggested sulfonyl group as a crucial functional group may improve the bioactivity of the target compound. It can be seen that compound 7a showed the strongest antifungi activity against the five phytopathogenic fungi.
|
|
Table 2 Inhibition effect of sulfides/sulfones at 100 μg/mL against five phytopathogenic fungi. |
4. Conclusion
In summary, a series of 2-(thioether/sulfone)-5-pyrazolyl-1, 3, 4-oxadiazole derivatives containing both pyrazole moiety and 1, 3, 4-oxadiazole moiety were designed and synthesized, and which antibacterial activity and antifungal activity were evaluated via turbidmeter test or the poison plate technique in vitro. Compounds 6c, 7a, 7b and 7c showed good inhibition effects against Xoo with the EC50 values ranging from 16.6 μg/mL to 65.7 μg/mL, which were better than those of commercial agricultural antibacterial bismerthiazol (92.6 μg/mL) and thiediazole copper (121.8 μg/mL). Meanwhile, compounds 7a, 7b, and 7c exerted good antifungal activities against fiveplant fungi, which were comparable tothatof HM. The results demonstrated that this kind of compounds can be further studied and developed as promising antifungal and antibacterial agents.
AcknowledgmentsWe acknowledge the financial support of the Key Technologies R & D Program (No. 2014BAD23B01), National Natural Science Foundation of China (No. 21372052), the Research Project of Chinese Ministry of Education (Nos. 213033A, 20135201110005), and Scientific Research Foundation for the Introduced Talents of Guizhou University (2015-34).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.06.055
| [1] | N. Kahriman, B. Yayli, A. Aktas, Z. Iske _fiyeli, F.S. Beris, N. Yayli, Synthesis, antibacterial and antioxidant activities of new 1-alkyl-4-(1-alkyl-4-oxo-1, 4-dihydroquinolin-2-yl) pyridinium bromides. Eur. J. Med. Chem. 69 (2013) 348–355. DOI:10.1016/j.ejmech.2013.08.050 |
| [2] | X. Zuo, N. Mi, Z.J. Fan, Q.X. Zheng, H.K. Zhang, H. Wang, Z.K. Yang, Synthesis of 4-methyl-1, 2, 3-thiadiazole derivatives via ugi reaction and their biological activities. J. Agric. Food Chem. 58 (2010) 2755–2762. DOI:10.1021/jf902863z |
| [3] | Q.Z. Zheng, X.M. Zhang, Y. Xu, K. Cheng, Q.C. Jiao, H.L. Zhu, Synthesis, biological evaluation, and molecular docking studies of 2-chloropyridine derivatives possessing 1, 3, 4-oxadiazole moiety as potential antitumor agents. Bioorg. Med. Chem. 18 (2010) 7836–7841. DOI:10.1016/j.bmc.2010.09.051 |
| [4] | M. Amir, H. Kumar, S.A. Khan, Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti-inflammatory and analgesic agents. Bioorg. Med. Chem. Lett. 18 (2008) 918–922. DOI:10.1016/j.bmcl.2007.12.043 |
| [5] | M.A. Ali, M. Shaharyar, A.A. Siddiqui, Synthesis, structural activity relationship and anti-tubercular activity of novel pyrazoline derivatives. Eur. J. Med. Chem. 42 (2007) 268–275. DOI:10.1016/j.ejmech.2006.08.004 |
| [6] | H.J. Park, K. Lee, S.J. Park, B. Ahn, J.C. Lee, H.Y. Cho, K.I. Lee, Identification of antitumor activity of pyrazole oxime ethers. Bioorg. Med. Chem. Lett. 15 (2005) 3307–3312. DOI:10.1016/j.bmcl.2005.03.082 |
| [7] | R. Sridhar, P.T. Perumal, S. Etti, G. Shanmugam, M.N. Ponnuswamy, V.R. Prabavathy, N. Mathivanan, Design, synthesis and anti-microbial activity of 1H-pyrazole carboxylates. Bioorg. Med. Chem. Lett. 14 (2004) 6035–6040. DOI:10.1016/j.bmcl.2004.09.066 |
| [8] | Z.B. Wu, D.Y. Hu, J.Q. Kuang, H. Cai, S.X. Wu, W. Xue, Synthesis and antifungal activity of N-(substituted pyridinyl)-1-methyl (phenyl)-3-(trifluoromethyl)-1H-pyrazole-4-carboxamide derivatives. Molecules 17 (2012) 14205–14218. DOI:10.3390/molecules171214205 |
| [9] | A. Tanitame, Y. Oyamada, K. Ofuji, M. Fujimoto, N. Iwai, Y. Hiyama, K. Suzuki, H. Ito, H. Terauchi, M. Kawasaki, K. Nagai, M. Wachi, J. Yamagishi, Synthesis and antibacterial activity of a novel series of potent DNA gyrase inhibitors. Pyrazole derivatives. J. Med. Chem. 47 (2004) 3693–3696. DOI:10.1021/jm030394f |
| [10] | X.L. Deng, J. Xie, Y.Q. Li, D.K. Yuan, X.P. Hu, L. Zhang, Q.M. Wang, M. Chi, X.L. Yang, Design, synthesis and biological activity of novel substituted pyrazole amide derivatives targeting EcR/USP receptor. Chin. Chem. Lett. 27 (2016) 566–570. DOI:10.1016/j.cclet.2016.02.009 |
| [11] | N. Nayak, J. Ramprasad, U. Dalimba, P. Yogeeswari, D. Sriram, Synthesis and antimycobacterial screening of new N-(4-(5-aryl-3-(5-methyl-1, 3, 4-oxadiazol-2-yl)-1H-pyrazol-1-yl) phenyl)-4-amide derivatives. Chin. Chem. Lett. 27 (2016) 365–369. DOI:10.1016/j.cclet.2016.01.015 |
| [12] | N.D. Vala, H.H. Jardosh, M.P. Patel, PS-TBD triggered general protocol for the synthesis of 4H-chromene, pyrano[4, 3-b]pyran and pyrano[3, 2-c]chromene derivatives of 1H-pyrazole and their biological activities. Chin. Chem. Lett. 27 (2016) 168–172. DOI:10.1016/j.cclet.2015.09.020 |
| [13] | S.L. Wang, Y.J. Shi, H.B. He, Y. Li, Y. Li, H. Dai, Synthesis and bioactivity of novel pyrazole oxime derivatives containing oxazole ring. Chin. Chem. Lett. 26 (2015) 672–674. DOI:10.1016/j.cclet.2015.04.017 |
| [14] | R. Zeun, G. Scalliet, M. Oostendorp, Biological activity of sedaxane a novel broad-spectrum fungicide for seed treatment. Pest Manag. Sci. 69 (2013) 527–534. DOI:10.1002/ps.3405 |
| [15] | L.H. Hand, H.J. Moreland, Surface water mineralization of isopyrazam according to OECD 309:observations on implementation of the new data requirement within agrochemical regulation. Environ. Toxicol. Chem. 33 (2014) 516–524. DOI:10.1002/etc.v33.3 |
| [16] | A. Gulkowska, I.J. Buerge, T. Poiger, Online solid phase extraction LC-MS/MS method for the analysis of succinate dehydrogenase inhibitor fungicides and its applicability to surface water samples. Anal. Bioanal. Chem. 406 (2014) 6419–6427. DOI:10.1007/s00216-014-8073-4 |
| [17] | S.S. Li, X.G. Liu, C. Chen, F.S. Dong, J. Xu, Y.Q. Zheng, Degradation of fluxapyroxad in soils and water/sediment systems under aerobic or anaerobic conditions. Bull. Environ. Contam. Toxicol. 95 (2015) 45–50. DOI:10.1007/s00128-015-1556-y |
| [18] | G.V.S. Kumar, Y.R. Prasad, S.M. Chandrashekar, Synthesis and pharmacological evaluation of some novel 4-isopropyl thiazole-based sulfonyl derivatives as potent antimicrobial and antitubercular agents. Med. Chem. Res. 22 (2013) 4239–4252. DOI:10.1007/s00044-012-0431-1 |
| [19] | M.A. Bhat, Synthesis and anti-mycobacterial activity of new 4-thiazolidinone and 1, 3, 4-oxadiazole derivatives of isoniazid. Acta Pol. Pharm. 71 (2014) 763–770. |
| [20] | D. Pal, R. Tripathi, D.D. Pandey, P. Mishra, Synthesis, characterization, antimicrobial, and pharmacological evaluation of some 2, 5-disubstituted sulfonyl amino 1, 3, 4-oxadiazole and 2-amino-disubstituted 1, 3, 4-thiadiazole derivatives. J. Adv. Pharm. Technol. Res. 5 (2014) 196–201. DOI:10.4103/2231-4040.143040 |
| [21] | M.M. Gamal El-Din, M.I. El-Gamal, M.S. Abdel-Maksoud, K.H. Yoo, C.H. Oh, Synthesis and in vitro antiproliferative activity of new 1, 3, 4-oxadiazole derivatives possessing sulfonamide moiety. Eur. J. Med. Chem. 90 (2015) 45–52. DOI:10.1016/j.ejmech.2014.11.011 |
| [22] | P. Li, L. Shi, X. Yang, L. Yang, X. Chen, F. Wu, Q. Shi, W.M. Xu, M. He, D.Y. Hu, B.A. Song, Design, synthesis, and antibacterial activity against rice bacterial leaf blight and leaf streak of 2, 5-substituted-1, 3, 4-oxadiazole/thiadiazole sulfone derivative. Bioorg. Med. Chem. Lett. 24 (2014) 1677–1680. DOI:10.1016/j.bmcl.2014.02.060 |
| [23] | P. Li, L. Shi, M. Gao, X. Yang, W. Xue, L.H. Jin, D.Y. Hu, B.A. Song, Antibacterial activities against rice bacterial leaf blight and tomato bacterial wilt of 2-mercapto-5-substituted-1, 3, 4-oxadiazole/thiadiazole derivatives. Bioorg. Med. Chem. Lett. 25 (2015) 481–484. DOI:10.1016/j.bmcl.2014.12.038 |
| [24] | W.M. Xu, S.Z. Li, M. He, S. Yang, X.Y. Li, P. Li, Synthesis and bioactivities of novel thioether/sulfone derivatives containing 1, 2, 3-thiadiazole and 1, 3, 4-oxadiazole/thiadiazole moiety. Bioorg. Med. Chem. Lett. 23 (2013) 5821–5824. DOI:10.1016/j.bmcl.2013.08.107 |
| [25] | P.Y. Wang, L. Zhou, J. Zhou, Z.B. Wu, W. Xue, B.A. Song, S. Yang, Synthesis and antibacterial activity of pyridinium-tailored 2, 5-substituted-1, 3, 4-oxadiazole thioether/sulfoxide/sulfone derivatives. Bioorg. Med. Chem. Lett. 26 (2016) 1214–1217. DOI:10.1016/j.bmcl.2016.01.029 |
| [26] | P.Y. Wang, M.N. Gao, L. Zhou, Z.B. Wu, D.Y. Hu, J. Hu, S. Yang, Synthesis and antibacterial activity of pyridinium-tailored aromatic amphiphiles. Bioorg. Med. Chem. Lett. 26 (2016) 1136–1139. DOI:10.1016/j.bmcl.2016.01.053 |
| [27] | X. Wang, J. Yin, L. Shi, G. Zhang, B.A. Song, Design, synthesis, and antibacterial activity of novel Schiff base derivatives of quinazolin-4(3H)-one. Eur. J. Med. Chem. 77 (2014) 65–74. DOI:10.1016/j.ejmech.2014.02.053 |
| [28] | T.K. Chattapadhyay, P. Dureja, Antifungal activity of 4-methyl-6-alkyl-2Hpyran-2-ones. J. Agric. Food Chem. 54 (2006) 2129–2133. DOI:10.1021/jf052792s |
 2017, Vol. 28
2017, Vol. 28